Abstract
Insulin is a life-saving medication for people with type 1 diabetes, but traditional insulin replacement therapy is based on multiple daily subcutaneous injections or continuous subcutaneous pump-regulated infusion. Nonphysiologic delivery of subcutaneous insulin implies a rapid and sustained increase in systemic insulin levels due to the loss of concentration gradient between portal and systemic circulations. In fact, the liver degrades about half of the endogenous insulin secreted by the pancreas into the venous portal system. The reverse insulin distribution has short- and long-term effects on glucose metabolism. Thus, researchers have explored less-invasive administration routes based on innovative pharmaceutical formulations, which preserve hormone stability and ensure the therapeutic effectiveness. This review examines some of the recent proposals from clinical and material chemistry point of view, giving particular attention to patients’ (and diabetologists’) ideal requirements that organic chemistry could meet.
Introduction
Patients with type 1 diabetes rely on the exogenous delivery of insulin because of impaired insulin secretion by the beta cells of the endocrine pancreas. In current practice, glycemic control is achieved through the use of basal and prandial insulin (multiple daily injections [MDIs]) or by continuous subcutaneous insulin infusion (CSII) using an external pump. Self-injecting insulin is unpleasant for patients, and the need for frequent self-monitoring of blood glucose (SMBG) by finger sticks presupposes motivation and implies pain, costs, technical skills, and psychological burden. Recent systematic reviews examined the comparative effectiveness of methods for insulin delivery and glucose monitoring and identified research priorities.Citation1,Citation2 Intensive insulin therapy delivered either by CSII or MDI was equally effective in lowering glycated hemoglobin (HbA1c) levels and resulted in similar rates of hypoglycemia in both adolescents and adults with type 1 diabetes.Citation2 Real-time continuous glucose monitoring (rt-CGM) was superior to SMBG in lowering HbA1c, without increasing the risk of hypoglycemia.Citation2 Although CSII and MDI without rt-CGM had similar effects on HbA1c, the addition of rt-CGM to CSII was associated with lower HbA1c levels than MDI/SMBG.Citation2 For type 1 diabetes, expert stakeholders ranked adolescents as the highest priority age group and artificial pancreas as the highest priority for future research. For glucose monitoring methods, all stakeholders prioritized rt-CGM for any patient with type 1 diabetes.Citation2 In patients with type 2 diabetes, CSII therapy was not superior to MDI.Citation3 Closed-loop insulin delivery, so-called artificial pancreas, combines continuous glucose sensor with insulin infusion pump using validated mathematical algorithms to drive CSII. These systems, which include the recent dual-hormone closed-loop delivery devices, could improve glycemic control and reduce the risk of hypoglycemia. One of the major disadvantages of insulin pump therapy is cost, which is higher than MDI (http://www.idf.org/debate-insulin-pump-therapy-matter-choice).
Whatever the regimen, the common drawback of both CSII and MDI remains that subcutaneous insulin is absorbed nonphysiologically into the systemic circulation with consequent peripheral hyperinsulinemia, weight gain, and risk of hypoglycemia. This review focuses on noninvasive approaches for insulin delivery with particular attention to systems potentially able to reproduce physiological insulin secretion.
Physiology of insulin secretion
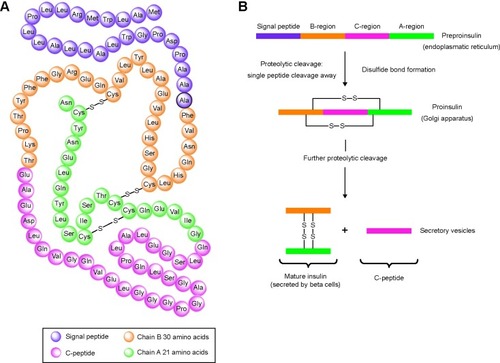
Circulating, monomeric insulin is composed of two polypeptide chains (the A and B chains consisting of 21 and 30 amino acids, respectively) and two disulfide bridges, which create the quaternary assembly of the molecule (http://www.betacell.org/content/articleview/article_id/8/) (Accessed on February 4, 2015).
Human insulin is synthesized as preproinsulin (110 amino acids) in the rough endoplasmic reticulum. Following removal of the first 24 amino acids (signal peptide) and packaging in the Golgi apparatus, insulin is stored as proinsulin in the immature secretory granules. The conversion of proinsulin into active insulin and C-peptide is catalyzed by the proteolytic activity of proinsulin convertase 1, proinsulin convertase 2, and carboxypeptidase H (). Insulin is secreted by the beta cells: every beta cell contains 10,000–13,000 secretory granules and a single insulin granule contains ~106 molecules of insulin.Citation4
Figure 1 Schematic representation of the amino acid sequence of the human preproinsulin (A) and the conversion of proinsulin into biologically active insulin and C-peptide (B).
