Abstract
Glaucoma is a common eye disease that can lead to irreversible vision loss if left untreated. The early diagnosis and treatment of primary open-angle glaucoma is challenging, and visual impairment in Chinese glaucoma patients is a serious concern. Most of these patients need more than one topical antiglaucoma agent to control their intraocular pressures (IOPs). In the People’s Republic of China, the daily cost of different glaucoma medication varies greatly, and the treatment habits differ throughout the country. Prostaglandin analogs (PGAs) are recommended as first-line monotherapy, because of their efficacy and low risk of systemic side effects. Fixed-combination drops, particularly PGA-based fixed combinations, have recently been developed and used in patients with progression or who have failed to achieve their target IOPs. Here, we reviewed the current literature on the use of bimatoprost-timolol fixed combination (BTFC) in the People’s Republic of China. BTFC has achieved good efficacy and tolerability in Chinese clinical trials. In addition, BTFC is more cost effective compared with other fixed combinations available in the People’s Republic of China. Fixed-combination drops may offer benefits, such as keeping the ocular surface healthy, convenience of administration, and improvement in long-term adherence and quality of life. Therefore, BTFC has great potential for the treatment of Chinese glaucoma patients. However, the long-term efficacy of BTFC, comparisons of BTFC with other fixed-combination drugs, and treatment adherence and persistence with treatment in Chinese patients are unknown and will require further study.
Keywords:
Outline of Chinese glaucoma patients and the management issues with antiglaucoma drops
Glaucoma is a chronic, progressive disease in which retinal ganglion cells disappear, with a subsequent gradual reduction in the visual field. A glaucoma-related, population-based study in Asia showed a higher prevalence of glaucoma in Asian patients, including a higher incidence of primary angle-closure glaucoma than in Caucasian patients, with primary open angle glaucoma (POAG) being the most commonly reported.Citation1 As reported, the prevalence of glaucoma in the People’s Republic of China is approximately 1.95%–3.60%.Citation2 The prevalence of POAG is 0.86%–2.85% in adults aged 50 years and older living in different places in the People’s Republic of China,Citation3–Citation7 and this is expected to rise substantially in the coming years as the population ages and the prevalence of myopia increases. There are an estimated 15 million glaucoma patients in the People’s Republic of China, including three million who are blind in one eye and 1.2 million who are blind in both eyes. The medical expense of treating glaucoma in the People’s Republic of China is about 4.8 billion USD annually; accordingly, the prevention and treatment of glaucoma has become an important public health issue in the People’s Republic of China.Citation8
The goal of glaucoma treatment is to reduce or prevent the loss of visual function and, thus, preserve quality of life. Lowering intraocular pressure (IOP) is currently the only therapeutic approach that preserves visual function.Citation9–Citation14
Due to the uneven economic and medical care conditions in the People’s Republic of China, POAG is often diagnosed at late stage. A previous Chinese survey showed that at diagnosis, 75% of patients already had severely damaged visual function with mid- or late-stage glaucoma in at least one eye.Citation15 The result of Beijing Eye Study conducted in 2001 revealed that in patients found to have POAG, this was a new diagnosis in 92.30% of these patients who were from rural areas and in 87.30% from urban areas.Citation16 Patients with newly discovered POAG had severely impaired visual function, and approximately one-fourth of these POAG patients (26.92% in rural and 23.64% in urban) had at least one eye with corrected visual acuity ≤0.3 (low visual acuity, according to the World Health Organization classification). The percentage of POAG patients with monocular blindness was 15.40% and 10.90% in rural and urban areas, respectively.Citation16 Because of the late diagnosis, these patients need a substantial reduction in IOP to reach their target IOP. In 2007, we performed a cross-sectional survey on the treatment adherence of the antiglaucoma drops to treat Chinese patients at nine hospitals in Beijing and Shanghai and found that 35% (70/202) of glaucoma or ocular hypertension (OHT) patients used one antiglaucoma drop and 65% (132/202) patients needed more than one antiglaucoma drops to control their IOP (Sun X et al, unpublished data, 2007). This cross-sectional survey was carried out in 2007 in nine major hospitals in Beijing and Shanghai, which are the most developed cities in the People’s Republic of China, and the results gave us an impression that most Chinese patients needed more than one medication to control their IOPs. With the increasing usage of prostaglandin analogs (PGAs) and PGA-timolol fixed combinations, the number of antiglaucoma eye drops used in Chinese glaucoma patients will change and need further study.
