Abstract
Background
Postoperative intra-abdominal adhesions are common complications after abdominal surgery. The exact molecular mechanisms that are responsible for these complications remain unclear, and there are no effective methods for preventing adhesion formation or reformation. The aim of the study reported here was to investigate the preventive effects and underlying potential molecular mechanisms of selective cyclooxygenase-2 (COX-2) inhibitors in a rodent model of postoperative intra-abdominal adhesions.
Materials and methods
The expression of COX-2 in postoperative intra-abdominal adhe-sions and normal peritoneal tissue was examined by immunohistochemistry and Western blot analysis. Assays were performed to elucidate the effect of COX-2 inhibition on hypoxia-induced fibroblast activity in vitro and on intra-abdominal adhesion formation in vivo.
Results
Hypoxia-induced COX-2 expression in peritoneal fibroblasts was increased in postoperative intra-abdominal adhesions. Inhibition of COX-2 attenuated the activating effect of hypoxia on normal peritoneal fibroblasts in vitro. Data indicate that selective COX-2 inhibitor prevents in vivo intra-abdominal adhesion by inhibition of basic fibroblast growth factor and transforming growth factor-beta expression, but not through an antiangiogenic mechanism. Furthermore, using selective COX-2 inhibitors to prevent intra-abdominal adhesions did not adversely affect the weight, bowel motility, or healing of intestinal anastomoses in a rat model.
Conclusion
These results show that hypoxia-induced COX-2 expression in peritoneal fibroblasts is involved in the formation of intra-abdominal adhesions. Inhibition of COX-2 prevents postoperative intra-abdominal adhesions through suppression of inflammatory cytokines.
Introduction
The development of postoperative intra-abdominal adhesions is one of the most common complications after abdominal surgery. Approximately 95% of patients undergoing abdominal surgery will develop adhesions.Citation1 Although adhesions are part of the wound-healing process, they may result in small bowel obstruction, postoperative abdominal pain, infertility, and other serious complications.Citation2 About 15% of patients with adhesions develop bowel obstructions and require lysis, with a resulting mortality of 5%–20% and a high rate of recurrence.Citation3 Thus, postoperative intra-abdominal adhe-sions represent a significant potential risk of additional complications. Accordingly, adhesions are a difficult problem for the surgeon and represent a significant public health cost.Citation4,Citation5 However, the exact molecular mechanisms by which this complication occurs remain unclear.Citation6 At present, there are no effective methods for preventing adhesion formation.Citation7,Citation8
The peritoneum is the serous membrane that covers most of the intra-abdominal organs and is composed of a layer of mesothelial cells with sub-mesothelial tissue that contains plentiful fibroblasts.Citation9 Surgical injuries to the peritoneal surface can result in adhesion formation, as a type of wound healing. The processes that result in either adhesion formation or normal peritoneal tissue repair are dependent on the function of fibroblasts.Citation6 These cells have multiple functions, such as extracellular matrix (ECM) reorganization, collagen synthesis, and wound contraction.Citation10
Following surgical injury to the peritoneum, inflammatory reactions at injury sites can result in the release of protein-enriched serosanguineous fluid and exudates, which cause congealing of the proteinaceous mass. If this congealed mass is not absorbed 3 to 5 days after formation, it will provide a scaffold for fibroblast proliferation and migration from underlying tissues, which can result in ECM deposition and the development of persistent adhesions.Citation11
Hypoxia, resulting from tissue injury, appears to play a role in the pathophysiology of wound healing and adhesion formation.Citation12 Induction of inflammatory markers and ECM proteins in normal peritoneal fibroblasts occurs in response to hypoxia.Citation12,Citation13 Moreover, fibroblasts from adhesions have been found to express cyclooxygenase-2 (COX-2), while normal peritoneal fibroblasts do not. Exposure of normal peritoneal fibroblasts to hypoxia induces COX-2 expression to levels seen in adhesion fibroblasts,Citation14 indicating inhibition of COX-2 may provide the opportunity to reduce postoperative adhesion formation, as COX-derived prostaglandins (PGs) have also been implicated in adhesion formation.
Several COX-2 inhibitors have been shown to have potent ability to prevent intra-abdominal adhesions in small animals. However, the precise mechanism by which this occurs remains poorly understood.Citation15–Citation19 The aim of the study reported here was to investigate the role of COX-2 in postoperative intra-abdominal adhesions and explore the preventive effects and underlying potential molecular mechanisms of selective COX-2 inhibitors in a rodent model of adhesions.
Materials and methods
Human tissue collection
As previously described,Citation20 a small piece of normal parietal peritoneal tissue from the anterior abdominal wall, lateral to the midline incision, or adhesion tissue was removed from patients who underwent laparotomy at the First Affiliated Hospital of the Medical College of Xi’an Jiaotong University. The latter excision was performed at the initiation of the surgery, after entry into the abdominal cavity. All patients gave informed written consent to tissue collection, and the protocol was approved by the Ethics Committee of Xi’an Jiaotong University.
Fibroblast isolation and culture
As described previously,Citation21 harvested tissue samples were immediately placed in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum, 200 μg/mL ampicillin, and 200 μg/mL streptomycin. Tissues were cut into small pieces and transferred into a sterile T-25 flask with dispase solution (Gibco®, Thermo Fisher Scientific, Waltham, MA, USA), and then incubated overnight at 37°C. After centrifu-gation for 5 minutes at 1,400× g, the samples were transferred to culture dishes in a 1:1 (v/v) mixture of Dulbecco’s Modified Eagle’s Medium/Ham’s F12. The cultures were incubated at 37°C in a humidified atmosphere with 5% CO2 for approximately 2 weeks. The selective COX-2 inhibitors celecoxib and valdecoxib (Sigma-Aldrich Co, St Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich Co) and then added into the culture medium for experiments as previously reported.Citation22,Citation23
Hypoxia conditions in cell culture
To evaluate the effects of hypoxia, cells were incubated under normoxic conditions until 65%–70% confluence and subsequently cultured in 2% O2 hypoxic conditions in a tri-gas incubator (HF100; Heal Force Development Ltd, Hong Kong, People’s Republic of China), as described previously.Citation24 Control cultures were placed in normoxic conditions. Cells were harvested at the 24-hour time point. All experiments were repeated three times.
