Abstract
Background
Cinnamic acid (CA) is a phytochemical originally derived from Cinnamomum cassia, a plant with numerous pharmacological properties. The intercalation of CA with a nanocarrier, zinc layered hydroxide, produces cinnamate-zinc layered hydroxide (ZCA), which has been previously characterized. Intercalation is expected to improve the solubility and cell specificity of CA. The nanocarrier will also protect CA from degradation and sustain its release. The aim of this study was to assess the effect of intercalation on the anti-inflammatory capacity of CA.
Methods
In this study, the anti-inflammatory activity of ZCA was investigated and compared with that of nonintercalated CA. Evaluations were based on the capacity of ZCA and CA to modulate the release of nitric oxide, prostaglandin E2, interleukin (IL)-6, tumor necrosis factor alpha (TNF-α), IL-1β, and IL-10 in lipopolysaccharide-induced RAW 264.7 cells. Additionally, the expression of proinflammatory enzymes, ie, cyclooxygenase-2, inducible nitric oxide synthase, and nuclear factor kappa B (NF-κB), were examined.
Results
Although both ZCA and CA downregulated nitric oxide, prostaglandin E2, tumor necrosis factor alpha, IL-1β, and IL-6, ZCA clearly displayed better activity. Similarly, expression of cyclooxygenase-2 and inducible nitric oxide synthase were inhibited in samples treated with ZCA and CA. The two compounds effectively inactivated the transcription factor NF-κB, but the anti-inflammatory cytokine, IL-10, was significantly upregulated by ZCA only.
Conclusion
The present findings suggest that ZCA possesses better anti-inflammatory potential than CA, while zinc layered hydroxide had little or no effect, and these results were comparable with the positive control.
Introduction
Inflammation is a beneficial host response to foreign attack or tissue injury that eventually leads to restoration of normal tissue structure and function.Citation1,Citation2 Acute inflammation is a controlled beneficial process, especially in response to infectious agents, but chronic inflammation is an obnoxious persistent phenomenon that may result in inflammatory disease.Citation3,Citation4 Detection of invaders through a wide range of foreign molecular patterns, which induce initiation of the protective innate immunity and inflammatory process, encompasses the first line of the body’s defense system. The major components of innate immunity are phagocytes (macrophages, neutrophils, and dendritic cells), complement system protein, and natural killer cells.Citation5,Citation6 Although the first immune cells to reach an area of attack are the neutrophils, macrophages mastermind the activation of the inflammatory reaction.Citation7,Citation8
Macrophages and other activated inflammatory cells secrete high amounts of prostaglandin PGE2 (PGE2), nitric oxide (NO), and cytokines, such as interleukin (IL)-6, tumor necrosis factor alpha (TNF-α), and IL-1β. Undoubtedly, host defense is complemented by these mediators, and their uncontrolled production can be a factor in the pathogenesis of many diseases, such as rheumatoid arthritis, atherosclerosis, sepsis, and pulmonary fibrosis.Citation9–Citation11 They may also induce cell and tissue damage.Citation12,Citation13 Since the production of these soluble factors is associated with macrophages, they are considered to play a critical role during inflammation by managing various immunopathological phenomena.Citation14,Citation15 Likewise, the presence of these mediators in the macrophages of inflammatory tissues with increased expression of their mRNAs, following exposure to an inflammatory stimulus, has been proven. Thus, inhibiting the production of these mediators remains a critical target in combating inflammatory diseases.Citation16,Citation17
Cinnamomum cassia, an evergreen plant of the Lauraceae family, has long been associated with the treatment of inflammatory disease, dyspepsia, blood circulation disturbances, and gastritis. Its extracts contain phenolics such as cinnamic alcohol, cinnamic aldehyde, coumarin, and cinnamic acid (CA).Citation18,Citation19 CA has recently demonstrated significant anti-inflammatory potential in both in vitro and in vivo experiments.Citation20 Apart from its antioxidant properties, CA has been found to reduce cell proliferation by 50% in melanoma, glioblastoma, and lung and prostate carcinoma cells.Citation21,Citation22 The compound also displayed antidiabetic activity in insulin-resistant FL83B cells.Citation23
Basically, the factors that determine the effect of a drug molecule are its natural therapeutic activity and its efficient delivery at the site of action.Citation24,Citation25 However, most conventional drugs or therapeutic agents suffer from nonspecificity of action, degradation of the drug before reaching the target site (low bioavailability or failure to traverse the blood–brain barrier), and poor solubility in the delivery medium. Drug intercalation with nanoparticles improves their efficacy by sustaining release and targeting the cell surface rising ligands connected with membrane disruption and endosomal uptake. They also permit drug release in the cell cytoplasm and shield the drug from enzymatic degradation.Citation26–Citation28 Use of inorganic nanoparticles in nanodrug development has gained considerable acceptance in recent times, due to their versatile features such as good biocompatibility, wide availability, rich surface functionality, and prospective capability of drug delivery.Citation29–Citation32
Intercalation of an active compound into inorganic nanoparticles such as zinc layered hydroxide (ZLH) has been successful due to the anionic exchange capacity of the nanocarrier. Apart from CA, many compounds of pharmacological importance have been successfully loaded into ZLH. These include gallic acid,Citation33,Citation34 nucleoside monophosphate, DNA,Citation35,Citation36 linoleic acid,Citation37 and sunscreen materials such as 4-amino benzoic acid.Citation38,Citation39 This study was designed to determine the effect of intercalation on the anti-inflammatory potential of CA by evaluating the production and expression of various inflammatory mediators, ie, NO, PGE2, TNF-α, IL-6, IL-1β, IL-10, cyclooxygenase-2 (COX-2), inducible NO synthase (iNOS), and nuclear factor kappa B (NF-κB), in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages.