The Chinese Glaucoma Society consensus (2014 version) state that glaucoma treatment should focus on lowering IOP to an individualized target to slow disease progression and thus ensure that visual impairment does not occur during the patient’s lifetime.Citation17 Among the various treatment options currently available, the Chinese Glaucoma Society guidelines recommended PGAs as the first-line treatment because they are administrated once daily and have a higher IOP-lowering efficacy and lower frequency of systemic side effects compared with other antiglaucoma drops.Citation17
There is more variation in the daily cost of glaucoma medications in the People’s Republic of China compared with that in developed countries.Citation18,Citation19 In the People’s Republic of China, the cost of a bottle of antiglaucoma eye drops ranges from 0.69 USD (0.5% timolol; Wuhan Wujin Drug Company, Wuhan, Hubei Province, People’s Republic of China) to 40.07 USD (0.005% latanoprost/0.5% timolol fixed combination [LTFC]; Xalacom, Pfizer, New York, NY, USA). Although the Chinese Glaucoma Society recommends PGAs as the first-line treatment for POAG, the relatively high price of PGAs has affected their market share in the People’s Republic of China, particularly in less-developed areas in the People’s Republic of China. Surveys on the Chinese glaucoma medication market performed in 2013 (IMS, China Hospital Audit, unpublished data, 2014 quarter 2) showed that in terms of number, beta-blockers accounted for 44% of all glaucoma medications, whereas PGAs accounted for only 14%. However, in terms of annual growth, PGAs increased by 29% compared with 10% for beta-blockers. These findings suggest that the PGAs are increasingly accepted as the first-choice treatment by Chinese ophthalmologists and glaucoma patients. According to the surveys conducted by Livingston Market Consultants in 2012 (Allergen, unpublished data, 2012), Chinese ophthalmologists in more developed cities have higher recognition of PGAs than do those in less developed cities. A total of 31% of physicians in more developed cities preferred PGAs as the first-choice glaucoma medicine compared with 13% in less developed cities. These differences accounted for the variation in glaucoma treatments across the People’s Republic of China.
In conclusion, the population of glaucoma patients in the People’s Republic of China is large, and the early diagnosis of POAG in the People’s Republic of China remains challenging. Visual impairment in Chinese glaucoma patients is serious, with most patients requiring more than one antiglaucoma eye drop to control their IOPs. Although the GCS recommends PGAs as the first-line treatment, there are large differences in glaucoma treatment patterns across the People’s Republic of China.
Overview of PGA-timolol combination therapies in the People’s Republic of China and the rationale for combination
Three types of PGAs are currently available and widely used in the People’s Republic of China: 0.03% bimatoprost (Lumigan®; Allergan, Irvine, CA, USA), 0.005% latanoprost (Xalatan®; Pfizer), and 0.004% travoprost (Travatan®; Alcon, Fort Worth, TX, USA). The latter two are prodrug esters that are converted into biologically active acids by corneal enzymes and then bind to the prostaglandin F2α receptor. Bimatoprost is viewed as a prostamide and might have a slightly different mode of action than the other two agents.Citation20 The IOP-lowing effect of bimatoprost is to improve aqueous humor drainage, primarily through the uveoscleral outflow pathwayCitation21 and partly through the trabecular outflow pathway.Citation22
Many patients are unable to achieve their target IOP using monotherapy alone, even with PGAs,Citation14,Citation23 and our study (Sun X et al, unpublished data, 2007), suggested 65% Chinese glaucoma patients need additional medications. Therefore, it is recommended that agents that decrease IOP via different pathways should be combined, one improving aqueous humor outflow and another reducing the production of aqueous humor. Timolol, as a beta-blocker, is the oldest eye drop for reducing the production of aqueous humor and is widely used around the world, while PGAs, as a new type of improved aqueous outflow agent, are also used in more and more countries and regions. When multiple medications are required, fixed combinations should be preferable than two or more separate instillations of agents and have several advantages over the same medications used concomitantly, including convenience, a simpler regimen, better quality of life, avoidance of washout, and decreased exposure to preservatives.Citation24–Citation26
Today, there are two PGA-timolol fixed combinations available in the People’s Republic of China, including LTFC, launched in the People’s Republic of China mainland on March 2009, and a bimatoprost-timolol fixed combination (BTFC) (Ganfort®; Allergan) launched in the People’s Republic of China mainland on June 2014.