Western blot analysis
Western blot analyses were performed as described previously.Citation25 Total protein was extracted by mammalian protein lysis buffer (Thermo Fisher Scientific). Equivalent amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were incubated with the following primary antibodies overnight at 4°C: anti-COX-2 antibody (Santa Cruz Biotechnology Inc, Dallas, TX, USA; 1:200 dilution), anti-COX-1 antibody (Santa Cruz Biotechnology Inc; 1:500 dilution), anti-hypoxia inducible factor-1 alpha (HIF-1α) antibody (Cell Signaling Technology, Inc, Danvers, MA, USA; 1:800 dilution), anti-vascular endothelial growth factor (VEGF) antibody (Santa Cruz Biotechnology Inc; 1:400 dilution), and anti-beta-actin (β-actin) antibody (Santa Cruz Biotechnology Inc; 1:1,000 dilution). The blots were detected by a secondary antibody coupled to horseradish peroxidase (HRP; Santa Cruz Biotechnology Inc) and an enhanced chemiluminescence system (EMD Millipore). The density of specific protein bands was determined by Image-Pro Plus software (v 5.0; Media Cybernetics, Inc, Rockville, MD, USA).
Quantification of prostaglandin E2
According to the manufacturer’s instructions, prostaglandin E2 (PGE2) concentrations in cell culture supernatant were quantified using a PGE2 enzyme-linked immunosorbent assay kit (R&D Systems, Inc, Minneapolis, MN, USA) after the number of cells was normalized to 0.5×106/mL in six-well culture plates.
Real-time reverse-transcription polymerase chain reaction
Total RNA was extracted from cells using TRIzol reagent (Invitrogen) and reverse transcribed using the PrimeScript™ RT Reagent Kit (TaKaRa, Dalian, China), according to the manufacturer’s instructions. The mRNA levels were measured using the SYBR® Green Real-Time PCR (polymerase chain reaction) Master Mix with PCR primer sequences shown in . Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal housekeeping gene control. Real-time reverse-transcription PCR was performed using an iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories Inc, Hercules, CA, USA). Fold changes in gene expression were quantified based on the ratio of target gene/GAPDH mRNA using the 2-ΔΔCt method.Citation26 The following PCR conditions were used: 5 seconds at 94°C, followed by 40 cycles at 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds.
Immunohistochemistry
Immunohistochemical staining was performed using a strept avidin-biotin complex (SABC) kit (Maixin Biotech. Co., Ltd, Fuzhou, People’s Republic of China) according to the manufacturer’s instructions. The tissue sections were incubated with primary antibodies for COX-1 (1:100 dilution), COX-2 (1:50 dilution), basic fibroblast growth factor (bFGF) (1:100 dilution), transforming growth factor-beta (TGF-β) (1:100 dilution), VEGF (1:100 dilution), and cluster of differentiation (CD) 34 (1:50 dilution) overnight at 4°C, then incubated with the appropriate biotinylated secondary antibody for 30 minutes at room temperature, followed by 30 minutes of incubation with streptavidin peroxidase (Dako LSAB™ + HRP kit). After rinsing, the results were visualized using 3,3′-diaminobenzidine (DAB) tetrahydrochloride and the slides were counterstained with hematoxylin.
Cell proliferation assays
Cell proliferation assays were performed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously.Citation27 Cells treated with DMSO served as a control. At various time points, 20 mL of a 5 mg/mL solution of MTT (Sigma-Aldrich Co) was added to each well, and cells were incubated for an additional 4 hours at 37°C. Following MTT incubation, 150 mL per well of DMSO was added and viable cells were counted by measuring absorbance at 490 nm using a spectrophotometer (Bio-Rad Laboratories Inc).
Lentivirus COX-2 short hairpin RNA transfection
The lentivirus vector short hairpin RNAs (shRNAs) were constructed and synthesized by Shanghai GeneChem Co, Ltd (Shanghai, People’s Republic of China). The shRNA sequences targeting human COX-2 were: 5′-TAA ACA CAG TGC ACT ACA TAC TTA TCA AGA GTA AGT ATG TAG TGC ACT GTG TTT TTT TTT C-3′ and 5′-TCG AGA AAA AAA AAC ACA GTG CAC TAC ATA CTT ACT CTT GAT AAG TAT GTA GTG CAC TGT GTT TA-3′. A lentiviral vector (pGCSIL-GFP) was used as a negative control. The virus titers produced were approximately 109 transducing μ/mL medium. Cells (0.2×106/well) seeded in six-well plates were transfected with lentivirus according to the manufacturer’s instructions. The cells were used for further experiments at 48 hours after transfection. COX-2 mRNA and protein levels were determined using reverse-transcription PCR and Western blotting, respectively.
Animals and formation of experimental adhesion
Male Sprague-Dawley rats weighing 200 to 250 g were purchased from the Animal Center at the College of Medicine, Xi’an Jiaotong University and housed individually in cages under constant temperature (22°C±2°C) with a 12-hour light–dark cycle. The animals were allowed access to water and standard rat chow ad libitum. All animal experimental protocols were approved by the Animal Care and Use Committee of the College of Medicine, Xi’an Jiaotong University.