The beta cell is electrically excitable: calcium-dependent exocytosis of the secretory granules is regulated by variations in plasma glucose concentration via changes in beta cell metabolism and closure of KATP channels.Citation4 In isolated islets, insulin secretion is biphasic with a first-phase component, which lasts a few minutes, and a slowly developing but sustained second-phase component. The first-phase secretion involves the docked granules (or possibly the so-called restless newcomers, ie, granules that are recruited by stimulation and immediately released), while the second-phase secretion involves the time- and ATP-dependent supply of new granules.Citation4 The secretory rate of beta cells in intact islets has been estimated ~15 granules per beta cell per second.Citation4
Assessing insulin secretion in vivo is quite more complex: 1) insulin is secreted in high-frequency pulses (recurring every 5–15 minutes) superimposed on slower, ultradian oscillations (every 80–120 minutes). Glucose administration mainly amplifies secretory burst amplitude/mass; 2) insulin pulses are secreted in the portal vein and underg ~40%–80% first-pass hepatic extraction with consequent waveform damping in the systemic circulation. The amplitude of insulin pulses is the principal determinant of hepatic insulin clearance; 3) plasma insulin levels in the peripheral circulation reflect hormone secretion, distribution, and degradation; 4) C-peptide is secreted in equimolar amounts with insulin but is more slowly catabolized than insulin. Unlike insulin, C-peptide is not extracted by the liver, thus C-peptide secretion rate can be estimated from plasma C-peptide levels; 5) proinsulin and insulin are not released equimolarly, and proinsulin clearance is lower than that of insulin. Circulating proinsulin accounts for ~10%–20% of normal fasting insulin, but it may be disproportionately increased in type 2 diabetes; however, highly specific immunoassay methods are required to differentiate between intact proinsulin and the specific and unspecific proinsulin derivatives.Citation5–Citation8
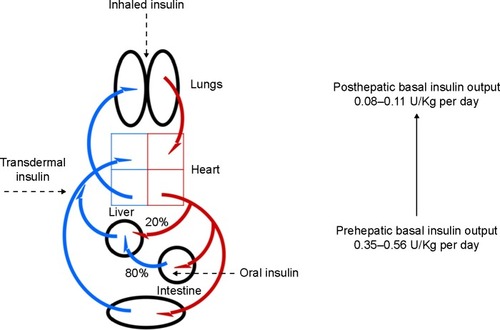
In the basal state, the range of the insulin concentration is ~100–1,000 pmol/L in the portal circulation vs 10–30 pmol/L in the systemic circulation. According to Meier et al the greater extraction of insulin within insulin secretory bursts is predicted by insulin receptor-binding kinetics; therefore, the pulse mass of insulin release dictates not only hepatic insulin delivery but also its fractional hepatic extraction (and in turn the delivery of insulin into the systemic circulation).Citation8
Insulin inhibits directly hepatic glucose production through inhibition of gluconeogenesis and glycogenolysis; indirect effects include inhibition of glucagon secretion, lipolysis in fat, and proteolysis in muscle. During a pancreatic clamp in the overnight-fasted, conscious dog, insulin infusion was switched from the hepatic portal vein to a peripheral vein; as a result, Edgerton et al obtained a doubling of the arterial insulin level and a 50% decrease in the insulin level within the hepatic sinusoids. The arterial plasma free fatty acids level and net hepatic free fatty acid uptake decreased by 40%–50%; hepatic glucose output increased more than twofold and remained elevated compared with that in the control group.Citation9 Insulin’s effects include reduction of glucagon secretion. Glucagon is a counterregulatory hormone that promotes glycogenolysis and inhibits glycogen synthesis in the liver, increases gluconeogenesis, and decreases glycolysis. The absolute levels or rather the ratios of glucagon to insulin are key regulators of glucose homeostasis and have been found to be elevated in patients with diabetes.Citation10
Exogenously administered insulin and alternative noninvasive routes of delivery
Exogenously administered insulin, usually by subcutaneous injection, is unable to mimic endogenously secreted insulin. In normal physiology, the liver is exposed to insulin concentrations two- to fourfold higher than those observed in the peripheral circulation. Subcutaneously injected insulin is unable to approach this portal-systemic insulin concentration gradient and results in impaired hepatic glucose suppression.Citation10 Relative insulin deficiency in the portal circulation of liver compromises 1) the regulation of the rate of hepatic glycogenolysis and gluconeogenesis during fasting and 2) paracrine suppression of glucagon secretion in the fed state. As a consequence, in the postprandial state, there is an inappropriate elevation of glucagon and depletion of glycogen stores.Citation10 Moreover, increasing insulin doses leads to peripheral hyperinsulinemia, which predisposes to hypoglycemia and weight gain.Citation10
After an overnight fast, the normal arterial plasma insulin level ranges from 5 μU/mL to 15 μU/mL, whereas the level of insulin in the portal vein is approximately threefold greater. The plasma insulin concentration in the liver sinusoids, which contain mixed arterial (20%) and portal (80%) blood, ranges from ~15 μU/mL to 40 μU/mL.Citation11 If fasting insulin secretion was totally absent, one could reproduce the peripheral fasting plasma insulin concentrations observed in nondiabetic subjects by infusing 0.35–0.56 U/kg per day intraportally, whereas 0.08–0.11 U/kg per day by peripheral insulin administration.Citation12 It is noteworthy that the dose–response curve for the effect of insulin on fasting hepatic glucose output is shifted to the left relative to the dose–response curve of peripheral insulin action. A doubling of insulin secretion inhibits hepatic glucose output by ~80%, while it increases glucose utilization by only 20%; the effect of insulin on glucose production is complete when insulin secretion increases threefold, while the effect of insulin on glucose utilization does not saturate even with the highest possible physiologic insulin levels.Citation11
Different routes of insulin administration (oral, pulmonary, transdermal, intranasal, ocular, vaginal, and rectal) have been explored.Citation13,Citation14 However, no alternative route of systemic insulin administration can reproduce a positive portal-systemic blood insulin gradient (). Unlike transdermal or pulmonary drug delivery routes, substances that are absorbed in the gastrointestinal tract are drained to the liver via the portal vein. The hepatic portal vein was formed by the junction of the superior mesenteric and splenic veins, with the inferior mesenteric vein joining at or near the angle of union; it drains venous blood from the gastrointestinal tract, spleen, pancreas, and gallbladder. Hence, oral insulin absorption into the portal vein could generate a high portal-systemic gradient, mimicking the endogenous secretion of insulin.
