Abstract
Aim
There is scarce information concerning the pharmacodynamic behavior of topical substances used in the physiotherapy setting. The aim of the present study was to estimate the formation and emptying of the diclofenac (DF) skin reservoir after passive, semiocclusive, and electrically assisted applications of DF.
Subjects and methods
Five different groups of healthy volunteers (ntotal=60, 23 male and 37 female), participated in this study. A 1% DF (Voltaren Emulgel) formulation (12 mg) was applied on the volar forearms on randomized defined circular skin areas of 7 cm2. DF was applied for 20 minutes under three different conditions at the same time. The presence of DF in the skin results in a reduction of the methyl nicotinate (MN) response. To estimate the bioavailability of DF in the skin, MN responses at different times following initial DF application (1.5, 6, 24, 32, 48, 72, 96, and 120 hours) were analyzed.
Results
At 1.5 hours after the initial DF application, a significant decrease in MN response was detected for the occluded and iontophoretic delivery. Passive application resulted in a decrease of the MN response from 6 hours post-DF application. The inhibition remained up to 32 hours post-DF application for the iontophoretic delivery, 48 hours for the occluded application, and 72 hours for the passive delivery. At 96 and 120 hours post-DF application none of the MN responses was inhibited.
Conclusion
The formation and emptying of a DF skin reservoir was found to be dependent on the DF-application mode. Penetration-enhanced delivery resulted in a faster emptying of the reservoir.
Introduction
Transdermal delivery of nonsteroidal anti-inflammatory drugs is a topical treatment routinely used in physiotherapy to reduce pain and inflammation in musculoskeletal disorders.Citation1–Citation4 In order to weaken the skin barrier and to achieve a clinically effective drug concentration in the target tissues, various transdermal enhancement techniques, such as sonophoresis, iontophoresis, and occlusion, are used.Citation5–Citation11 However, little information is available concerning the efficacy of the treatment protocol and the penetration profiles of the substances used in the physiotherapy setting. Indeed, most clinical studies have complex treatment protocols, and are unable to relate clinical outcome with drug penetration. A recent meta-analysis (2012) suggested a better clinical outcome for iontophoretically delivered substances.Citation12 Despite methodological flaws (eg, combined therapies, lack of control for passive diffusion and occlusion), definite conclusions regarding the possible penetration enhancement of electrically assisted delivery cannot be inferred.Citation12
The building up of a stratum corneum (SC) reservoir is an important component of the pharmacodynamics of topically applied substances. Rougier et al used the reservoir effect of the SC after 30 minutes of application to predict the total amount absorbed.Citation13 Moreover, the human SC has the capacity to store topically applied substances through a prolonged time.Citation14 The existence of a reservoir within the SC has been documented for several topically applied solutes such as corticosteroids,Citation14–Citation18 caffeine,Citation19,Citation20 nicotine,Citation21 diclofenac (DF),Citation22 and chemical ultraviolet filters.Citation23 Roberts et al emphasized that reservoir properties can also be attributed to the viable epidermis and dermis. Specifically, DF-binding capacities in dermal tissue have been reported.Citation24
Lambrecht et al estimated DF skin bioavailability by quantifying the reduction of methyl nicotinate (MN)-induced vasodilatation using chromametry and laser Doppler flowmetry. They detected a significant reduction in MN response 90 minutes after different application modalities of DF. Open application resulted in a 32% reduction in MN response, application under occlusion accounted for a reduction of 66%, and the iontophoretic application resulted in a 65% reduction.Citation11 At that time, one could not discriminate between the occlusive and electrically assisted delivered DF. To obtain more insight into the pharmacodynamics and reservoir properties of DF, the aim of the present study was to evaluate the bioavailability in the skin of DF at longer time intervals after DF application using different application modalities. Therefore, three different forms of application (passive, occlusive, iontophoretic) were examined in order to detect the anti-inflammatory effect and thereby get a better understanding of the skin-penetrating and reservoir-building behavior of DF as a function of time. MN responses were quantified by skin colorimetry at different time points (1.5, 6, 24, 32, 48, 72, 96, and 120 hours) after initial DF application.
Subjects and methods
Subjects
Five different groups, (group 1, n=13 [1.5 hours]; group 2, n=12 [6 hours]; group 3, n=12 [24, 32 hours]; group 4, n=14 [48, 72 hours] and group 5, n=9 [96, 120 hours]) of healthy volunteers (n=60, 23 male and 37 female) not treated with any drugs participated in this study. The volunteers were Caucasians and had healthy skin. The mean age of the subjects was 21.4±4.1 years. Approval by the ethical committee of the Vrije Universiteit Brussel was obtained for this study (2002/136). Before testing, all subjects were informed of the research protocol and signed an informed consent. During the duration of the experiments, the volunteers were asked to maintain their daily activities, but to abstain from swimming and extensive showering.