Animals were anesthetized by inhalation of methoxyflurane for all procedures. Prior to incision, the abdomen was shaved and skin sterilized with Betadine. As described previously,Citation28 a 2–3 cm midline abdominal incision was made and approximately 2–3 cm2 of the anterior cecal surface and the abdominal wall facing the treated cecum were lightly abraded 40 times with a swab to generate petechial hemorrhages. The cecum was placed back into its original position. For the hyaluronan group, 1 mL of medical hyaluronan gel (Qingdao Haitao Biochemical Co., Ltd., Qingdao People’s Republic of China) was daubed on the abraded area. The other group received no treatment. The abdomen was closed in two layers using interrupted 3-0 Vicryl® sutures. A sham operation group was prepared in the same manner, but without abrasion.
Celecoxib (40 mg/kg) was orally administered to the animals twice daily for 7 days as described previously.Citation15 Parecoxib (1 mg/kg) was injected by the tail vein daily for 7 days as described previously.Citation29 The other group of animals was given normal saline solution as a control.
Adhesion evaluation
Seven days after the surgery, the rats were anesthetized, and intra-abdominal adhesion formation was evaluated according to a modified classification system described by Katada et al.Citation30 Assessment was performed by a single researcher blinded to the treatment data of the study. The tenacity was quantified based on the severity of adhesions, and assigned to one of five categories: 0 (no adhesion), 1 (mild, localized, and easy to separate), 2 (moderate), 3 (severe, very widespread, and difficult to separate), and 4 (the most severe). The extent was quantified as a percentage of abrasion with adhesions present and assigned to one of five categories: 0 (no adhesion), 1 (≤25%), 2 (26%–50%), 3 (51%–75%), and 4 (≥75%). The area was scaled as the number of tissues involving adhesion and assigned to one of five categories: 0 (no adhesion), 1 (only the tissues with abrasion), 2 (one other tissue, including the small intestine, liver, omentum, and spleen), 3 (two other tissues), and 4 (three or more other tissues). The final scores ranged from 0 to 12.
Picrosirius red staining for collagen
Picrosirius red staining for collagen was achieved using 0.1% picrosirius red (Direct Red 80; Sigma-Aldrich Co) and counterstained with Weigert’s hematoxylin (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The percentage of positively stained areas was evaluated by Image-Pro Plus software (Media Cybernetics, Inc., Bethesda, MD, USA) in eight randomly selected fields, and the average of the eight values was taken as the collagen content in the adhesions.
Hydroxyproline determination
Hydroxyproline content was determined by hydroxyproline assay kit (Sigma-Aldrich Co) according to the manufacturer’s instructions. Tissue hydroxyproline levels, which were used as an indicator of adhesion severity, were presented as micrograms of hydroxyproline per gram of protein.
Measurement of gastrointestinal transit, anastomotic bursting pressure, and cotton pellet granuloma
Rats were divided randomly into four groups (n=8 per group). Operations were performed as described previously.Citation31 Briefly, all animals underwent general anesthesia; laparotomy; descending intestinal resection (15 cm proximal to the ileocecal junction); and subsequent anastomosis using eight single-layer, everting, interrupted 6/0 monofilament biodegradable sutures. Sterile cotton pellets (30±3 mg) were implanted intraperitoneally in the rats as described by Fu et al.Citation32 The animals then received treatment similar to the rat adhesion formation model. Rats were fed a standard diet and allowed water ad libitum. They were checked for wound healing and passage of stool.
On the 7th day, each rat was administered by gavage a 0.2 mL solution of 1.5% carboxy methylcellulose sodium salt and 5% charcoal as a marker. After 30 minutes, the rats were sacrificed and the cotton pellet removed, dried overnight at 70°C, and weighed. The increased weight of the pellet was taken to be a measure of the weight of granulomas. The gastrointestinal tract was removed. The distance traveled by the charcoal plug from the pylorus to the cecum was determined and expressed as a percentage of the total length of the small intestine. The anastomotic segments approximately 4 cm in length (with the anastomosis in the middle) were resected. The anastomotic bursting pressure was quantified as previously described.Citation31
Statistical analyses
The results are expressed as the mean ± standard error or the median. An independent Student’s t-test was performed to analyze the statistical significance between two groups. Differences among groups were evaluated by Kruskal–Wallis variance analysis, followed by the posthoc Mann–Whitney U- test with SPSS software (v 13.0). P<0.05 was considered statistically significant.
Results
Expression of HIF-1α, COX-1, COX-2, and VEGF in peritoneal adhesion tissues
To determine the possible role of hypoxia-induced COX-2 expression or angiogenesis in intraperitoneal adhesion formation, the expression of HIF-1α, COX-1, COX-2, and VEGF in the surgical cases was explored using Western blotting (). The results show that HIF-1α, COX-2, and VEGF were markedly increased in peritoneal adhesion tissues compared with in normal peritoneum. However, no significant changes were found in the expression of COX-1 protein between adhesion tissues and normal peritoneum. To evaluate COX-2 mRNA levels, real-time reverse-transcription PCR analysis was performed on 17 adhesive tissues and 20 normal peritoneum tissues. The relative level of COX-2 mRNA was increased in the adhesion tissues compared with in normal peritoneum tissues (P<0.05) (). Previous studiesCitation30 have demonstrated that PGE2 is one of the primary COX-2-derived mediators responsible for adhesion formation. Therefore, we focused on PGE2 content in tissues, and found that PGE2 levels in peritoneal adhesions were higher than in normal peritoneum specimens ().
Figure 1 Expression of anti-hypoxia inducible factor-1 alpha (HIF-1α), cyclooxygenase (COX)-1, COX-2, and vascular endothelial growth factor (VEGF) in peritoneal adhesion tissues.