Materials and methods
Materials
The RAW 264.7 cell line was sourced from the American Type Culture Collection (ATCC, Manassas, VA, USA), cultured and maintained in Dulbecco’s modified Eagle’s medium, and supplemented with 10% fetal bovine serum and 1% antibiotics. LPS and dexamethasone were sourced from Nacalai Tesque Inc (Tokyo, Japan). CA was supplied by Acros (Geel, Belgium), while ZLH and cinnamate-ZLH (ZCA) were synthesized and characterized in the Institute of Advanced Technology, Universiti Putra Malaysia.Citation39 PGE2 and an enzyme-linked immunosorbent assay kit were purchased from R&D Systems (Minneapolis, MN, USA). All Western blotting apparatus and reagents were from Bio-Rad (St Louis, MO, USA). The primary antibodies iNOS, COX-2, NF-κB, and appropriate secondary antibodies (goat anti-rabbit and goat anti-mouse) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Enhanced chemiluminescence substrates were manufactured by Nacalai Tesque Inc, while the polyvinylidene fluoride membrane was from Bio-Rad.
Synthesis of nanocomposite and characterization
ZLH and ZCA were synthesized and characterized in the Institute of Advance Technology, Universiti Putra Malaysia. ZLH was synthesized by a conventional coprecipitation method using aqueous solutions of zinc nitrate hexahydrate.Citation40 ZnO was used as the parent material in the synthesis of ZCA through a newly developed direct method.Citation39 Hydrolysis of ZnO formed a layer of Zn(OH)2, while the reaction of Zn ion species, hydroxyls, water, and cinnamate anions in the solution generated ZCA. Basal spacing of the nanocomposite obtained from power X-ray diffraction was 23.9Å following intercalation of cinnamate between the interlayer spaces of ZLH. Fourier transform infrared analysis showed that the nanocomposite possesses the absorption characteristics of both ZLH and pure CA. However, elemental analysis showed that 40.4% w/w of CA was contained in the nanohybrid, and the intercalated compound exhibited excellent ultraviolet A and B capacity. ZnO exhibited a slow absorption rate, reaching an optimal uptake at 8 cm3/g, but after intercalating CA into ZLH layers, the adsorption uptake reached 37 cm3/g. In addition, various types of medium were used to show slow and saturated release at low concentrations as evidence of retention of cinnamate in the ZLH interlayers. The resultant ZCA was found to be an effective and safe sunscreen agent.Citation39
Cell viability assay
Cell viability was determined by the ability of mitochondrial reductase to convert 3-(4,5-dimethythiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) (MTT) to formazan dye. Overnight culture of RAW 264.7 cells in 96-well plates was done at a density of 1.5×104 cells/100 μL in each well. This was followed by treating the cells with ZCA, CA, and ZLH for 24 hours, after which 10 μL of MTT at a concentration of 5 mg/mL was added to each well and kept in a 5% CO2 incubator for 4 hours at 37°C. The MTT solution was then removed and 100 μL of dimethyl sulfoxide was added to each well to lyse the cells. A Lambda 35 microplate reader (Perkin-Elmer, Boston, MA, USA) was used to measure absorbance at 570 nm.
Determination of NO production
RAW 264.7 cells were seeded in 24-well culture plates at a density of 2×105 cells/well in 1 mL of culture medium followed by overnight incubation. Confluent cells were treated with varying concentrations of ZCA, CA, and ZLH (2.5, 5 and 10 μg/mL) for one hour. The cells were then challenged with a 1 μg/mL concentration of LPS and incubated for a further 24 hours. Accumulated nitrite in the culture medium was measured as a representative of NO production using Griess reagent (0.1% N-1-[naphthyl] ethylenediamine dihydrochloride and 1% sulphanilamide and 5% H3PO4). Next, 100 μL of cell supernatant and an equal amount of Griess reagent were plated in each well of a 96-well plate. They were incubated for 10 minutes followed by absorbance reading at 550 nm.
Determination of cytokine production
The inhibitory effect of ZCA on the production of IL-6, IL-1β, TNF-α, and IL-10 were determined by enzyme-linked immunosorbent assay using medium collected from the treated cells. RAW 264.7 cells were seeded into a six-well plate at a density of 2×105 cells/well and incubated overnight. The cells were then treated with ZCA, CA, and ZLH (2.5, 5 and 10 μg/mL) for one hour. This was followed with addition of 1 μg/mL LPS for 24 hours to induce inflammation. Culture supernatants were assayed according to the protocol of the enzyme-linked immunosorbent assay kit (R&D Systems) to measure the amount of IL-6, IL-1β, TNF-α, and IL-10 produced in each sample. The experiment was carried out in triplicate.
Determination of PGE2 level
RAW 264.7 cells plated at a density of 2×105 in six-well plates were incubated overnight. Confluent cells were then treated with ZCA, CA, or ZLH at concentrations of 2.5, 5, and 10 μg/mL for one hour, followed by induction of inflammation with LPS for another 24 hours. The concentration of PGE2 was determined using a PGE2 immunoassay kit (R&D Systems) according to the manufacturer’s guidelines.