The measurements were performed under standardized temperature (20°C±2°C) and relative humidity (45%±5%) conditions. Before every DF application and each measurement session, the volunteers participated in a 30-minute acclimatization period. For each time interval tested, five randomized circular skin areas (7 cm2) were demarcated on the subjects’ volar forearms, for the following treatment and/or control modes: 1) open application (without occlusion), 2) passive diffusion under a semiocclusive humid sponge, 3) iontophoretic application, 4) standard MN response, and 5) untreated skin side. This meant that either five skin areas for groups 1 and 2 or ten skin areas for groups 3, 4, and 5 (testing two time intervals) were randomly demarcated on both arms. This procedure enabled a single application of MN on every skin site at different time intervals, avoiding the occurrence of tachyphylaxis.
Bioavailability test
DF skin bioavailability was assessed by quantifying the reduction of the MN response, as proposed by Lambrecht et al.Citation11 Topical application of MN provokes an increase of local cutaneous blood flow (erythema).Citation25 When DF is present in the skin, the nicotinate response is depressed in a concentration-dependent way.Citation26 Treffel and Gabard demonstrated a good correlation between the in vivo inhibition of the MN-induced inflammation and the in vitro determination (chromatographic analysis) of nonsteroidal anti-inflammatory drug-concentration levels in the SC.Citation27 Lambrecht et al evaluated the inhibition of MN-induced erythema with both laser Doppler flowmetry and the colorimetric method, which resulted in equivalent conclusions. Therefore it was decided that the chromametric evaluation be used.Citation11,Citation28 In the present study, the inhibition of the MN response with sum of the a* parameter (Σa*) was evaluated up to 50 minutes post-MN application, making the protocol more feasible and bearable for the subjects.
Instruments
Skin-surface colorimetric measurements were performed with a Minolta CR 200 chromameter operating in the International Commission on Illumination L*a*b* color space. For the quantification of skin-surface color and erythema, the a* parameter is especially useful, as it represents the chromaticity between red/magenta and green (negative values indicate green, while positive values indicate magenta).Citation29,Citation30 Chromameter measurements were carried out before DF application, prior to MN application, and every 5 minutes until 50 minutes post-MN application for the different reservoir-estimation time points. Since different groups of subjects were involved, an untreated skin area was included in order to be able to correct for blank values.
Materials
For treatment modes 1, 2, and 3, a commercially available 1% DF (Voltaren® Emulgel®; Novartis Pharma, Bern, Switzerland) formulation (12 mg) was applied. The product was gently rubbed into the skin using a gloved finger. The MN test was applied using paper-filter disks that were saturated in a 0.005 M aqueous MN solution and applied on the circular demarcated skin areas for 30 seconds. After removal of the filter disk, excess solution was gently wiped away using a tissue paper.
Application modes
DF was applied for 20 minutes under three different conditions: 1) open application (without occlusion), 2) passive diffusion under a semiocclusive humid sponge, and 3) iontophoretic application (direct current, 0.2 mA/cm2, cathode). To reproduce physiotherapeutic conditions, skin occlusion was performed using electrode sponges as applied in the clinical therapeutic setting. Two marked skin areas were not treated with DF: the application side for the standard MN-induced response and the blank untreated skin side.
Reservoir estimation
The bioavailability of DF in the SC under the three conditions was assessed by quantification of MN induced erythema at 1.5, 6, 24, 48, 72, 96, and 120 hours post-DF application.
Calculations and statistics
To estimate the presence of DF in the skin, MN responses at the different time periods following initial DF application (1.5, 6, 24, 32, 48, 72, 96, and 120 hours) were compared. The Σa* corrected for blank values (untreated skin) up to 50 minutes post-MN application was used as an indicator for the magnitude of the MN response. Normality was evaluated using the Kolmogorov–Smirnov goodness-of-fit test. At the different time periods following initial DF application, the skin response to the different application modalities was compared using analysis of variance with Bonferroni correction for post hoc tests. Statistical significance between any application mode and the standard MN response was used as an indication for the presence of DF in the skin at that particular time point following initial DF application. The significance level was set at 5%.