Transdermal delivery of insulin
The skin is composed of three layers: the stratum corneum, epidermis, and dermis. It not only provides both a physical and a chemical barrier against foreign invaders but also functions as an active immune organ. The stratum corneum consists of nonnucleated protein-enriched corneocytes (anchored together by means of corneodesmosomes) and lipid-enriched intercellular domains. The epidermis is a multilayered, epithelial tissue divided into the basal cell layer, the spinous cell layer, and the granular cell layer. The papillary dermis and reticular dermis contain collagen and elastic fibers, proteoglycans, and glycoproteins; the dermal vasculature, lymphatics, nerves, sweat glands, and hair roots are embedded within the fibrous tissue. The skin immune system includes keratinocytes and Langerhans cells in the epidermis; fibroblasts, dendritic cells, and mast cells in the dermis; and T- and B-lymphocytes in the skin-draining lymph nodes. Easy access to this skin immune system has been considered an attractive site for vaccination.Citation15
The transdermal permeation of insulin is now extensively investigated in in vitro and in vivo studies conducted in animal models and human subjects. Several techniques have been explored to reduce the skin barrier properties. Penetration enhancers act by various mechanisms such as: 1) increased drug solubility (chemical enhancers), 2) optimization of the formulation (chemical modification, encapsulation within carrier systems), 3) increased diffusion coefficients (microdermabrasion, laser ablation, chemical and biochemical enhancers, ultrasound, electroporation, microneedles), and 4) provision of additional driving force (ultrasound, iontophoresis, electroporation).Citation16,Citation17 Most approaches to increasing skin permeability seek to breach the stratum corneum barrier without damaging viable epidermis. However, viable epidermis also poses a significant barrier to transdermal diffusion of small hydrophilic molecules and macromolecules even in the absence of stratum corneum.Citation18 The application of the photomechanical waves allowed 6 kDa protein molecules to cross the stratum corneum and to reach the systemic circulation;Citation19 after photomechanical insulin delivery in a streptozotocin-diabetic rat model, blood glucose decreased (80%±3%) and remained below 200 mg/dL for more than 3 hours.Citation19 Skin permeation after laser ablation was proportional to the treated energy: electron micrographs of pig skin treated by erbium:yttrium–aluminum–garnet (Er:YAG) laser showed a disarrangement of the stratum corneum surface, wider intervening spaces between the corneocyte aggregates,Citation20 and some of the corneocytes were disrupted probably as a result of laser-induced photomechanical stress. The authors conclude that major prerequisite of a permeation-enhancing method is that the skin recover its normal barrier properties following removal of the enhancement method, but the stratum corneum and epidermis could completely recover within 3–4 days after laser treatment based on different fluences used.Citation20 Most recent approaches toward transdermal insulin delivery include: 1) the conductive polymer nanotube transdermal patch, specifically designed for hydrophilic drugs and insulin;Citation21 2) the iontophoresis treatment with liposomes encapsulating insulin;Citation22 3) the transferosomal (highly deformable vesicles) drug delivery system (gel) that has demonstrated prolonged hypoglycemic effect in alloxan-induced diabetic rats after transdermal administration;Citation23 4) the dissolving polymer microneedle patches that enable stable encapsulation of insulin and produce a significant hypoglycemic effect in diabetic rats, with a relative pharmacological availability and relative bioavailability of 92.2% and 92.3%, respectively.Citation24 When the conductive polymer nanotube transdermal patch was loaded with insulin and delivered ex vivo at a potential of −1.2 V, the cumulative amount of insulin released through porcine skin over 24 hours was substantially less than control dye molecules.Citation21 In streptozotocin-induced type 1 diabetic rats, blood glucose levels decreased slowly after iontophoretic administration of liposomes encapsulating insulin (0.66 mg/kg =18.3 IU/kg), reducing to ~20% of basal levels 18 hours after iontophoresis; these lower blood glucose levels were maintained for up to 24 hours after administration.Citation22 Over 24 hours after transdermal application of optimized transferosomal gel (containing 2.24 mg insulin) in alloxan-induced diabetic rats, mean blood glucose level was lower by about 30% as compared to that in untreated rats.Citation23 In diabetic rats, the insulin-loaded microneedles reduced blood glucose levels to ~40% of basal levels at 120 minutes after administration; the relative pharmacological availability and relative bioavailability of insulin were both ~92%.Citation24 Although the field has continued to evolve and improve over time, efficacy studies of transdermal insulin delivery to human subjects are preliminaryCitation18,Citation25 and there are a number of safety aspects that need to be addressed. Safety issues may be associated with strategies that overcome the stratum corneum barrier properties, including pain, local reactions (irritant or allergic contact dermatitis), and infections.Citation17
Inhaled insulin