Western blot analysis
Confluent RAW 264.7 cells were treated with ZCA, CA, or ZLH (2.5, 5, and 10 μg/mL) for one hour and then challenged with LPS 1 μg/mL for 24 hours. Thereafter, the cells were scraped and centrifuged at 1,200 rpm for 10 minutes at 4°C. The supernatant was discarded, and phosphate-buffered saline was added followed by centrifugation at 1,200 rpm for 10 minutes at 4°C. Ice-cold cell lysis buffer were added to the cells for 30 minutes with intermittent shaking. Finally, the cells were centrifuged at 14,000 rpm for 30 minutes at 4°C. Protein concentrations in the samples were estimated using a bicinchoninic acid protein assay kit with bovine serum albumin as the standard. Total proteins (20 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 10% polyacrylamide gel and transferred to a polyvinylidene fluoride membrane. Blocking was done with 5% skimmed milk in PBST (1% v/v Tween-20 in phosphate-buffered saline, pH 7.2) for one hour. The membranes were thereafter incubated with anti-mouse iNOS, anti-mouse NF-κB, and anti-mouse COX-2 (Santa Cruz Biotechnology) in a 1:1,000 concentration at 4°C overnight. The membranes were then washed six times in PBST at regular intervals for one hour. This was followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse, immunoglobulin G secondary antibodies (Santa Cruz Biotechnology) at a concentration of 1:2,000 for one hour. Membranes were finally washed six times in PBST for 10 minutes each. Chemiluminescence substrate (Thermo Scientific, Rockford, IL, USA) was used to reveal the blots detected with Chemidoc™ XRS (Bio-Rad). Intensity of the blots was finally analyzed using Bio-Rad Lab image software.
Statistical analysis
The statistical significance of differences between the various experimental values and control values was determined using the Student’s t-test. The data are expressed as the mean ± standard deviation, and the results were selected from at least three independent experiments performed in triplicate. P-values of 0.05 or less were considered to be statistically significant.
Results
Effect of ZCA, CA, and ZLH on cell viability
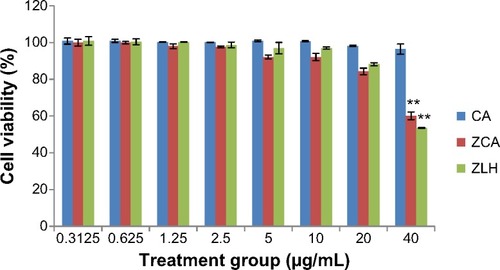
The cytotoxicity potential of compounds, ie, CA intercalated with ZLH (ZCA) which is the nanocomposite of interest, CA and the nanocarrier system, ZLH, were tested in RAW 264.7 cells. As shown in , incubation of cells with ZCA showed a 40% reduction in cell viability at 40 μg/mL, but cell viability was above 80% when ZCA 20 μg/mL was incubated with confluent cells. While coincu-bation of cells with ZLH 40 μg/mL yielded 53% cell viability, other concentrations showed more than 80% cell viability. However, CA did not display any toxicity even at 40 μg/mL. Nevertheless, the three concentrations (2.5, 5.0, and 10.0 μg/mL) were chosen for subsequent assays and analysis.
Figure 1 Viability of RAW 264.7 cells after 24 hours of treatment with ZCA, CA, and ZLH.
Abbreviations: CA, cinnamic acid; ZCA, cinnamate-zinc layered hydroxide; ZLH, zinc layered hydroxide.
Effects of ZCA on LPS-induced NO production
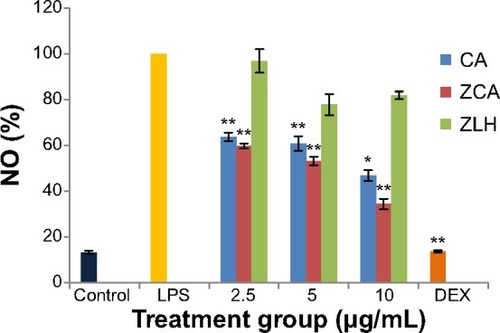
The inhibitory effect of ZCA on the production of NO was investigated using LPS-activated RAW 264.7 macrophages. Inhibition of NO by ZCA occurred in a dose-dependent manner (47.66%, 52.52%, and 65.12% at 2.5, 5, and 10 μg/mL, respectively, as shown in ). The activity displayed by CA was lower than that of ZCA when the compound was incubated with LPS-stimulated RAW 264.7 macrophages. CA achieved 36.31%, 39.198%, and 53.27% inhibition of NO production at 2.5, 5, and 10 μg/mL, respectively. ZLH did not show any significant inhibitory activity. However, dexamethasone, which served as the positive control, was found to inhibit the production of NO by 86.24%.
Figure 2 Inhibitory effects of ZCA on LPS-induced NO production in RAW 264.7 macrophages.
Abbreviations: CA, cinnamic acid; LPS, lipopolysaccharide; NO, nitric oxide; ZCA, cinnamate-zinc layered hydroxide; ZLH, zinc layered hydroxide; DEX, dexamethasone.
Effect of ZCA on PGE2 biosynthesis
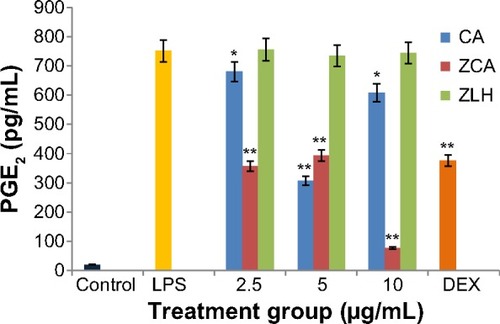
The downregulatory activity of ZCA on production of the proinflammatory marker PGE2 was investigated using LPS-activated RAW 264.7 cells. Enzyme-linked immunosorbent assay results showed a significant increase in levels of PGE2 in the culture supernatants of RAW 264.7 cells stimulated with LPS (7,611.62 pg/mL) compared with nonstimulated cells (197.65 pg/mL). Addition of ZCA to the mixture significantly inhibited the production of PGE2 at different concentrations, as shown in . The activity of ZCA in samples treated with 10.0 μg/mL was the highest, ie, 89.98% (762.34 pg/mL) inhibition (P<0.01), while inhibition was 53.14% (3,566.70 pg/mL) at 5 μg/mL and 48.35% (3,931.35 pg/mL) at 2.5 μg/mL. CA showed comparatively lower effects, ie, 10.65% (6,800.70 pg/mL), 20.16% (6,077.59 pg/mL), and 57.73% (3,069.84 pg/mL) inhibition at 2.5, 5.0, and 10.0 μg/mL, respectively. ZLH exhibited the lowest inhibitory activity, ie, 0.78% (7,551.19 pg/mL), 3.50% (7,345.56 pg/mL), and 2.27% (7,438.53 pg/mL). The inhibitory effect of the positive control drug, dexamethasone, was 49.94% (3,760.05 pg/mL).