BTFC, a fixed combination of 0.03% bimatoprost and 0.5% timolol, was approved in May 2006 by the European Medicines Agency for IOP reduction in patients with open-angle glaucoma or OHT. At present, its use and marketing has been approved in more than 30 countries and regions worldwide.
Efficacy studies of bimatoprost and BTFC, including comparative studies
Efficacy of bimatoprost
Several studies, conducted in different countries, have assessed the efficacy of PGA monotherapies on POAG and OHT. In 2005, Holmstrom et al conducted a meta-analysis reviewing the efficacy of three PGAs as the monotherapy and showed that the mean IOP reduction was 26.7%, 28.7%, and 30.3% for latanoprost, travoprost, and bimatoprost, respectively, compared with 22.2% for timolol. In terms of the proportion of patients that achieved a target IOP, bimatoprost appeared to be the most efficacious agent for any target IOP between 13 and 20 mmHg, although the travoprost studies were too few to permit meaningful comparisons.Citation27
To study the efficacy of bimatoprost in Chinese patients, we conducted a prospective, open-label, multicenter clinical study in the People’s Republic of China. A total 263 Chinese patients with POAG and OHT who needed initial or additional IOP-lowering were recruited in this study and were treated with bimatoprost 0.03% once daily in the evening. IOP was measured between 8:00 am and 10:00 am before treatment and after 3 months of treatment. The data revealed that bimatoprost achieved a similar IOP reduction in Chinese patients to those from other countries, as shown in . Treatment-naïve patients achieved a reduction in IOP of 8.0±3.7 mmHg (32%) 3 months after commencing bimatoprost monotherapy. In patients who had previously received various therapy regimens, an additional IOP reduction, ranging from 1.9±2.8 mmHg (9.5%) to 6.4±6.1 mmHg (24.8%), was achieved after switching to bimatoprost monotherapy or bimatoprost combination therapy.Citation28
Table 1 Mean IOP change from baseline, by bimatoprost therapy, in Chinese patients at the 3-month visit
A few clinical studies published in Chinese journals have compared the efficacy of the three PGAs available in the People’s Republic of China and found no significant difference in IOP reduction among these agents in Chinese patients. In 2005, Jin et al reported a single-center, randomized, investigator-masked, parallel-group clinical trial. A total of 56 patients with POAG or OHT received bimatoprost or latanoprost once daily in the evening. IOPs were measured at 8:30 am, 11:30 am, 2:00 pm, and 4:30 pm, before and after 6 weeks of treatment. After 6 weeks, the daily average IOP reduction was 6.95±3.24 mmHg (29.9%) in the bimatoprost group, while the IOP reduction was 8.18±3.89 mmHg (34.3%) in the latanoprost group. The greatest IOP reductions occurred at 8:30 am in both groups. There was no statistically significant difference between the two groups.Citation29 In 2006, Kong et al reported a single-center, randomized, investigator-masked, parallel-group clinical trial in which 102 POAG or OHT patients received one of three PGAs including bimatoprost, latanoprost and travoprost once daily at 8:00 pm IOPs were measured at four time points (8:30 am, 11:00 am, 1:30 pm, and 4:00 pm). The greatest IOP reductions occurred at 8:30 am in all three groups. After 4 weeks, the IOP at 8:30 am decreased from 24.57±3.68 mmHg to 15.29±2.67 mmHg, a reduction of 37.8%, in the latanoprost group, while the IOP decreased from 24.54±2.95 mmHg to 16.29±3.11 mmHg, a reduction of 33.6%, in the travoprost group, and the IOP decreased from 25.41±3.63 mmHg to 16.00±4.45 mmHg, a reduction of 37%, in bimatoprost group. They found no statistically significant difference in effectiveness among the three PGA monotherapies.Citation30 In 2011, Huang et al reported a case-controlled observational clinical study. A total of 63 POAG patients enrolled in the study and their 24-hour IOPs (every 2 hours) were measured, before and 4 weeks after PGA monotherapy. The daily average IOP in the latanoprost group decreased from 18.9±2.1 mmHg to 15.3±2.7 mmHg, and the extent of the decrease was 19%. The IOP in travoprost group decreased from 18.6±1.9 mmHg to 15.3±2.1 mmHg, with a decrease of 19.4%. The IOP in the bimatoprost group decreased from 18.6±1.9 mmHg to 14.9±1.9 mmHg, with a decrease of 19.9%. The IOP reductions in the three groups had no significant difference.Citation31 The low decreases of IOP in this study might have been due to IOP baseline levels before treatment that were lower than in other studies. The circadian variations in IOP in the three groups did not differ significantly.