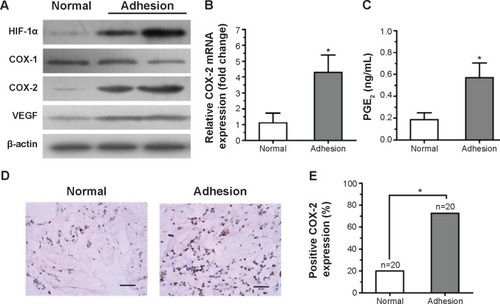
To further confirm these results, COX-2 expression in the surgical cases was explored. Intensive immunohistochemical staining of COX-2 was detected in the cytoplasm of fibroblasts in peritoneal adhesions, whereas there was only rare staining in normal peritoneal tissues ().
Overexpression of COX-2 was detected in 72.5% of peritoneal adhesion samples compared with in only 20% of normal peritoneum samples (P<0.05) (). The results show that the levels of COX-2 protein in peritoneal adhesion tissues were significantly elevated compared to in normal peritoneal tissues.
Hypoxia increased COX-2 expression and activity in fibroblasts from the normal peritoneum to levels similar to those in adhesion fibroblasts
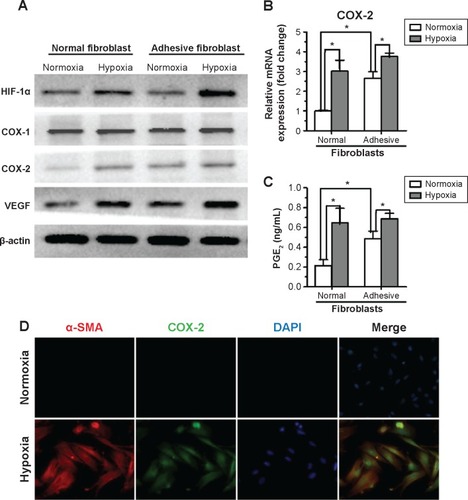
To determine the effect of hypoxia on activation of primary fibroblasts from human normal peritoneum or adhesion fibroblasts, cells were cultured for 24 hours under hypoxic conditions and assessed for protein expression. The results show that hypoxia significantly increased HIF-1α, COX-2, and VEGF levels in normal peritoneal fibroblasts to levels similar to those in adhesion fibroblasts under normoxia. However, hypoxia had no effect on COX-1 expression (). Similar results were observed with COX-2 mRNA levels in normal and adhesion fibroblasts by real-time reverse-transcription PCR (). Furthermore, PGE2 levels in the culture medium showed that PGE2 was significantly increased in normal fibroblasts and slightly increased in adhesion fibroblasts under hypoxia (). Immunofluorescence analysis revealed that COX-2 levels, which were paralleled by levels of alpha-smooth muscle actin (α-SMA), a molecular marker of activation, were more stable and predominantly expressed in normal fibroblasts under hypoxia compared to under normoxia (). These data suggest that hypoxia increases COX-2 expression and activity in fibroblasts from the normal peritoneum to levels similar to those in peritoneal adhesions.
Figure 2 Hypoxia promotes cyclooxygenase (COX)-2 expression and function in fibroblasts from the normal peritoneum.
COX-2 inhibition decreases hypoxic activation of fibroblasts from the normal peritoneum or activated fibroblasts from adhesive tissue
To further determine the role of COX-2 in the activation of fibroblasts from the normal peritoneum in response to hypoxia, a small interfering RNA (siRNA) specific for COX-2 in fibroblasts was used. Downregulation of COX-2 expression was confirmed using Western blotting (). Although the expression of HIF-1α increased in both COX-2 siRNA and control siRNA cells under hypoxia, no significant changes were found in the expression of α-SMA and VEGF under hypoxia in the COX-2 siRNA group (). In contrast, α-SMA and VEGF levels were significantly elevated under hypoxia compared to under normoxia in the control siRNA group.
Figure 3 Cyclooxygenase (COX)-2 inhibition attenuates the activated effect of hypoxia on fibroblasts from normal peritoneum.
Abbreviations: α-SMA, alpha-smooth muscle actin; HIF-1α, anti-hypoxia inducible factor-1 alpha; OD, optical density; VEGF, vascular endothelial growth factor; h, hours.
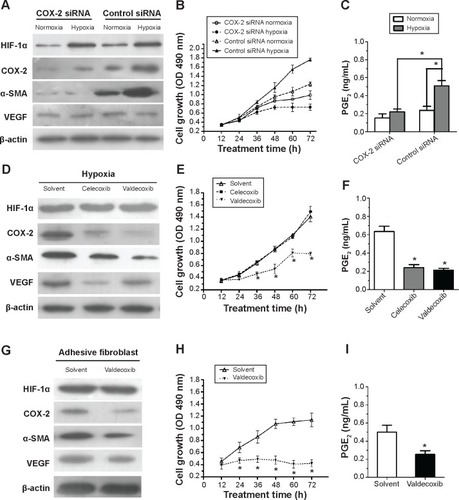
To explore the role of COX-2 on cell proliferation under normoxia and hypoxia, fibroblasts were seeded onto 96-well plates and at various time points the proliferative rates of fibroblasts were determined by MTT assay (). The results show that the proliferation of fibroblasts increased in response to hypoxia compared with in response to normoxia in the control siRNA group (P<0.05). However, inhibition of COX-2 by siRNA completely inhibited the increased cell proliferation caused by hypoxia. Additionally, elevated PGE2 levels induced by hypoxia in cell culture supernatants were significantly decreased in the COX-2 siRNA group compared to in the control siRNA group (P<0.05) ().