Figure 3 Inhibitory effect of ZCA on LPS-induced production of PGE2 in RAW 264.7 macrophages.
Abbreviations: CA, cinnamic acid; DEX, dexamethasone; LPS, lipopolysaccharide; PGE2, prostaglandin E2; ZCA, cinnamate-zinc layered hydroxide; ZLH, zinc layered hydroxide.
Effect of ZCA on LPS-induced production of proinflammatory cytokines
The concentrations of IL-6, TNF-α, and IL-1β (suggestive of proinflammatory cytokine activity) and IL-10 (suggestive of anti-inflammatory activity) in the treated RAW 264.7 cells are shown in , respectively. The results indicate that concurrent ZCA or CA treatment with LPS-stimulated cells significantly inhibited levels of IL-6, TNF-α, and IL-1β. Although production of IL-6 was reduced by both ZCA and CA in a similar manner, the inhibitory activity of ZCA in the synthesis of TNF-α was comparatively higher. While CA exhibited 15.60%, 15.83%, and 19.96% inhibition at 2.5, 5 and 10 μg/mL, respectively, ZCA inhibited TNF-α production by more than 40% in all treatments. The highest augmentation of IL-1β inhibition was observed in samples treated with ZCA at a concentration of 10 μg/mL. Although inhibitory activity of ZLH was not significant when compared with nanocomposite, ZCA and free drug CA.
Figure 4 (A–D) Effect of ZCA on IL-6, TNF-α, IL-1β, and IL-10 production in LPS stimulated RAW 264.7 macrophages.
Abbreviations: CA, cinnamic acid; DEX, dexamethasone; IL, interleukin; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor alpha; ZCA, cinnamate-zinc layered hydroxide; ZLH, zinc layered hydroxide.
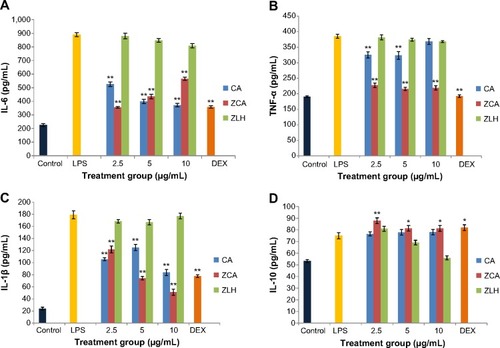
In the case of anti-inflammatory cytokine activity, ZCA treatment potentiated a significant rise in levels of IL-10 in RAW 264.7 macrophages. In comparison with LPS-stimulated groups, ZCA treated samples were highly significant (P<0.01), with 17.17% upregulation at 10 μg/mL, while its activity at 2.5 and 5 μg/mL was less significant (P<0.05), with upregulation of 8.31% and 8.43%, respectively. Although CA upregulated the IL-10 cytokine at all three concentrations applied, the effect of CA was not significant when compared with samples stimulated with LPS alone. In contrast, treatment with ZLH did not improve production of IL-10.
Effects of ZCA on LPS-induced COX-2, iNOS and NF-κB expression
The effect of ZCA on the protein expression of iNOS, COX-2, and NF-κB was investigated using LPS-induced RAW 264.7 macrophages. From the Western blotting data shown in , nonstimulated RAW 264.7 cells lacked iNOS activity. When the cells were induced with LPS, some level of iNOS expression was observed. On the other hand, expression of iNOS protein was significantly reduced by ZCA and CA when incubated with LPS-stimulated RAW 264.7 cells.
Figure 5 Inhibitory effect of ZCA on LPS-induced iNOS, COX-2, and NF-κB expression in LPS stimulated RAW 264.7 macrophages.
Abbreviations: CA, cinnamic acid; Cont, control; COX-2, cyclooxygenase-2; DEX, dexamethasone; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; NF-κB, nuclear factor kappa B; ZCA, cinnamate-zinc layered hydroxide; ZLH, zinc layered hydroxide.
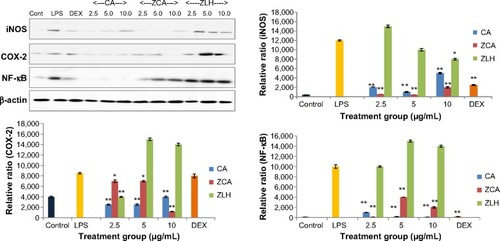
Additionally, while COX-2 was hardly detected in non-stimulated cells, high expression of the enzyme was observed on Western blotting analysis in samples treated with ZLH at 5.0 and 10.0 μg/mL (). Both ZCA and CA exhibited low expression of COX-2 in contrast with the expression observed in samples treated with LPS alone. RAW 264.7 cells incubated with LPS alone showed a high intensity of NF-κB, while samples pretreated with ZCA and CA before LPS stimulation showed downregulated expression of NF-κB. Although CA inhibited NF-κB to a better extent than the intercalated compound, the activities of the two compounds were highly significant (P<0.01), as shown in the analysis. Again, high expression of NF-κB was observed in samples treated with the nanocarrier, ZLH. Nevertheless, dexamethasone inhibited NF-κB in a manner comparable with that of CA.