In summary, the three PGAs cause similar IOP reductions in Chinese POAG or OHT patients. However, all those comparative studies were single-center studies, and the cases were limited and the observation done for a short time. The long-term efficacy of the three PGAs in Chinese patients needs further studies in the future.
Efficacy of BTFC
A multicenter, observational, noncontrolled, open-label study throughout Europe that enrolled 5,556 patients showed that BTFC lowered the mean IOP from baseline by 5.4 mmHg over the 12-week study period. In patients with no previous treatment (n=311), BTFC reduced IOP by 9.1 mmHg, corresponding to a reduction from baseline of 36.4%. In patients that had received prior therapy of a PGA, a beta-blocker, or another fixed combination, BTFC reduced IOP by a further 24.5%, 25.9%, and 21.4%, respectively. In addition, BTFC achieved long-term IOP-lowing efficacy.Citation32,Citation33
Several clinical studies conducted in European countries have reported the superior efficacy of BTFC compared with bimatoprost monotherapyCitation25,Citation33–Citation35 or latanoprost monotherapy.Citation32,Citation36 There has been no comparative study conducted in the People’s Republic of China on the efficacy of BTFCs against PGA monotherapies to date.
To assess the efficacy of BTFC in Chinese patients, we carried out a multicenter, randomized, double-masked, parallel controlled study. Patients with POAG or OHT who were insufficiently responsive to monotherapy using either topical beta-blockers or PGAs were randomized into one of two treatment groups, in a 1:1 ratio, at eleven Chinese ophthalmic centers. The BTFC group received one drop of a BTFC (fixed combination of 0.03% bimatoprost and 0.5% timolol), followed by one drop of vehicle, for masking, once daily at 7:00 pm, and the unfixed-combination group received one drop of 0.03% bimatoprost followed by one drop of 0.5% timolol (Allergan) once daily at 7:00 pm. The results are shown in . The intent-to-treat (ITT) population included all randomized patients and was used to analyze patient demographics and treatment efficacy. The per-protocol population was the subset of the ITT population with no major protocol deviations. In the ITT population, 93.4% of patients (113/121) in the BTFC group completed the study compared with 91.2% (104/114) in the unfixed-combination group. In the ITT population, after 4 weeks of treatment, the mean change in mean IOP (± standard deviation) from baseline was −9.38±4.66 mmHg in the BTFC group compared with −8.93±4.25 mmHg in the unfixed-combination group (P<0.01). The difference in the change from baseline of mean IOP between the two treatment groups (BTFC minus unfixed combination) was −0.556 mmHg (P=0.330).Citation15 These results indicate that the administration of BTFC to Chinese POAG or OHT patients is not inferior to concurrent dosing using the individual components.
Table 2 The comparison of efficacy of BTFC and unfixed combinations in Chinese patients
The efficacies of three PGA-timolol combinations have been compared in previous studies conducted in European countries. The results of several prospective, randomized studies supported a significantly higher IOP-lowering effect with BTFC compared with LTFC. Centofanti et al reported a prospective, multicenter, investigator-masked clinical study in Italy. POAG or OHT patients were randomized to receive either BTFC (n=47) or LTFC (n=35) topical therapy once nightly for 12 weeks. The mean IOP reduction was significantly greater in the BTFC group than in the LTFC group (−21.4% vs −13.7%) (P<0.001). A higher percentage of patients in the BTFC group showed a mean IOP reduction from baseline, ≥15% (72.3% vs 40.0%) and ≥20% (61.7% vs 17.1%), compared with patients in the LTFC group.Citation37 In a 4-week trial of 36 patients in Spain, BTFC was associated with superior IOP reduction compared with LTFC at all three time points at week 4.Citation38 Similarly, in a 12-week crossover trial of 54 patients in Spain, BTFC was associated with significantly superior IOP-lowering compared with LTFC at six of seven time points at week 12.Citation39
Several prospective randomized studies demonstrated that BTFC causes a greater reduction in IOP than does a travoprost-timolol fixed combination (TTFC). Macky performed a hospital-based prospective randomized study in an Egyptian population to compare the efficacy of BTFC and TTFC. The results showed that BTFC elicited a greater significant mean IOP reduction from baseline than did TTFC at each visit (P<0.001). The mean IOP reduction values were 11.34 and 6.42 mmHg at 2 weeks (P=0.000), and 11.17 and 7.89 mmHg at 6 months (P=0.001) for BTFC and TTFC, respectively.Citation40 Centofanti et al carried out a randomized, double-masked, crossover trial in an Italian population to compare BTFC with TTFC.Citation41 BTFC was more effective overall at lowering IOP. In particular, BTFC caused a significantly greater mean IOP reduction compared with TTFC, at three of five diurnal time points at 3 month. The mean diurnal IOP was 14.7 mmHg after 3 months with BTFC compared with 15.4 mmHg with TTFC.