Two different selective COX-2 inhibitors were administered to fibroblasts in the presence of hypoxia. As shown in , 10 μM celecoxib or valdecoxib, both highly selective COX-2 inhibitors, had negative effects on COX-2 expression. On the other hand, both celecoxib and valdecoxib reduced α-SMA and VEGF expression in hypoxia-activated fibroblasts. Blockade of COX-2 activity by celecoxib and valdecoxib decreased cell proliferation under hypoxia similar to the inhibition of COX-2 expression by siRNA (). PGE2 levels in cell culture supernatants were significantly downregulated after 48 hours of celecoxib and valdecoxib treatment under hypoxia compared to in untreated controls (). Taken together, these findings strongly indicate that COX-2 inhibition decreases the hypoxic activation of fibroblasts from the normal peritoneum.
Valdecoxib significantly decreased COX-2, α-SMA, and VEGF expression (), inhibited cell proliferation (), and reduced PGE2 levels in adhesion fibroblasts ().
Selective COX-2 inhibitors prevent intra-abdominal adhesions in a rat model
There were no visible differences in weight between the five groups and all animals appeared healthy. The control animals developed bowel-to-parietal peritoneum, omentum-to-parietal peritoneum, bowel-to-bowel, and omentum-to-bowel adhesions. Animals that underwent sham operation surgery barely developed any intra-abdominal adhesions. In contrast, the animals treated with selective COX-2 inhibitors (ie, celecoxib and parecoxib) had fewer intra-abdominal adhesions than controls. However, intra-abdominal adhesions were only slightly fewer in the animals treated with hyaluronan ().
Figure 4 Cyclooxygenase (COX)-2 inhibition attenuates the activated effect of hypoxia on fibroblasts from normal peritoneum.
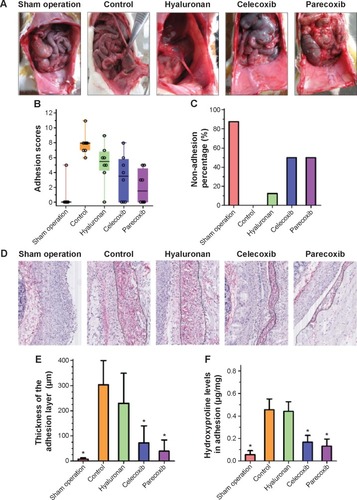
Adhesion scores for the macroscopic classification were found to be significantly different between the five groups (). In the celecoxib group and parecoxib group, the macroscopic classification scores were lower than in the control or hyaluronan group. However, the parecoxib group had lowest adhesion scores of all groups. With regards to preventing adhesions, there was a significant difference between two COX-2 selective inhibitor treatment groups (celecoxib and parecoxib), the control group (P<0.05), and the hyaluronan group (P<0.05) ().
As shown in , the collagen content of adhesions was significantly decreased in the celecoxib group and the parecoxib group compared to in the control group. Moreover, there were significant decreases in hydroxyproline levels in both COX-2 selective inhibitor treatment groups compared to in the control group (P<0.05) and the hyaluronan group (P<0.05) (). Both the macroscopic and objective data suggest that selective COX-2 inhibitors prevent intra-abdominal adhesions in the rat model.
The effect of selective COX-2 inhibitors on intra-abdominal adhesion prevention possibly results from inhibition of bFGF and TGF-β expression
HIF-1α expression in adhesion tissues was increased in the four groups that underwent mechanical injury, regardless of the interventions (). Compared with the control group, both celecoxib and parecoxib decreased COX-2 expression in peritoneal adhesion tissue (). COX-2 expression was only slightly decreased in the hyaluronan group. These results are consistent with the results of Western blotting and COX-2 mRNA levels (). Furthermore, mRNA levels of type I collagen and α-SMA were significantly decreased by celecoxib or parecoxib treatment when compared with the control (). Using the presence of α-SMA as a marker of fibroblast activation, we determined that α-SMA was downregulated after celecoxib or parecoxib treatment compared with in the control and hyaluronan groups. These results provide additional evidence that the inhibition of intra-abdominal adhesion indeed involves fibroblast growth inhibition ().
Figure 5 Selective cyclooxygenase (COX)-2 inhibitors decreased COX-2 expression in injured peritoneum.
Abbreviation: HIF-1α, anti-hypoxia inducible factor-1 alpha.
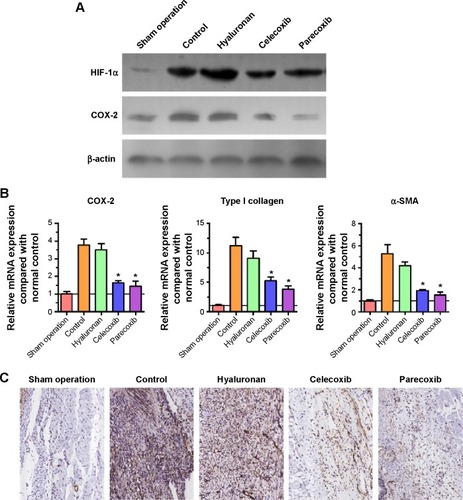
Immunohistochemical analysis showed that the expression of COX-2 in rat intra-abdominal adhesion tissue was increased after treatment with the selective COX-2 inhibitors in the control group compared with the normal peritoneum in the sham operation group (). The sections from peritoneal adhesions stained intensely positive for inflammatory cytokines bFGF and TGF-β in the control group, confirming the presence of an inflammatory process ( and C). The expression of bFGF and TGF-β were significantly lower in both the celecoxib and parecoxib treatment groups than in the control group and the hyaluronan group.
Figure 6 Immunohistochemical analysis of cyclooxygenase (COX)-2 (A), basic fibroblast growth factor (bFGF) (B), transforming growth factor-beta (TGF-β) (C), vascular endothelial growth factor (VEGF) (D), and cluster of differentiation (CD) 34 (E) in intra-abdominal adhesion tissues from groups that underwent peritoneum and cecum abrasion, and normal peritoneum in the sham operation group.