Discussion
The role of macrophages in many inflammatory processes highlights their importance in both innate and adaptive immunity.Citation41,Citation42 Macrophages are associated with many disease conditions, such as inflammation, infection, atherosclerosis, lupus, cancer, and diabetes.Citation43 In this study, the RAW 264.7 macrophage was selected to evaluate the potential of CA-ZLH intercalation to improve the anti-inflammatory properties of CA. Some recent studies have reported the anti-inflammatory activity of CA.Citation44 However, its effect on the production of NO, PGE2, and cytokines in LPS-stimulated RAW 264.7 cells is yet to be reported. Apart from a report on the potential of ZCA as a sunscreen agent with an ultraviolet protection effect,Citation39 there is no report on the anti-inflammatory activity of the nanocomposite (ZCA) in LPS-induced inflammation.
The LPS endotoxin triggers an extensive injury to the macrophage. Apart from a reduced capacity to produce antigen, synthesis of important mediators, such as reactive oxygen species, free radicals, cytokines, and bioactive lipids, are altered.Citation45–Citation47 In recent times, treatment of inflammatory disorders has been largely based on inhibiting the action or synthesis of important mediators that prompt the host’s response to attack. Many therapeutic agents, such as nonsteroidal anti-inflammatory drugs, steroids, and histamine, were developed based on this strategy. Data acquired from the assays undertaken in this research confirm that LPS endotoxin activates significant production of NO, PGE2, and inflammatory cytokines (IL-6, TNF-α, IL-1β), in RAW 264.7 cells.
However, the data reported in the previous sections show the enhanced anti-inflammatory potential of the ZCA nanocomposite when compared with CA. Synthesis of ZCA involved loading of only 40.4% of CA between the ZLH interlayers, but ZCA reduced the production of inflammatory markers in a manner comparable with that of the positive control. Samples treated with ZCA at all concentrations showed low levels of NO, providing clear evidence that the intercalation enhanced the anti-inflammatory activity of CA. The inhibitory effect of ZCA on production of NO may be associated with downregulation of iNOS expression by the same compound (). NO production is strongly linked with the continuous synthesis of iNOS, which breaks down arginine to NO and citrulline. Increased NO production is observed in various diseases, and NO synthase inhibitors have successfully reversed many classic signs and symptoms of inflammation.Citation48,Citation49 Mulligan et al, using experimental rats with immune complex-induced lung injury, found that inhibitors of NO synthesis have protective properties in this model.Citation50 In contrast, L-arginine, which is a precursor of NO, complicates tissue injury. Similarly, the effect of inflammatory bowel disease was reduced in experimental animals with induced colitis by administration of two different NO synthase inhibitors, ie, N-nitro-L-arginine methyl ester and aminoguanidine.Citation48,Citation49 Therefore, any compound that is capable of inhibiting iNOS, like ZCA, may be a potential anti-inflammatory agent.
Further, the effect of ZCA in inhibiting production of PGE2 was not only significant at the lowest concentration but comparatively better than CA and even dexamethasone (). COX-2 is a key mediator in the inflammatory process, and is responsible for production of PGE2 from arachidonic acid. Downregulation of COX-2 is therefore a condition for the inhibition of PGE2, which is expressed in all processes that lead to major signs of inflammation, ie, swelling, redness, and pain.Citation51,Citation52 Hence, in and there is a strong relationship between the activity of ZCA, where the expression of COX-2 was significantly inhibited by the nanocomposite. Administration of N-398, a COX-2 inhibitor, after treatment with carrageenan lowered the production of PGE2 and the same pattern was observed in COX-2 knockout mice. Deficiency of COX-2 was also observed to reduce PGE2 by 75%.Citation53,Citation54 The COX-2/PGE2 pathway is the major target for designing pain-relieving drugs for osteoarthritis, but most nonsteroidal anti-inflammatory drugs inhibit the activity of COX-1/COX-2, which may result in unwanted side effects. Limited side effects are some of the advantages of drugs such as celecoxib that exclusively target COX-2.Citation55–Citation57 ZCA is a potential anti-inflammatory agent that can selectively block the synthesis of the inducible enzyme, COX-2.
The inhibitory effect of ZCA on the secretion of TNF-α in and a similar downregulation of IL-1β in (5.0 μg/mL and 10.0 μg/mL) were better than with CA. The carrier system did not have any inhibitory effects on these cytokines, which dispels any possibility of ZLH contributing to the anti-inflammatory activity of ZCA. Proinflammatory cytokines like TNF-α, IL-1β, and IL-6 are small molecules that modulate inflammation and immunity. Bacterial LPS activates macrophages to secrete TNF-α, and the released TNF-α or LPS then triggers production of IL-6 and IL-1β.Citation58,Citation59 TNF-α induces many physiological changes, including inflammation, septic shock, and cytotoxicity.Citation60 However, anti-TNF therapy triggers a rapid improvement in many serological parameters and histological characteristics of the synovium.Citation61–Citation63 Blockade of TNF-α activity has also been linked with improved survival in animal models of sepsis and shock.Citation64–Citation66
The inhibition of TNF-α and other cytokines may be connected with low expression of NF-κB in samples treated with ZCA and CA. as shown in . NF-κB is a key transcription factor that coordinates the expression of proinflammatory cytokines and enzymes.Citation7,Citation67 Treatment with anti-TNF-α antibody after LPS activation inhibited TNF-α and NF-κB in an in vivo animal model; moreover, the kinetics of the NF-κB activation and TNF-α secretion triggered by LPS in this model were similar.Citation68,Citation69 This shows that any treatment that affects the expression of NF-κB is likely to influence the production of proinflammatory cytokines. Agents that block proximal cytokines such as TNF-α and IL-1β also restrict activation of NF-κB, thereby inhibiting the inflammatory cascade.Citation70,Citation71
It has been established that IL-10 influences the inflammatory process by suppressing inflammatory mediators, including proinflammatory cytokines, adhesion molecules, and antigen-presenting molecules in neutrophils, monocytes/ macrophages, and T-cells.Citation72,Citation73 IL-10 blocks the expression of proinflammatory cytokines such as IL-6, TNF-α, and IL-1β in activated macrophages. In specific terms, it upregulates other endogenous anticytokines and inhibits proinflammatory cytokine receptors.Citation59,Citation74 Therefore, it can counter-regulate the release and roles of proinflammatory cytokines at multiple levels. Given that inflammatory proteins are transcriptionally regulated by NF-κB, it has been proposed that IL-10 may express a major part of its anti-inflammatory activity by inhibiting the transcription factor.Citation75–Citation77 As a result, upregulation of IL-10 may be associated with inhibition of NF-κB by ZCA and CA.