The inhalation route of biopharmaceutical drug delivery has the advantage of rapid absorption due to the large surface area (~145 m2) and the close proximity of the air and blood compartments. However, deep lung delivery of insulin is influenced by several factors, first of all aerodynamic particle size, particle speed, and ventilatory parameters.Citation26,Citation27 Biopharmaceuticals with a mass median aerodynamic diameter of 1–3 μm reach the alveolar surfaces where they may undergo different fates: clearance by alveolar macrophages, binding to surfactant, and/or enzymatic degradation; alveolar absorption of proteins is slow when their molecular weight is high. Moreover, patient’s ability to perform an appropriate inspiration maneuver is another determinant for reproducible delivery of drug to the distal part of the lung. The efficiency of delivery for an inhaled drug depends on the fraction of dose delivered from the device, the fraction deposited in the alveolar region, and the bioavailable fraction that is absorbed.Citation27 Inhalers generally belong to four categories: metered dose inhalers, spacers and holding chambers, dry powder inhalers, and nebulizers.Citation28 Inhaled insulin has been extensively studied and first inhalable formulation (Exubera) gained market approval in 2006 but was withdrawn in 2007 due to low cost-effectiveness.Citation29 Other inhaled insulin devices were developed (such as AERx Diabetes Management System, AIR, and Technosphere), which differed in the formulation (liquid or powder) and the delivery device. However, the development of these products was discontinued, except for Technosphere insulin that is delivered via a breath-powered inhaler system and received FDA approval in 2014.Citation30 Inhaled insulin showed low efficiency of delivery (10%–12%), which was further affected by pulmonary diseases and smoking,Citation26,Citation27 and induced higher levels of circulating antibodies than comparator insulins given by subcutaneous routes, especially in patients with type 1 diabetes.Citation31 Moreover, it was associated with an increased incidence of cough and a greater decrease in diffusing capacity of the lung for carbon monoxide than subcutaneous insulin in patients with type 1 diabetes.Citation32 Nevertheless, in a recent study that evaluated pulmonary function changes in diabetes patients receiving inhaled Technosphere insulin, changes in lung function were judged not clinically meaningful since they were small, occurred early after therapy initiation, and remained nonprogressive over 2 years.Citation33
Pharmacokinetics studies showed that the total insulin exposure for inhaled insulin was comparable to that of subcutaneous regular insulin, but the exposure time was shorter; inhaled insulin was found to be as clinically effective as injected short-acting insulin.Citation34,Citation35 In large-scale studies, inhaled insulin was effective, well tolerated and well accepted in patients with type 1 diabetes.Citation36–Citation39 However, the limited bioavailability of inhaled insulin, which implies considerably higher costs, together with the uncertainties about the efficacy and safety, induced many sponsors to terminate inhaled insulin programs.Citation26,Citation27,Citation31–Citation33,Citation36–Citation39
Oral insulin delivery
The oral route is the most preferred form of chronic drug administration. Oral medication for diabetic patients treated throughout their lives represents a crucial demand in order to improve their quality of life and to assure adherence to the treatment regimen. Moreover, oral insulin is delivered directly to the liver via portal circulation and could generate a high portal-systemic gradient replicating the endogenous secretion of insulin.Citation40 Nevertheless, effective oral insulin delivery remains challenging because of poor bioavailability.Citation41,Citation42 In the gastrointestinal tract, the absorption of protein and peptide molecules is hampered by physical and biochemical barriers (epithelium, variable pH, enzymatic proteolysis, efflux pumps, and first-pass elimination by liver); solubility, molecular weight, and partition coefficient are the major physicochemical concerns dictating drug dissolution and permeability through the gastrointestinal barrier.Citation41 The pharmacokinetic and pharmacodynamic properties of oral insulin formulations change as a function of the site (along the gastrointestinal tract) and pathway (cellular or paracellular) of absorption. The fast absorption has been attributed to paracellular pathways, whereas the slow absorption to cell pathways (endocytosis via enterocytes or M-cells).Citation40,Citation43–Citation45
The application of nanotechnologies in drug delivery is expected to achieve multiple goals: i) shielding the entrapped drugs from the gastrointestinal environment so that they can reach intact the site of absorption; ii) enhancing drug water solubility; iii) enhancing the intestinal permeability of drugs once carried by nanoparticles (NPs) that are chiefly taken up by M-cells, known for their high transcytotic capacity and low lysosomal hydrolase activity; and iv) reducing dosing frequency because of the controlled and sustained release of the nanoencapsulated drugs, and thus improving treatment adherence.Citation41
To improve oral bioavailability of insulin, different types of delivery systems and functional excipients have been explored: capsules, tablets, microparticles, micelles, liposomes, solid lipid NPs and NPs of biodegradable polymers, hydrogels, film patches, superporous matrices, intestinal patches, charge-coupled micromagnet microparticles, polymeric adhesives, protease inhibitors, insulin aggregation inhibitors, permeation enhancers, etc.Citation40,Citation41,Citation46 Depending on their constitutive materials and physicochemical characteristics, NPs may allow formulators to design different release profiles and to achieve local or systemic targeting of the encapsulated drug.Citation41 Patents published on oral insulin delivery formulations have been recently reviewed.Citation47
Currently, the clinicaltrial.gov registry lists two recruiting clinical studies aimed at evaluating safety/efficacy of oral insulin in patients with type 1 diabetes (NCT01973920, Oshadi Icp, Oshadi Drug Administration, and NCT02094534, ORMD-0801 capsules, Oramed Pharmaceutical/Hadassah Medical Organization), two completed studies (NCT01120912, Oshadi oral insulin, and NCT00867594, ORMD-0801), and one unknown study (NCT01035801, Drug: IN-105, Biocon Limited).