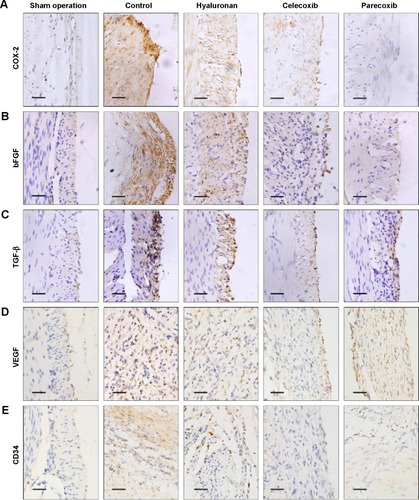
Because a previous studyCitation15 claimed that post-operation intra-abdominal adhesion formation was possibly dependent on angiogenesis, immunohistochemical staining for a vital angiogenesis factor, VEGF, and an endothelial cell marker, CD34, was performed to identify new blood microvessels. In the control group sections, the expression of VEGF was significantly higher than in those of the sham operation group, which had negative or very low levels (). However, compared with the control group, the level of VEGF expression was not changed in the parecoxib treatment group, but was decreased in the celecoxib treatment group ().
Compared to normal peritoneum in the sham operation group, the adhesion tissues in the control group were well vascularized, containing a large number of microvessels (). The microvessel density in the hyaluronan treatment group was not different to that in the control animals. The microvessel density in the celecoxib-treated, but not the parecoxib-treated, mice was less than that in the control mice, in spite of the significant effect of parecoxib on prevention of intra-abdominal adhesion formation and inhibition of bFGF and TGF-β expression ().
Selective COX-2 inhibitors prevented intra-abdominal adhesion, but not postoperative complications
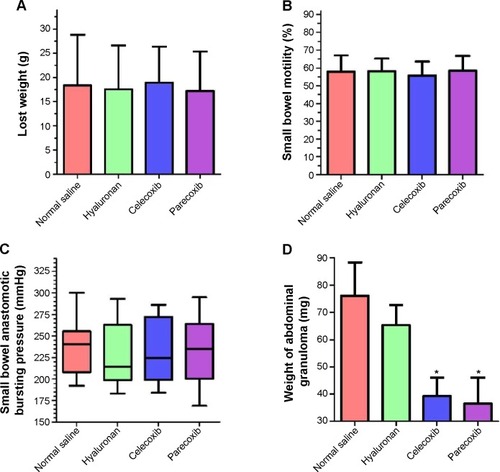
The effects of two selective COX-2 inhibitors on postoperative recovery and inflammation were observed in a rat intestinal anastomosis model. No deaths or abdominal wound complications were observed. Weight loss in all rats was assessed on day 7 before the animals were euthanized; there was no difference between the four groups (). Thirty minutes before sacrifice, all animals were gavaged with charcoal. The propelled distance of the charcoal within the gastrointestinal tract in the mice was measured. As shown in , there was no difference in the propulsive function of the gastrointestinal tract between the groups. Additionally, the intestinal anastomotic bursting pressures shown in were not significantly different between the four groups. Both of the two selective COX-2 inhibitors, especially parecoxib, significantly decreased granuloma weight (approximately 50%) compared to that of the normal saline group (P<0.05) and the hyaluronan group (P<0.05) (). However, there was no significant difference in granuloma weight between the normal saline group and the hyaluronan group (P>0.05). These results indicate that inhibition of COX-2 may decrease inflammation, but does not affect postoperative recovery.
Figure 7 Use of selective cyclooxygenase (COX)-2 inhibitors to prevent intra-abdominal adhesion did not contribute to postoperative complications.
Discussion
In the current study, it was shown that hypoxia-induced COX-2 expression in peritoneal fibroblasts was involved in postoperative intra-abdominal adhesion formation. Inhibition of COX-2 resulted in decreased intra-abdominal adhesions in vivo. The data are consistent with the expression of COX-2 observed in adhesion fibroblasts.Citation33 Treatment with selective COX-2 inhibitor has been shown previously to reduce the formation of postoperative adhesions in a rat model.Citation15,Citation34 However, in the current study, it was further shown that in vitro COX-2 inhibition decreased the effect of hypoxic activation on fibroblasts from the normal peritoneum. We provide evidence that the effect of selective COX-2 inhibitors on prevention of intra-abdominal adhesion possibly results from inhibition of bFGF and TGF-β expression, but not through an antiangiogenic mechanism. Moreover, the use of selective COX-2 inhibitors to prevent intra-abdominal adhesion did not result in postoperative complications in the rat model.
Hypoxia, resulting from tissue injury, has been suggested to play an important role in wound healing,Citation35,Citation36 and may directly participate in the development of postoperative adhesions.Citation37 Previous in vitro and in vivo studiesCitation12,Citation21 have documented the gross and histological changes that may be triggered by hypoxia in peritoneal wounds. Moreover, it has been reported that exposure of normal peritoneal fibroblasts to hypoxia irreversibly induces TGF-β1 and type I collagen to levels seen in adhesion fibroblasts.Citation21 Here, we present compelling evidence that abnormal COX-2 expression in adhesive tissue via a hypoxia-dependent manner results in fibroblast activation.