NF-κB is known for its unique role in coordinating the immune and inflammatory responses.Citation78 They are identifiable with the homodimer or heterodimer structure of the Rel proteins. In resting cells, NF-κB forms an inactive complex with inhibitory κB (IκB) proteins like IκBα, IκBβ, and IκBe.Citation79 Upon recognition of LPS by toll-like receptor-4 and MD2, its accessory protein attached to immune cells and activates the IκB kinase (IκK) complex, which phosphorylates cytoplasmic IκBs.Citation80,Citation81 Subsequently, ubiquitin-mediated degradation of IκBs occurs, and NF-κB is liberated and moves into the nucleus. The binding of NF-κB to the κB sequences regulates the transcription of immune and inflammatory genes such as COX-2, iNOS, and inflammatory cytokines (IL-6, IL-1β, TNF-α, IL-10).Citation82 Since the expression levels of these proinflammatory mediators are regulated by NF-κB, our findings suggest that their production is transcription-ally inhibited by ZCA by blocking of the NF-κB signaling pathway.
Finally, findings from ELISA and western blot analysis revealed that ZLH exhibited a slight upregulation of inflammatory enzymes and NF-κB activation in LPS stimulated RAW cells. Although, ZLH has been found to have a low toxicity profile, studies have shown that some nanomaterials have the potential to stimulate inflammatory reactions. Notably, ZnO, which can serve as precursor for ZLH, has been reported to cause marginal production of IL-6 and IL-8 in HK-2 and HEK 293 cells. Release of ions by ZnO materials has been proposed as the likely mechanism of cytotoxicity.Citation39,Citation83 Studies have also shown that nanoparticles generate reactive oxygen species through interaction with the plasma membrane, but the cell has a self-repair mechanism to maintain its integrity, and overexpression of inflammatory mediators may not be associated with production of reactive oxygen species.Citation84 The increased level of inflammatory mediators beyond the negative control thresholds may be due to a synergistic action between LPS and ZLH. Nonetheless, ZLH on its own was not toxic at the doses applied.
Conclusion
The present study is an example of fundamental research towards developing ZCA as a strong anti-inflammatory nanomedicine. Our results suggest that ZCA has better inhibitory activity for LPS-stimulated proinflammatory mediators than that of the free drug, CA. Therefore, ZCA possess therapeutic potential for the regulation and modulation of macrophage activation, and may serve as effective alternative treatment for a number of inflammation-mediated diseases.
Acknowledgments
This research was supported by a grant from the Research Universiti Grant Scheme (GP/IPB/2013/9425802), Universiti Putra Malaysia, Selangor, Malaysia.
Disclosure
The authors report no conflicts of interest in this work.
References
- Cook-MillsJMDeemTLActive participation of endothelial cells in inflammationJ Leukoc Biol200577448749515629883
- KvietysPRGrangerDNRole of reactive oxygen and nitrogen species in the vascular responses to inflammationFree Radic Biol Med201252355659222154653
- KaplanskiGMarinVMontero-JulianFMantovaniAFarnarierCIL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammationTrends Immunol2003241252912495721
- LippitzBECytokine patterns in patients with cancer: a systematic reviewLancet Oncol2013146e218e22823639322
- MedzhitovRJanewayCJrInnate immunityN Engl J Med2000343533834410922424
- ElinavEStrowigTKauALNLRP6 inflammasome regulates colonic microbial ecology and risk for colitisCell2011145574575721565393
- AderemAUlevitchRJToll-like receptors in the induction of the innate immune responseNature2000406679778278710963608
- van EdenWSpieringRBroereFvan der ZeeRA case of mistaken identity: HSPs are no DAMPs but DAMPERsCell Stress Chaperones201217328129222139593
- GuzikTJKorbutRAdamek-GuzikTNitric oxide and superoxide in inflammationJ Physiol Pharmacol200354446948714726604
- LathamKAWhittingtonKBZhouRQianZRosloniecEFEx vivo characterization of the autoimmune T-cell response in the HLA-DR1 mouse model of collagen-induced arthritis reveals long-term activation of type II collagen-specific cells and their presence in arthritic jointsJ Immunol200517473978398515778354
- CaudrillierAKessenbrockKGillissBMPlatelets induce neutrophil extracellular traps in transfusion-related acute lung injuryJ Clin Invest201212272661267122684106
- KasamaTMiwaYIsozakiTOdaiTAdachiMKunkelSLNeutrophil-derived cytokines: potential therapeutic targets in inflammationCurr Drug Targets Inflamm Allergy20054327327916101533
- ZawrotniakMRapala-KozikMNeutrophil extracellular traps (NETs) – formation and implicationsActa Biochim Pol201360327728423819131
- ZeilhoferHUBruneKAnalgesic strategies beyond the inhibition of cyclooxygenasesTrends Pharmacol Sci200627946747416876882
- HwangSHWeckslerATWagnerKHammockBDRationally designed multitarget agents against inflammation and painCurr Med Chem201320131783179923410172
- HeoSJYoonWJKimKNEvaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophagesFood Chem Toxicol2010488–92045205120457205
- KimIDHaBJPaeoniflorin protects RAW 264.7 macrophages from LPS-induced cytotoxicity and genotoxicityToxicol In Vitro20092361014101919540912
- HeZDQiaoCFHanQBAuthentication and quantitative analysis on the chemical profile of cassia bark (cortex cinnamomi) by high-pressure liquid chromatographyJ Agric Food Chem20055372424242815796573
- SungYYYoonTJangJYParkSJJeongGHKimHKInhibitory effects of Cinnamomum cassia extract on atopic dermatitis-like skin lesions induced by mite antigen in NC/Nga miceJ Ethnopharmacol2011133262162821035532
- LiaoJCDengJSChiuCSAnti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivoEvid Based Complement Alternat Med2012201242932022536283
- LiuLHudginsWRShackSYinMQSamidDCinnamic acid: a natural product with potential use in cancer interventionInt J Cancer19956233453507628877
- SinghTSMitraSInteraction of cinnamic acid derivatives with serum albumins: a fluorescence spectroscopic studySpectrochim Acta A Mol Biomol Spectrosc201178394294821247795
- HuangD-WSzu-ChuanSCaffeic acid and cinnamic acid ameliorate glucose metabolism via modulating glycogenesis and gluco-neogenesis in insulin-resistant mouse hepatocytesJ Funct Foods201241358366
- SlowingIIVivero-EscotoJLWuCWLinVSMesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriersAdv Drug Deliv Rev200860111278128818514969
- ColillaMGonzálezBVallet-RegíMMesoporous silica nanoparticles for the design of smart delivery nanodevicesBiomater Sci201312114134
- MaedaHWuJSawaTMatsumuraYHoriKTumor vascular permeability and the EPR effect in macromolecular therapeutics: a reviewJ Control Release2000651–227128410699287
- FarokhzadOCLangerRImpact of nanotechnology on drug deliveryACS Nano200931162019206243
- KamalyNXiaoZValenciaPMRadovic-MorenoAFFarokhzadOCTargeted polymeric therapeutic nanoparticles: design, development and clinical translationChem Soc Rev20124172971301022388185
- BarbeCBartlettJKongLSilica particles: a novel drug–delivery systemAdv Mater2004162119591966
- BauerLABirenbaumNSMeyerGJBiological applications of high aspect ratio nanoparticlesJ Mater Chem2004144517526
- OzkanMQuantum dots and other nanoparticles: what can they offer to drug discovery?Drug Discov Today20049241065107115582795
- YangPGaiSLinJFunctionalized mesoporous silica materials for controlled drug deliveryChem Soc Rev20124193679369822441299
- HusseinMZGhotbiMYYahayaAHRahmanMZASynthesis and characterization of (zinc-layered-gallate) nanohybrid using structural memory effectMater Chem Phys20091131491496
- BarahuieFHusseinMZFakuraziSZainalZDevelopment of drug delivery systems based on layered hydroxides for nanomedicineInt J Mol Sci20141557750778624802876
- ChoyJHKwakSYParkJSJeongYJPortierJIntercalative nano-hybrids of nucleoside monophosphates and DNA in layered metal hydroxideJ Am Chem Soc1999121613991400
- Hussein Al AliSHAl-QubaisiMHusseinMZIsmailMZainalZHakimMNComparative study of Mg/Al-and Zn/Al-layered double hydroxide-perindopril erbumine nanocomposites for inhibition of angio-tensin-converting enzymeInt J Nanomedicine201274251426222904631
- ChoyJHShinJLimSYOhJMOhMHOhSCharacterization and stability analysis of zinc oxide nanoencapsulated conjugated linoleic acidJ Food Sci2010756N63N6820722942
- CursinoACGardolinskiJEWypychFIntercalation of anionic organic ultraviolet ray absorbers into layered zinc hydroxide nitrateJ Colloid Interface Sci20103471495520378120
- MohsinSMHusseinMZSarijoSHFakuraziSArulselvanPHinTYSynthesis of (cinnamate-zinc layered hydroxide) intercalation compound for sunscreen applicationChem Cent J2013712623383738
- SaifullahBArulselvanPEl ZowalatyMEDevelopment of a highly biocompatible antituberculosis nanodelivery formulation based on para-aminosalicylic acid-zinc layered hydroxide nanocompositesScientific World Journal2014201440146025050392
- ChouJJLiSKleeCBBaxASolution structure of Ca(2+)-calmodulin reveals flexible hand-like properties of its domainsNat Struct Biol200181199099711685248
- SunYXMinthonLWallmarkAWarkentinSBlennowKJanciauskieneSInflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s diseaseDement Geriatr Cogn Disord200316313614412826739
- HagemannTBiswasSKLawrenceTSicaALewisCERegulation of macrophage function in tumors: the multifaceted role of NF-κBBlood2009113143139314619171876
- LiaoJCDengJSChiuCSAnti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivoEvid Based Complement Alternat Med2012201242932022536283
- ChouTCFuEShenECChitosan inhibits prostaglandin E2 formation and cyclooxygenase-2 induction in lipopolysaccharide-treated RAW 264.7 macrophagesBiochem Biophys Res Commun2003308240340712901883
- HusseinHHussainHSalbiahMSMuhainiOLowYLBehPKUse of drugs for rheumatological and bone disordersFaridahAMSivasampuSLianLMHazimahHKokLCChinniahRJMalaysian Statistics on Medicines 2007Kuala Lumpur, MalaysiaMinistry of Health Malaysia2010
- KimIDHaBJPaeoniflorin protects RAW 264.7 macrophages from LPS-induced cytotoxicity and genotoxicityToxicol In Vitro20092361014101919540912