Results
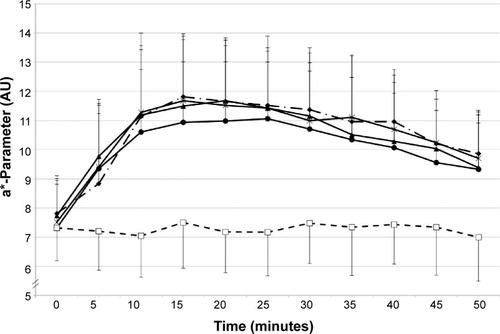
Analysis of the skin color (a* parameter) after MN application for the different application modes (open application, occlusion, and iontophoresis) and at the different reservoir-estimation times (1.5, 6, 24, 32, 48, 72, 96, and 120 hours) clearly showed changes in MN response as a function of the application mode and reservoir-estimation time. As an example, kinetics of the skin-color parameters are given in and . At 1.5 hours post-DF application, a clear inhibition was visible for application under occlusion and under iontophoresis, while open DF application did not provoke an inhibition in MN response (). At 96 hours post-DF application, inhibition of the MN response was no longer perceived ().
Figure 1 a*-Parameter changes over time 1.5 hours after the initial application of DF.
Abbreviations: DF, diclofenac; MN, methyl nicotinate.
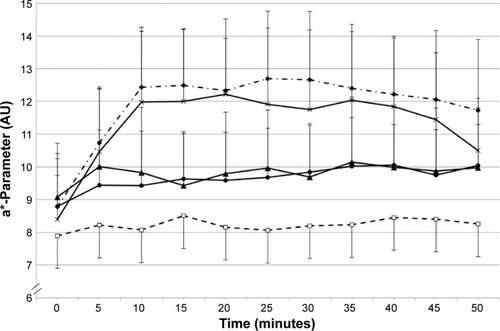
Figure 2 a*-Parameter changes over time 96 hours after the initial application of DF.
Abbreviations: DF, diclofenac; MN, methyl nicotinate.
Differences between application modes at the different reservoir-estimation times were assessed using the Σa* values calculated from the different kinetics (). At 1.5 hours after the initial DF application, a significantly decreased response was detected for the occluded and iontophoretically delivered DF only. From 6 hours up to 32 hours post-DF application, a significantly reduced MN response was detected at all DF-pretreated skin sites. At 48 hours post-DF application, only the passive and occluded applications resulted in a significant reduction. At 72 hours post-DF application, only the response after the passively delivered DF remained significant. From 96 hours on, no differences between the MN responses were detected (see ).
Table 1 Comparison of the total responses (Σa*; means ± standard deviation)
Discussion
The applied method – inhibition of a physiological reaction (MN) due to the presence of the inhibitor (DF) in the skin reservoir – enabled us to estimate penetration kinetics, DF skin-reservoir building, and emptying properties of three different application conditions up to 120 hours after initial DF application. In contrast with other reservoir protocols, an active substance-liberating action, such as occlusion or increased hydration, was not required.Citation24 This may be a confirmation of the presence of DF in the viable tissue (dermal compound), since MN-induced vasodilatation is inhibited immediately.Citation24
The application modality influenced the formation and the emptying of the reservoir. Penetration-enhancing factors, such as occlusion and current, induced faster formation and emptying of the reservoir compared to an open (passive) DF application. The presence of the reservoir for the passive DF application after 6 hours only is in contrast with earlier findings from our laboratory.Citation11 Lambrecht et alCitation11 presented the data of the MN response as calculated areas under the curve, relating the inhibition of MN response throughout a 65-minute time period. They found a 32% reduction of the MN response 1.5 hours after application, reaching borderline significance (P=0.04). The 25% reduction in MN response in the present study did not reach significance. Consequently, the weaker inhibition potential at 1.5 hours post-DF application noticed in both studies indicates lower bioavailability at that moment, due to a slower percutaneous penetration compared to the other application modes. According to the literature, the formation of a skin reservoir for topically applied substances is determined by a variety of factors (eg, lipid/water-solubility, protein-binding capacity, percutaneous absorption, compound concentration, clearance, application time, and application mode).Citation11,Citation14–Citation16,Citation23,Citation24 Our results support the previous findings of Vickers, showing that increased drug diffusivity in the SC provoked by penetration enhancers results in faster formation of the reservoir.Citation14 More specifically, Takahashi et al suggested increased diffusivity of DF into the SC from vehicles containing urea compared to vehicles without urea. The latter was explained by the hydration-enhancement effect of urea on the SC.Citation31