COX-2 expression increases in response to various types of tissue injury, resulting in elevated levels of local PGs.Citation38 Peritoneal tissue damage during operations can result in induction of COX-2 expression during the healing process, with subsequent persistence of COX-2 expression by adhesion fibroblasts.Citation33 The current results show that exposure of normal peritoneal fibroblasts to hypoxia significantly increases COX-2 expression and the enzymatic activity product PGE2. Based on previously published evidence and the current findings, we speculate that inhibition of COX-2 results in reversal of the activation of normal peritoneal fibroblasts in response to hypoxia, which may help prevent adhesion formation. This notion is further supported by the fact that the genetic or pharmacologic inhibition of COX-2 by shRNA or selective COX-2 inhibitors, respectively, decreased activation of normal peritoneal fibroblasts under hypoxia, suggesting that inhibition of COX-2 may be a potential target for the prevention of intra-abdominal adhesions.
A large amount of evidenceCitation39,Citation40 has demonstrated that COX-2 is involved in pathologic inflammation and angio-genesis in various diseases. A previous reportCitation15 claimed that COX-2 inhibitors, through their antiangiogenic properties, inhibit intra-abdominal adhesions in an experimental murine model. In the current study, it was found that, although both COX-2 inhibitors (celecoxib and parecoxib) have anti-adhesion properties, the VEGF levels and microvessel densities were unchanged in the parecoxib-treated group compared to in the control group. The inflammatory factors bFGF and TGF-β were significantly decreased in response to COX-2 inhibition. Hence, we conclude that COX-2 inhibition prevents postoperative intra-abdominal adhesions by attenuating hypoxia-induced activation of peritoneal fibroblasts rather than anti-angiogenesis. Because wound healing is dependent on angiogenesis, there previously existed awareness that agents with antiangiogenic effect have been precluded as anti-adhesion agents because of their possibly negative effects on wound healing.Citation16,Citation19 Our results may help to eliminate this doubt of the inhibition of COX-2 as a prevention strategy for adhesions.
Parecoxib, which is a water soluble and injectable prodrug of valdecoxib,Citation41 can be used perioperatively when patients are unable to take oral medications. Because inhibition of postoperative intestinal motility can result in an increased number of adhesions,Citation42 special attention was paid to small bowel motility and intestinal anastomotic healing in the experiment animal; it was found that there was no adverse effect of parecoxib on post-operation recovery. Furthermore, parecoxib is approved through much of Europe for short-term perioperative pain control.Citation43 Thus, we believe the COX-2 inhibitor parecoxib is a safe medicine for preventing the formation of postoperative adhesions.
Limitations and future directions
Unfortunately, the results of this study are not robust and should be further validated. A major weakness of this in vivo study is its reliance on one small animal model of adhesion. Moreover, there is a lack of accurate methods to evaluate severity of adhesions. Clinical trials need to be performed to confirm the beneficial properties and suitable dose of parecoxib in reducing adhesions.
Conclusion
We found that hypoxia-induced COX-2 expression in peritoneal fibroblasts is involved in postoperative intra-abdominal adhesion formation. The current results suggest that COX-2 inhibition can prevent postoperative intra-abdominal adhe-sions by decreasing hypoxia-induced activation of peritoneal fibroblasts rather than by anti-angiogenesis. This study indicates that inhibition of COX-2 may represent a promising preventive strategy for postoperative intra-abdominal adhesions.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (number 81201824), the Scientific and Technological Development Research Project Foundation by Shaanxi Province (number 2007K12-02), and the Fundamental Research Funds for the Central Universities in Xi’an Jiaotong University (number 2013jdhz33). We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Supplementary material
Table S1 Real-time PCR primer sequence
Disclosure
The authors declare no conflicts of interest in this work.
References
- ten BroekRPIssaYvan SantbrinkEJBurden of adhesions in abdominal and pelvic surgery: systematic review and met-analysisBMJ2013347f558824092941
- FevangBTFevangJLieSASøreideOSvanesKVisteALong-term prognosis after operation for adhesive small bowel obstructionAnn Surg2004240219320115273540
- DuronJJSilvaNJdu MontcelSTAdhesive postoperative small bowel obstruction: incidence and risk factors of recurrence after surgical treatment: a multicenter prospective studyAnn Surg2006244575075717060768
- MavrosMNVelmahosGCLeeJLarentzakisAKaafaraniHMMorbidity related to concomitant adhesions in abdominal surgeryJ Surg Res2014192228629225151471
- OkabayashiKAshrafianHZacharakisEAdhesions after abdominal surgery: a systematic review of the incidence, distribution and severitySurg Today201444340542023657643
- ArungWMeurisseMDetryOPathophysiology and prevention of postoperative peritoneal adhesionsWorld J Gastroenterol201117414545455322147959
- DiamondMPWexnerSDdiZeregGSAdhesion prevention and reduction: current status and future recommendations of a multinational interdisciplinary consensus conferenceSurg Innov201017318318820798093
- WardBCPanitchAAbdominal adhesions: current and novel therapiesJ Surg Res201116519111120036389
- WangNLiQZhangLMesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6PLoS One201278e4376822912904