In human type 1 diabetes, the administration dose of oral insulin reported in the literature ranged widely, from 50 units to 2,400 units.Citation48–Citation51 The addition of ORMD-0801 oral insulin capsules (8 mg insulin) three times daily to subcutaneous insulin injection regimens of eight patients with type 1 diabetes resulted in a synergistic reduction in blood glucose concentrations, which was most prominent during the early evening hours.Citation48 Kapitza et al investigated the pharmacokinetic and pharmacodynamic properties of an oral insulin formulation (300 units combined with 400 mg monosodium N-(4-chlorosalicyloyl)-4-aminobutyrate in two capsules) and compared it with subcutaneously injected regular human insulin in ten patients with type 2 diabetes. Maximum plasma insulin concentration was significantly higher, and time was significantly shorter with oral insulin administration. Relative bioavailability of oral insulin for the 0–1-hour, 0–2-hour, and 0–6-hour periods were 26%±28%, 7%±4%, and 2%±1%, respectively. Respective values for biopotency were 55%±92%, 12%±9%, and 3%±1%.Citation49 Under fasting conditions, variability in absorption was high (coefficient of variation 60%–70%), but could increase further with prandial administration.Citation49 The time-action of another orally administered enteric insulin capsule formulation (insulin doses 50 units, 100 units, and 200 units) was evaluated in 12 healthy Chinese subjects using euglycemic glucose clamps. The mean time periods for maximal metabolic effects for 50 units, 100 units, and 200 units were 250±118 minutes, 170±58 minutes, and 236±132 minutes, respectively; the onset of action was at 38±10 minutes, 41±18 minutes, and 65±58 minutes, respectively. Thus, the time-action profile was similar to that of the comparator NPH insulin. The biopotency of this formulation was uncertain due to the high variability.Citation50 A Phase I clinical trial assessed the safety and tolerability of single ascending doses of oral insulin 106 (NNC 0148-0000-0106) in healthy subjects, and of a single-dose level of oral insulin 106 in subjects with type 1 and type 2 diabetes. Insulin 106 in a GIPET® I gastro-resistant tablet was administered orally as tablets of 300 nmol, 900 nmol, 1,800 nmol, 3,600 nmol, and 7,200 nmol. The time to maximum serum concentration ranged from 0.75 hours to 1.88 hours, and the mean serum half-life values increased from 0.64 hours to 2.17 hours over the tested dose range in fasting state. The interindividual variability in the pharmacokinetic endpoints of oral insulin 106 was large with a coefficient of variation of ~200% for its area under the serum insulin concentration time curve. The pharmacodynamic results demonstrated a rapid onset of action with a large interindividual variability in glucose infusion rate endpoints (coefficient of variation 95%–445%).Citation51
Carriers for oral delivery of insulin
Most materials used in the formulation of oral insulin NPs and tested in animals were polymers. Hydrophilic or hydrophobic polymers often were synthesized as microparticles or NPs. Polymers such as poly(lactide-co-glycolide (PLGA), poly(lactide) (PLA), poly(ε-caprolactone) (PCL), chitosan (CS) and its derivatives, dextran, solid lipids, poly(allylamine), and poly(acrylic acid) have been used due to their well-established safety. Using various polymeric materials and formulation processes allows to modulate the physicochemical properties of NPs, extent of drug loading, and drug release profile.Citation40
The most widely used polymeric materials were based on PLGA as poly-hydroxyethyl-aspartamide,Citation52 cyclodextrin and polymethacrylic acid,Citation53 PLGA/HP55,Citation54 concanavalin A-PLGA conjugate,Citation55 antacid co-encapsulated PLGA,Citation56 folate-decorated PLGA NPs,Citation57 insulin-sodium oleate-PLGA complex.Citation58 Chitosan-based materials were formulated as chitosan–insulin NPsCitation59–Citation61 or conjugated with alginate,Citation62–Citation64 poly(γ-glutamic acid),Citation65 lauryl succinyl,Citation66 poly(methyl methacrylate),Citation67 and iron oxide NPs.Citation68 Solid lipid NPs containing insulin were formulated with cetyl palmitateCitation69 and stearic acid–octaarginineCitation70 in order to protect insulin from enzymes. Many of these polymeric NPs were effective in lowering the blood glucose level in animal models; however, much larger doses of insulin were required in comparison with subcutaneous administration. Using some of these promising NP systems, insulin doses required to reduce blood glucose concentration by 50% range from 30 IU/kg to 100 IU/kg; they are higher than the typical dose of 1 IU/kg needed to induce the same reduction in blood glucose level via the subcutaneous injection.Citation40 Moreover, it has been suggested that long-term administration of high doses of insulin may induce mitogenic changes in the gastrointestinal epithelium, as insulin is also a growth factor.Citation40 Thus, the current polymer NPs should be improved in order to reduce the amount of insulin required to control blood glucose levels.Citation40
Gold nanoparticles (AuNPs) are being investigated as novel carriers for oral insulin delivery owing to their excellent biocompatibility and low cytotoxicity. AuNPs are stable metal NPs with unique physical, chemical, optical, and electronic properties: large surface-to-volume ratio for easy conjugation of a variety of ligands, practical nanoscale assembly, inert nature, extreme resistance to oxidation, enhanced permeability and retention effect, surface plasmon resonance phenomenon, size- and shape-dependent electronic, and optical properties. To use the AuNPs in vivo for a long retention time avoiding the action of the reticular endothelial system, their surface can be modified with antibiofouling agents, such as polyethylene glycol, and more stable bonds can be created by self-assembling molecules with thiol groups onto gold surfaces.Citation71