The results concerning the penetration-enhancement effect of iontophoresis are in line with the results of Curdy et al on the in vivo uptake of piroxicam after passive, occlusive, and iontophoretic administration. Only after iontophoresis were enhanced drug uptakes found at 30, 60, and 125 minutes following the initial application at different depths in the SC. In contrast with our results, Curdy et al found no significant difference between passive delivery and the application under occlusion. A possible explanation could be the low lipophilicity of piroxicam resulting in a low passive uptake into the SC.Citation32 Fang et al postulated that the route and mechanism for the iontophoretic delivery of DF through the skin might be different compared to passive delivery. Therefore, the importance of the SC as a rate-limiting barrier is reduced for iontophoretic delivery of DF.Citation33
Our results indicate that the emptying of the reservoir is influenced by the application mode. After iontophoretic delivery, the DF reservoir was present up to 32 hours following initial application. However, after occlusive and passive delivery of DF, the reservoir was present up to 48 and 72 hours, respectively. It is well established that diffusivity influences clearance from the reservoir, with faster emptying as a function of increasing diffusivity. Increasing SC hydration as well as iontophoresis have been shown to be effective methods for enhancing the percutaneous penetration of DF.Citation33 The present data fit in the model proposed by Roberts et al with a shorter lag time resulting in a faster reservoir emptying.Citation24 Equally, based on the results of Rougier et alCitation13 one can assume that the application with the fastest reservoir-buildup conditions may result in a greater delivery of active substances to the viable tissues, which may have an effect on the clinical outcomes of the physiotherapeutic treatment.
Within the limitations of our experimental design, we can hypothesize that the faster emptying of the reservoir after iontophoretic delivery compared to application under occlusion may be an indication for a higher diffusivity during the current-assisted application, leading to a superior delivery under iontophoretic circumstances.
Limitations of the present study are the lack of information on absolute DF quantities entering the viable skin and the fact that the methods used did not allow differentiation between an epidermal reservoir and a dermal reservoir. Further research estimating in vivo tissue concentrations after different modes of application are required for further elaboration of the pharmacodynamics of topical applied substances in physiotherapeutic practice.
Conclusion
This study measured the penetration kinetics and reservoir properties of DF after a single topical passive, occlusive, and electrical assisted application in a realistic physiotherapeutic setting. The results indicate that the contribution of occlusive and passive penetration in the iontophoretic delivery can be substantial. The prompt inhibition of the vasoactive reaction may be an indication for a dermal DF reservoir. The formation and emptying of the reservoir was found to be dependent on the application mode.
Disclosure
The authors report no conflicts of interest in this work.
References
- KaelinDLOhTHLimPABranderVABiundoJJJrRehabilitation of orthopedic and rheumatologic disorders. 4. Musculoskeletal disordersArch Phys Med Rehabil2000813 Suppl 1S73S77 quiz S78–S8610721764
- RovenskýJMicekováDGubzováZTreatment of knee osteoarthritis with a topical non-steroidal antiinflammatory drug. Results of a randomized, double-blind, placebo-controlled study on the efficacy and safety of a 5% ibuprofen creamDrugs Exp Clin Res2001275–620922111951579
- MachenJWhitefieldMEfficacy of a proprietary ibuprofen gel in soft tissue injuries: a randomised, double-blind, placebo-controlled studyInt J Clin Pract200256210210611926695
- BaşkurtFOzcanAAlgunCComparison of effects of phonophoresis and iontophoresis of naproxen in the treatment of lateral epicondylitisClin Rehabil20031719610012617384
- PierreMBDos Santos Miranda CostaILiposomal systems as drug delivery vehicles for dermal and transdermal applicationsArch Dermatol Res2011303960762121805180
- MeshaliMAbdel-AleemHSakrFNazzalSEl-MalahYEffect of gel composition and phonophoresis on the transdermal delivery of ibuprofen: in vitro and in vivo evaluationPharm Dev Technol20111629310120100032
- ClijsenRBaeyensJPBarelAOClarysPInfluence of the timing of ultrasound application on the penetration of corticosteroidsSkin Res Technol2013191e279e28222712560