- ElliottCGWangJGuoXPeriostin modulates myofibroblast differentiation during full-thickness cutaneous wound repairJ Cell Sci2012125Pt 112113222266908
- ReijnenMMBleichrodtRPvan GoorHPathophysiology of intra-abdominal adhesion and abscess formation, and the effect of hyaluronanBr J Surg200390553354112734857
- AmblerDRFletcherNMDiamondMPSaedGMEffects of hypoxia on the expression of inflammatory markers IL-6 and TNF-a in human normal peritoneal and adhesion fibroblastsSyst Biol Reprod Med201258632432923043632
- SaedGMZhangWDiamondMPMolecular characterization of fibroblasts isolated from human peritoneum and adhesionsFertil Steril200175476376811287032
- SaedGMMunkarahARAbu-SoudHMDiamondMPHypoxia upregulates cyclooxygenase-2 and prostaglandin E(2) levels in human peritoneal fibroblastsFertil Steril200583Suppl 11216121915831295
- GreeneAKAlwaynIPNoseVPrevention of intra-abdominal adhesions using the antiangiogenic COX-2 inhibitor celecoxibAnn Surg2005242114014615973112
- CahillRAPrevention of intra-abdominal adhesions using the antiangiogenic COX-2 inhibitor celecoxibAnn Surg20062442327328 author reply 32816858202
- KimYIComparative study for preventive effects of intra-abdominal adhesion using cyclo-oxygenase-2 enzyme (COX-2) inhibitor, low molecular weight heparin (LMWH), and synthetic barrierYonsei Med J20135461491149724142656
- ArungWJehaesFCheramyJPEffects of parecoxib on the prevention of postoperative peritoneal adhesions in ratsJ Invest Surg201326634034623927529
- CahillRASheehanKMScanlonRWMurrayFEKayEWRedmondHPEffects of a selective cyclooxygenase 2 inhibitor on colonic anastomotic and skin wound integrityBr J Surg200491121613161815505871
- SaedGMKrugerMDiamondMPExpression of transforming growth factor-beta and extracellular matrix by human peritoneal mesothelial cells and by fibroblasts from normal peritoneum and adhesions: effect of TisseelWound Repair Regen200412555756415453838
- FletcherNMJiangZLDiamondMPAbu-SoudHMSaedGMHypoxia-generated superoxide induces the development of the adhesion phenotypeFree Radic Biol Med200845453053618538674
- PaulissenSMvan HamburgJPDavelaarNAsmawidjajaPSHazesJMLubbertsESynovial fibroblasts directly induce Th17 patho-genicity via the cyclooxygenase/prostaglandin E2 pathway, independent of IL-23J Immunol201319131364137223817417
- Wiktorowska-OwczarekAThe effect of valdecoxib on the production of growth factors evoked by hypoxia and bacterial lipopolysaccharide in HMEC-1 cellsAdv Clin Exp Med201322679580024431307
- LeiJHuoXDuanWα-Mangostin inhibits hypoxia-driven ROS-induced PSC activation and pancreatic cancer cell invasionCancer Lett2014347112913824513179
- LiXWangZMaQSonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancerClin Cancer Res201420164326433824947933
- SchmittgenTDLivakKJAnalyzing real-time PCR data by the comparative C(T) methodNat Protoc2008361101110818546601
- LiXMaQXuQSDF-1/CXCR4 signaling induces pancreatic cancer cell invasion and epithelial-mesenchymal transition in vitro through non-canonical activation of Hedgehog pathwayCancer Lett2012322216917622450749
- PeytonCCKeysTTomblynSHalofuginone infused keratin hydrogel attenuates adhesions in a rodent cecal abrasion modelJ Surg Res2012178254555222901798
- Schuh-HoferSTayefehMReuterUDirnaglUArnoldGEffects of parecoxib on plasma protein extravasation and c-fos expression in the ratHeadache200646227628516492237
- KatadaJSaitoHOhashiASignificance of cyclooxygenase-2 induced via p38 mitogen-activated protein kinase in mechanical stimulus-induced peritoneal adhesion in miceJ Pharmacol Exp Ther2005313128629215576468
- AaronsCBCohenPAGowerAStatins (HMG-CoA reductase inhibitors) decrease postoperative adhesions by increasing peritoneal fibrinolytic activityAnn Surg2007245217618417245169
- FuFHouYJiangWWangRLiuKEscin: inhibiting inflammation and promoting gastrointestinal transit to attenuate formation of postoperative adhesionsWorld J Surg2005291216141620 discussion 1621–162216311848
- SaedGMMunkarahARDiamondMPCyclooxygenase-2 is expressed in human fibroblasts isolated from intraperitoneal adhesions but not from normal peritoneal tissuesFertil Steril20037961404140812798889
- GuvenalTCetinAOzdemirHYanarOKayaTPrevention of postoperative adhesion formation in rat uterine horn model by nimesulide: a selective COX-2 inhibitorHum Reprod20011681732173511473974
- RuthenborgRJBanJJWazirATakedaNKimJWRegulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1Mol Cells201437963764324957212
- LokmicZMusyokaJHewitsonTDDarbyIAHypoxia and hypoxia signaling in tissue repair and fibrosisInt Rev Cell Mol Biol201229613918522559939
- SaedGMDiamondMPEffects of interferon-gamma reverse hypoxia-stimulated extracellular matrix expression in human peritoneal and adhesion fibroblastsFertil Steril200685Suppl 11300130516616105
- CesarioARoccaBRutellaSThe interplay between indoleamine 2,3-dioxygenase 1 (IDO1) and cyclooxygenase (COX)-2 in chronic inflammation and cancerCurr Med Chem201118152263227121517752
- SalvadoMDAlfrancaAHaeggströmJZRedondoJMProstanoids in tumor angiogenesis: therapeutic intervention beyond COX-2Trends Mol Med201218423324322425675
- AlhouayekMMuccioliGGCOX-2-derived endocannabinoid metabolites as novel inflammatory mediatorsTrends Pharmacol Sci201435628429224684963
- HullettBSalmanSO’HalloranSJPeirceDDaviesKIlettKFDevelopment of a population pharmacokinetic model for parecoxib and its active metabolite valdecoxib after parenteral parecoxib administration in childrenAnesthesiology201211651124113322450476
- OuaïssiMGaujouxSVeyrieNPost-operative adhesions after digestive surgery: their incidence and prevention: review of the literatureJ Visc Surg20121492e104e11422261580
- AthanasakisKPetrakisIVitsouEPimenidouAKyriopoulosJA cost-effectiveness analysis of parecoxib in the management of postoperative pain in the Greek health care settingClin Ther20133581118112423867113