In 2006, insulin-capped bare AuNPs (Au-Ins) and aspartic acid-capped AuNPs (Au-Asp-Ins) were delivered in diabetic Wistar rats by both oral and intranasal (transmucosal) routes and significantly lowered blood glucose levels.Citation72 Oral administration of both Au-Ins (50 IU/kg) and Au-Asp-Ins (50 IU/kg) formulations reduced blood glucose levels by 19% and 31%, respectively. Nasal administration of both Au-Ins (20 IU/kg) and Au-Asp-Ins (20 IU/kg) induced a maximum reduction of 50% and 55%, respectively. Moreover, the formulation Au-Ins released insulin more slowly than the Au-Asp-Ins formulation. The maximum blood glucose reduction occurred 180 minutes and 120 minutes after administration of the Au-Ins and the Au-Asp-Ins formulations, respectively. Thus, by comparing the maximum blood glucose-reducing response to the two formulations (oral and intranasal), the insulin uptake was higher and faster in the intranasal delivery protocol. Membrane permeability of nanogold-insulin formulations across nasal mucosal cells was greater than across gastrointestinal mucosa. Indeed, the diabetic rats received 20 IU/kg of insulin in the intranasal experiment vs 50 IU/kg after oral administration. The maximum blood glucose reduction after the Au-Asp-Ins intranasal administration (55%) was comparable to that observed after the subcutaneous administration (53%). This finding suggested that the transmucosal delivery of insulin by AuNPs could be an effective alternative to subcutaneous delivery.Citation72
Recently, chondroitin sulfate (CS)-capped AuNPs were synthesized. CS, a sulfated glycosamino-glycan composed of N-acetylgalactosamine and glucuronic acid, was used as a stabilizer in the synthesis of AuNPs, and insulin was embedded in the CS-capped AuNPs structure (AuNPs/INS, ~120 nm mean diameter, narrow size distribution, and negative zeta potential).Citation73 AuNPs/INS remained stable during the test period and its cytotoxicity against Caco-2 cells was negligible. In the diabetic rat model, oral administration of the AuNPs/INS formulation reduced blood glucose level up to 32.1%. The reduction in the blood glucose level was higher than previous reported results using insulin-loaded sodium borohydride reduced AuNPs (18%).Citation72 Moreover, the mean insulin concentration 120 minutes after oral administration of AuNP/INS was 6.61-fold higher than that of insulin solution-treated group, thus suggesting that AuNPs had a role in the permeation of insulin.
Thus, several NP systems have been developed for oral insulin delivery with promising results in experimental models, but their long-term efficacy and safety must be demonstrated in animals and human beings. Furthermore, so far, the low bioavailability showed by various noninvasive insulin delivery have required huge insulin doses as compared to subcutaneously injected insulin: the additional cost adversely affects cost-effectiveness as with inhaled insulin. Finally, the management of both fasting and postprandial blood glucose levels requires different time-action profiles of oral formulations (rapid and basal) that ensure the predictability and reproducibility of insulin release. No oral insulin formulation is commercially available, and there have been very few clinical trial reports with human data.
Conclusion and future strategies
Oral delivery of insulin has the chance to improve the quality of life of patients with diabetes. There are therefore several attempts to develop oral carrier systems to resist gastrointestinal tract conditions and to preserve insulin integrity. The literature suggests that insulin carriers based on biodegradable polymers and AuNPs are promising perspectives to prepare formulations for oral insulin delivery. However, current research efforts are still allocated to the preclinical stages and clinical data reports only made up 4% of all the oral insulin publications. From the pharmacological point of view, the main barriers faced in oral insulin delivery are 1) unpredictable and erratic absorption and 2) the extremely low and variable bioavailability and bioefficacy. Furthermore, improved clinical trial designs should consider short-term and long-term outcomes, active comparators, the effect of food on insulin absorption, and response reproducibility.Citation74
Noninvasive insulin delivery is an attractive target to reduce the perceived burden of conventional subcutaneous insulin treatment. Developing efficient drug delivery systems requires large-scale, multidisciplinary research efforts that combine biological and polymer sciences, pharmaceutical biotechnology, and conjugation chemistry. Research initiatives should focus on promoting most promising projects coordinated between national and international programs through a joint effort of governments, universities, and industries. From the manufacturing point of view, the production of safety materials is essential for sustainable development of oral insulin formulations. The investigation of the toxicological features and the impact of nanomaterials on the body as well as on the environment are important aspects, which cannot be overlooked.Citation75
Disclosure
The authors report no conflicts of interest in this work.
References
- YehHCBrownTTMaruthurNComparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysisAnn Intern Med201215733634722777524
- YehHCLauBDGoldenSHDonnerTBrownTTBassEBInsulin Delivery and Glucose Monitoring Methods: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 57Rockville, MDAgency for Healthcare Research and Quality (US)2013
- JeitlerKHorvathKBergholdAContinuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysisDiabetologia20085194195118351320
- RorsmanPBraunMRegulation of insulin secretion in human pancreatic isletsAnnu Rev Physiol20137515517922974438
- PørksenNGrøfteTGreisenJHuman insulin release processes measured by intraportal samplingAm J Physiol Endocrinol Metab20022823E695E70211832375
- TuraAPaciniGKautzky-WillerALudvikBPragerRThomasethKBasal and dynamic proinsulin-insulin relationship to assess beta-cell function during OGTT in metabolic disordersAm J Physiol Endocrinol Metab20032851E155E16212670835
- CaumoALuziLFirst-phase insulin secretion: does it exist in real life? Considerations on shape and functionAm J Physiol Endocrinol Metab20042873E371E38515308473
- MeierJJVeldhuisJDButlerPCPulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humansDiabetes20055461649165615919785
- EdgertonDSLautzMScottMInsulin’s direct effects on the liver dominate the control of hepatic glucose productionJ Clin Invest2006116252152716453026
- AronoffSLBerkowitzKBSheinerBWantLGlucose metabolism and regulation: beyond insulin and glucagonDiabetes Spectr200417183190
- LeRoithDTaylorSIOlefskyJMDiabetes Mellitus: A Fundamental and Clinical Text3rd edPhiladelphiaLippincott Williams & Wilkins2004
- FerranniniEPhysiology of glucose homeostasis and insulin therapy in type 1 and type 2 diabetesEndocrinol Metab Clin North Am2012411253922575405
- VermaAKumaNMalviyaRSharmaPKEmerging trends in noninvasive insulin deliveryJ Pharm2014201419 article ID 378048
- ElçiogluHKSezerADNanoparticle insulin drug delivery – applications and new aspectsSezerADApplication of Nanotechnology in Drug DeliveryRijekaInTech2014237256
- LeeMYShinMCYangVCTranscutaneous antigen delivery systemBMB Rep2013461172423351379
- PrausnitzMRLangerRTransdermal drug deliveryNat Biotechnol200826111261126818997767
- Tuan-MahmoodTMMcCruddenMTTorrisiBMMicroneedles for intradermal and transdermal drug deliveryEur J Pharm Sci201350562363723680534
- AndrewsSNJeongEPrausnitzMRTransdermal delivery of molecules is limited by full epidermis, not just stratum corneumPharm Res20133041099110923196771
- LeeSMcAuliffeDJMulhollandSEDoukasAGPhotomechanical transdermal delivery of insulin in vivoLasers Surg Med200128328228511295766
- FangJYLeeWRShenSCWangHYFangCLHuCHTransdermal delivery of macromolecules by erbium: YAG laserJ Control Rel200410017585
- NguyenTMLeeSLeeSBConductive polymer nanotube patch for fast and controlled ex vivo transdermal drug deliveryNanomedicine (Lond)20149152263227224405462
- KajimotoKYamamotoMWatanabeMNoninvasive and persistent transfollicular drug delivery system using a combination of liposomes and iontophoresisInt J Pharm20114031–2576520970487
- MalakarJSenSONayakAKSenKKFormulation, optimization and evaluation of transferosomal gel for transdermal insulin deliverySaudi Pharm J201220435536323960810
- LingMHChenMCDissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic ratsActa Biomater20139118952896123816646
- McVeyEHirschLSutterDEPharmacokinetics and postprandial glycemic excursions following insulin lispro delivered by intradermal microneedle or subcutaneous infusionJ Diabetes Sci Technol20126474375422920798
- MastrandreaLDInhaled insulin: overview of a novel route of insulin administrationVasc Health Risk Manag20106475820234779
- McElroyMCKirtonCGliddonDWolffRKInhaled biopharmaceutical drug development: nonclinical considerations and case studiesInhal Toxicol201325421923223480198
- DolovichMBDhandRAerosol drug delivery: developments in device design and clinical useLancet201137797701032104521036392
- BaileyCJBarnettAHWhy is Exubera being withdrawn?BMJ20073351156
- KuglerAJFabbioKLPhamDQNadeauDAInhaled Technosphere insulin: a novel delivery system and formulation for the treatment of types 1 and 2 diabetes mellitusPharmacotherapy201535329831425809179
- FinebergSEKawabataTTKrasnerASFinebergNSInsulin antibodies with pulmonary delivery of insulinDiabetes Technol Ther20079suppl 1S102S11017563298
- GargSKMathieuCRaisNTwo-year efficacy and safety of AIR inhaled insulin in patients with type 1 diabetes: an open-label randomized controlled trialDiabetes Technol Ther200911suppl 2S5S1619772449
- RaskinPHellerSHonkaMPulmonary function over 2 years in diabetic patients treated with prandial inhaled Technosphere insulin or usual antidiabetes treatment: a randomized trialDiabetes Obes Metab201214216317321951325
- RaveKBottSHeinemannLTime-action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulinDiabetes Care20052851077108215855570
- ForstTHohbergCSchöndorfTTime-action profile and patient assessment of inhaled insulin via the Exubera device in comparison with subcutaneously injected insulin aspart via the FlexPen deviceDiabetes Technol Ther2009112879219848574
- QuattrinTBelangerABohannonNJSchwartzSLEfficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes: results of a 6-month, randomized, comparative trialDiabetes Care200427112622262715504996
- SkylerJSHollanderPAJovanovicLInhaled Human Insulin Type 1 Diabetes Study GroupSafety and efficacy of inhaled human insulin (Exubera) during discontinuation and readministration of therapy in adults with type 1 diabetes: a 3-year randomized controlled trialDiabetes Res Clin Pract200882223824618824271
- MosesRGBartleyPLuntHSafety and efficacy of inhaled insulin (AERx iDMS) compared with subcutaneous insulin therapy in patients with Type 1 diabetes: 1-year data from a randomized, parallel group trialDiabet Med200926326026719317821
- ComuladaALRenardENakanoMEfficacy and safety of AIR inhaled insulin compared to insulin lispro in patients with type 1 diabetes mellitus in a 6-month, randomized, noninferiority trialDiabetes Technol Ther200911suppl 2S17S2519772445