- AggarwalGDhawanSHarikumarSLFormulation, in vitro, and in vivo evaluation of matrix-type transdermal patches containing olanzapinePharm Dev Technol201318491692521913873
- TurnerNGKaliaYNGuyRHThe effect of current on skin barrier function in vivo: recovery kinetics post-iontophoresisPharm Res1997149125212579327457
- LuksurapanWBoonhongJEffects of phonophoresis of piroxicam and ultrasound on symptomatic knee osteoarthritisArch Phys Med Rehabil201394225025523063790
- LambrechtRClarysPClijsenRBarelAODetermination of the in vivo bioavailability of iontophoretically delivered diclofenac using a methyl nicotinate skin inflammation assaySkin Res Technol200612321121616827697
- ClijsenRTaeymansJBaeyensJBarelAClarysPThe effects of iontophoresis in the treatment of musculoskeletal disorders – a systematic review and meta-analysisDrug Deliv Lett201223180194
- RougierADupuisDLotteCRoguetRThe measurement of the stratum corneum reservoir. A predictive method for in vivo percutaneous absorption studies: influence of application timeJ Invest Dermatol198584166683965580
- VickersCFStratum corneum reservoir for drugsAdv Biol Skin1972121771894579191
- PelchrzimRWeigmannHJSchaeferHDetermination of the formation of the stratum corneum reservoir for two different corticosteroid formulations using tape stripping combined with UV/VIS spectroscopyJ Dtsch Dermatol Ges200421191491916281609
- ClarysPGabardBBarelAOA qualitative estimate of the influence of halcinonide concentration and urea on the reservoir formation in the stratum corneumSkin Pharmacol Appl Skin Physiol1999121–2858910325587
- PershingLKBakhtianSPonceletCECorlettJLShahVPComparison of skin stripping, in vitro release, and skin blanching response methods to measure dose response and similarity of triamcinolone acetonide cream strengths from two manufactured sourcesJ Pharm Sci20029151312132311977107
- PellandaCOttikerEStrubCTopical bioavailability of triamcinolone acetonide: effect of dose and application frequencyArch Dermatol Res2006298522123016858572
- Chambin-RemoussenardOTreffelPBechtelYAgachePSurface recovery and stripping methods to quantify percutaneous absorption of caffeine in humansJ Pharm Sci19938211109911018289121
- ZeschASchaeferHStüttgenGThe quantitative distribution of percutaneously applied caffeine in the human skinArch Dermatol Res19792663277283526050
- BenowitzNLLakeTKellerKHLeeBLProlonged absorption with development of tolerance to toxic effects after cutaneous exposure to nicotineClin Pharmacol Ther19874211192203595064
- RobertsMSCrossSEA physiological pharmacokinetic model for solute disposition in tissues below a topical application sitePharm Res19991691392139810496655
- TeichmannAJacobiUWeigmannHJSterryWLademannJReservoir function of the stratum corneum: development of an in vivo method to quantitatively determine the stratum corneum reservoir for topically applied substancesSkin Pharmacol Physiol2005182758015767768
- RobertsMSCrossSEAnissimovYGFactors affecting the formation of a skin reservoir for topically applied solutesSkin Pharmacol Physiol200417131614755122
- WilkinJKFortnerGReinhardtLAFlowersOVKilpatrickSJStreeterWCProstaglandins and nicotinate-provoked increase in cutaneous blood flowClin Pharmacol Ther19853832732774028621
- TreffelPGabardBFeasibility of measuring the bioavailability of topical ibuprofen in commercial formulations using drug content in epidermis and a methyl nicotinate skin inflammation assaySkin Pharmacol1993642682758198812
- TreffelPGabardBIbuprofen epidermal levels after topical application in vitro: effect of formulation, application time, dose variation and occlusionBr J Dermatol199312932862918286226
- ClarysPAlewaetersKLambrechtRBarelAOSkin color measurements: comparison between three instruments: the Chromameter(R), the DermaSpectrometer(R) and the Mexameter(R)Skin Res Technol20006423023811428962
- WeatherallILCoombsBDSkin color measurements in terms of CIELAB color space valuesJ Invest Dermatol19929944684731402005
- AleSILaugierJPMaibachHISpacial variability of basal skin chromametry on the ventral forearm of healthy volunteersArch Dermatol Res1996288127747778950459
- TakahashiKSuzukiTSakanoHMizunoNEffect of vehicles on diclofenac permeation across excised rat skinBiol Pharm Bull19951845715757655430
- CurdyCKaliaYNNaikAGuyRHPiroxicam delivery into human stratum corneum in vivo: iontophoresis versus passive diffusionJ Control Release2001761–2737911532314
- FangJWangRHuangYWuPCTsaiYPassive and iontophoretic delivery of three diclofenac salts across various skin typesBiol Pharm Bull200023111357136211085366