- PilichosCJKouerinisIAZografosGCThe effect of nitric oxide synthases inhibitors on inflammatory bowel disease in a rat modelIn Vivo200418451351615369194
- YangYTomkovichSJobinCCould a swimming creature inform us on intestinal diseases? Lessons from zebrafishInflamm Bowel Dis201420595696624577115
- MulliganMSHevelJMMarlettaMAWardPATissue injury caused by deposition of immune complexes is L-arginine dependentProc Natl Acad Sci USA19918814633863421648737
- FunkCDProstaglandins and leukotrienes: advances in eicosanoid biologyScience200129455481871187511729303
- LeeKWBodeAMDongZMolecular targets of phytochemicals for cancer preventionNat Rev Cancer201111321121821326325
- LangenbachRLoftinCLeeCTianoHCyclooxygenase knockout mice: models for elucidating isoform-specific functionsBiochem Pharmacol19995881237124610487525
- RicciottiEFitzGeraldGAProstaglandins and inflammationArterioscler Thromb Vasc Biol2011315986100021508345
- MitchellJAAkarasereenontPThiemermannCFlowerRJVaneJRSelectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenaseProc Natl Acad Sci U S A1993902411693116978265610
- JeffreyJEAspdenRMCyclooxygenase inhibition lowers prostaglan-din E2 release from articular cartilage and reduces apoptosis but not proteoglycan degradation following an impact load in vitroArthritis Res Ther200796R12918096078
- MangalDUbohCEJiangZSomaLRInterleukin-1β inhibits synthesis of 5-lipooxygenase in lipopolysaccharide-stimulated equine whole bloodProstaglandins Other Lipid Mediat201410892224530239
- BeutlerBCeramiAThe common mediator of shock, cachexia, and tumor necrosisAdv Immunol1988422132312834923
- ZhangJ-MAnJCytokines, inflammation and painInt Anesthesiol Clin2007452273717426506
- DoufasAGTianLPadrezKAExperimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apneaPLoS One201381e5480723382975
- CamussiGLupiaEThe future role of anti-tumour necrosis factor (TNF) products in the treatment of rheumatoid arthritisDrugs19985556136209585859
- SinghJSuruchiAAntiTNF-α strategy: present status of this therapeutic paradigmIndian J Pharmacol20043611014
- AppelEWallachDChimeric proteins, their preparation and pharmaceutical compositions containing themUS Patent8,092,806 issued1102012
- AppoloniODupontEVandercruysMAndriensMDuchateauJVincentJLAssociation of tumor necrosis factor-2 allele with plasma tumor necrosis factor-alpha levels and mortality from septic shockAm J Med2001110648648811331061
- VincentJLAfelimomabInt J Clin Pract200054319019310829362
- ZídekZAnzenbacherPKmoníckováECurrent status and challenges of cytokine pharmacologyBr J Pharmacol2009157334236119371342
- TheoharidesTCAlysandratosKDAngelidouAMast cells and inflammationBiochim Biophys Acta201218221213321185371
- LuoJLMaedaSHsuLCYagitaHKarinMInhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regressionCancer Cell20046329730515380520
- RuffellBAffaraNICoussensLMDifferential macrophage programming in the tumor microenvironmentTrends Immunol201233311912622277903
- TakPPFiresteinGSNF-κB: a key role in inflammatory diseasesJ Clin Invest2001107171111134171
- DiDonatoJAMercurioFKarinMNF-κB and the link between inflammation and cancerImmunol Rev2012246137940022435567
- MooreKWde Waal MalefytRCoffmanRLO’GarraAInterleukin-10 and the interleukin-10 receptorAnnu Rev Immunol20011968376511244051
- PaluckaKBanchereauJCancer immunotherapy via dendritic cellsNat Rev Cancer201212426527722437871
- KiguchiNKobayashiYKishiokaSChemokines and cytokines in neuroinflammation leading to neuropathic painCurr Opin Pharmacol2012121556122019566
- SchotteliusAJMayoMWSartorRBBaldwinASJrInterleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA bindingJ Biol Chem199927445318683187410542212
- HoentjenFSartorRBOzakiMJobinCSTAT3 regulates NF-κB recruitment to the IL-12p40 promoter in dendritic cellsBlood2005105268969615251981
- OsbornOOlefskyJMThe cellular and signaling networks linking the immune system and metabolism in diseaseNat Med201218336337422395709
- O’SullivanBThompsonAThomasRNF-κB as a therapeutic target in autoimmune diseaseExpert Opin Ther Targets200711211112217227228
- BaeuerlePABaltimoreDI kappa B: a specific inhibitor of the NF-kappa B transcription factorScience198824248785405463140380
- ZandiEChenYKarinMDirect phosphorylation of IκB by IKKα and IKKβ: discrimination between free and NF-κB-bound substrateScience19982815381136013639721103
- NagaiYAkashiSNagafukuMEssential role of MD-2 in LPS responsiveness and TLR4 distributionNat Immunol20023766767212055629
- TianBBrasierARIdentification of a nuclear factor kappa B-dependent gene networkRecent Prog Horm Res2003589513012795416
- KermanizadehAVranicSBolandSAn in vitro assessment of panel of engineered nanomaterials using a human renal cell line: cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicityBMC Nephrol2013149623617532
- FröhlichEThe role of surface charge in cellular uptake and cytotoxicity of medical nanoparticlesInt J Nanomedicine20127557723144561