- ChenMCSonajeKChenKJSungHWA review of the prospects for polymeric nanoparticle platforms in oral insulin deliveryBiomaterials2011329826983821925726
- DiabRJaafar-MaalejCFessiHMaincentPEngineered nanoparticulate drug delivery systems: the next frontier for oral administration?AAPS J201214468870222767270
- PatelACholkarKMitraAKRecent developments in protein and peptide parenteral delivery approachesTher Deliv20145333736524592957
- WoitiskiCBNeufeldRJVeigaFCarvalhoRAFigueiredoIVPharmacological effect of orally delivered insulin facilitated by multilayered stable nanoparticlesEur J Pharm Sci2010413–455656320800679
- DamgeCSochaMUbrichNMaincentPPoly(epsilon-caprolactone)/eudragit nanoparticles for oral delivery of aspart-insulin in the treatment of diabetesJ Pharm Sci201099287988919691099
- SonajeKLinKJWeySPBiodistribution, pharmacodynamics and pharmacokinetics of insulin analogues in a rat model: oral delivery using pH-responsive nanoparticles vs subcutaneous injectionBiomaterials201031266849685820619787
- FontePAraujoFReisSSarmentoBOral insulin delivery: how far are we?J Diabetes Sci Technol20137252053123567010
- NavgireSDSatputeASPandeySPatilATRecent patents on oral insulin deliveryRecent Pat Drug Deliv Formul20148320220524981288
- EldorRArbitECorcosAKidronMGlucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot studyPLoS One201384e5952423593142
- KapitzaCZijlstraEHeinemannLCastelliMCRileyGHeiseTOral insulin: a comparison with subcutaneous regular human insulin in patients with type 2 diabetesDiabetes Care20103361288129020185734
- LiJWangYHanLSunXYuHYuYTime-action profile of an oral enteric insulin formulation in healthy Chinese volunteersClin Ther201234122333233823195963
- Novo NordiskA Trial Investigating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of NNC 0148-0000-0106 in Healthy Subjects and Subjects with Type 1 and Type 2 Diabetes2012 Available from: http://novotrials.com/website/pdf/registry/bin_20120423-015136-794.pdf
- LicciardiMPitarresiGCavallaroGGiammonaGNanoaggregates based on new poly-hydroxyethyl-aspartamide copolymers for oral insulin absorptionMol Pharm20131051644165423331253
- SajeeshSSharmaCPCyclodextrin-insulin complex encapsulated polymethacrylic acid based nanoparticles for oral insulin deliveryInt J Pharm20063251–214715416859846
- WuZMLingLZhouLYNovel preparation of PLGA/HP55 nanoparticles for oral insulin deliveryNanoscale Res Lett20127129922682064
- HurkatPJainAJainAShilpiSGulbakeAJainSKConcanavalin A conjugated biodegradable nanoparticles for oral insulin deliveryJ Nanopart Res2012141219
- SharmaGvan der WalleCFRavi KumarMNAntacid co-encapsulated polyester nanoparticles for peroral delivery of insulin: development, pharmacokinetics, biodistribution and pharmacodynamicsInt J Pharm20134409911022227604
- JainSRathiVVJainAKDasMGoduguCFolate-decorated PLGA nanoparticles as a rationally designed vehicle for the oral delivery of insulinNanomedicine (Lond)201271311133722583576
- SunSLiangNPiaoHYamamotoHKawashimaYCuiFInsulin-S.O (sodium oleate) complex-loaded PLGA nanoparticles: formulation, characterization and in vivo evaluationJ Microencapsul201027647147820113168
- MukhopadhyayPSarkarKChakrabortyMBhattacharyaSMishraRKunduPPOral insulin delivery by self-assembled chitosan nanoparticles: in vitro and in vivo studies in diabetic animal modelMater Sci Eng C Mater Biol Appl20133337638225428084
- ElsayedAAl-RemawiMQinnaNFaroukAAl-Sou’odKABadwanAAChitosan-sodium lauryl sulfate nanoparticles as a carrier system for the in vivo delivery of oral insulinAAPS Pharm Sci Tech2011123958964
- MakhlofATozukaYTakeuchiHDesign and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin deliveryEur J Pharm Sci201142544545121182939
- SarmentoBRibeiroAVeigaFSampaioPNeufeldRFerreiraDAlginate/chitosan nanoparticles are effective for oral insulin deliveryPhar Res2007241221982206
- KadirAMokhtarMTWongTWNanoparticulate assembly of mannuronic acid- and guluronic acid-rich alginate: oral insulin carrier and glucose binderJ Pharm Sci2013102124353436324258282
- PaulWSharmaCPSynthesis and characterization of alginate coated zinc calcium phosphate nanoparticles for intestinal delivery of insulinProcess Biochem201247882886
- SonajeKChenYJChenHLEnteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin deliveryBiomaterials201031123384339420149435
- RekhaMRSharmaCPSynthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorptionJ Control Release2009135214415119331862
- CuiFQianFZhaoZYinLTangCYinCPreparation, characterization, and oral delivery of insulin loaded carboxylated chitosan grafted poly(methyl methacrylate) nanoparticlesBiomacromolecules20091051253125819292439
- KebedeASinghAKRaiPKControlled synthesis, characterization, and application of iron oxide nanoparticles for oral delivery of insulinLasers Med Sci201328257958722581389
- SarmentoBMartinsSFerreiraDSoutoEBOral insulin delivery by means of solid lipid nanoparticlesInt J Nanomedicine20072474374918203440
- ZhangZHZhangYLZhouJPLvHXSolid lipid nanoparticles modified with stearic acid-octaarginine for oral administration of insulinInt J Nanomedicine201273333333922848162
- RuggeriGCovolanVLBernabòMMetal nanostructures with magnetic and biodegradable properties for medical applicationsJ Braz Chem Soc201324191200
- JoshiHMBhumkarDRJoshiKPokharkarVSastryMGold nanoparticles as carriers for efficient transmucosal insulin deliveryLangmuir200622130030516378435
- ChoHJOhJChooMKHaJIParkYMaengHJChondroitin sulfate-capped gold nanoparticles for the oral delivery of insulinInt J Biol Macromol201463152024444886
- ZijlstraEHeinemannLPlum-MorschelLOral insulin reloaded: a structured approachJ Diabetes Sci Technol20148345846524876606
- BakshiMSinghHBAbhilashPCThe unseen impact of nanoparticles: more or less?Curr Sci2014106350352