Rigollet et al reported a randomized, prospective, single-blinded study in Spain, All three combinations (BTFC, TTFC, and LTFC) were effective at lowering IOP, but at 12 months, LTFC and BTFC were more effective than TTFC.Citation42
In 2012, Aptel et al conducted a meta-analysis to review the IOP-lowering effects and tolerability of three PGA-timolol fixed combinations. From direct comparisons, IOP reduction was significantly greatest with BTFC, at 9:00 am, 4:00 pm, and over the mean diurnal curve compared with LTFC (mean difference =0.90–1.48 mmHg) (P<0.001) and at all time points compared with TTFC (mean difference =0.66–0.90 mmHg) (P<0.001).Citation43 In the same year, Cheng et al published another meta-analysis that reviewed the efficacy of six fixed combinations and reported the relative reduction in mean diurnal IOP was 34.9% for TTFC, 34.3% for BTFC, and 33.9% for LTFC.Citation44
Most previous studies in non-Chinese showed that BTFC appears to be slightly more efficacious than LTFC or TTFC; however, there have been no comparative studies of PGA-timolol fixed combinations in the People’s Republic of China.
Safety and tolerability
PGAs have distinctive local adverse reactions, including conjunctival hyperemia, eyelash bristling/lengthening, eyelid pigmentation, iris pigmentation, and upper eyelid deepening. No systemic adverse reactions have been linked to PGA eye drop usage.Citation45
Adverse events associated with PGAs
A meta-analyses of several systematic reviewsCitation46 showed that conjunctival hyperemia occurred significantly less often with latanoprost than with travoprost (odds ratio =0.512) or with bimatoprost (odds ratio =0.32). In other meta-analyses based on systematic reviews of patient-reported data, conjunctival hyperemia was more likely to occur with bimatoprost than with latanoprost (relative risk =1.70) or travoprost (relative risk =1.19).Citation47
In 2011, Inoue et al investigated the frequency of eyelid pigmentation and eyelash bristles after the use of different PGAs in Japan. The study included 250 patients who were diagnosed with POAG, normal-tension glaucoma, or OHT. The patients were administered one of the PGAs in only one eye for more than 3 months and were followed up at the Inoue Eye Hospital from January to June 2011. The data revealed that eyelash lengthening/number increase occurred 54%, 46%, and 26% more often in the eye treated with bimatoprost, travoprost, and latanoprost, respectively, compared with untreated eyes. The differences between individual drops were not significant.Citation48
All of the PGAs had similar effects on eyelid pigmentation. Specifically, eyelid pigmentation changes occurred in 6%, 4%, and 6% of subjects who received bimatoprost, travoprost, and latanoprost respectively. The differences among agents were not significant.Citation48
Deepening of the upper eyelid sulcus (DUES) is another adverse effect of PGAs, and the occurrence of DUES was first reported with bimatoprost in 2004.Citation49 The incidence of DUES differs among the various PGAs. Prostaglandin F2-alpha can inhibit fat production.Citation50 Therefore, it was thought that PGAs reduced orbital adipose tissue mass to cause DUES. In a study by Inoue et al, 250 POAG or OHT patients were treated in one eye with PGAs, and the other eye was left untreated for more than 3 months. Photographs of the face were taken, and DUES was evaluated using a scoring system.Citation51 DUES was reported objectively (photograph) and subjectively (questionnaire) in 24.0% and 12.0%, 50.0% and 24.0%, 60.0% and 40.0% of the patients in the latanoprost, travoprost, and bimatoprost groups, respectively. It occurred more frequently (objectively and subjectively) in the bimatoprost group compared with the latanoprost group (P<0.001).
Adverse events associated with BTFC
Several studies in Europe and the United States showed that fixed combinations led to a lower hyperemia risk than did unfixed combinations and their respective PGA monotherapies.Citation25,Citation32,Citation34,Citation37 A meta-analysis that reviewed the incidence of hyperemia was significantly less with LTFC and BTFC than with the individual PGAs (relative risk =0.66 and 0.61, respectively; P=0.05 and P<0.001, respectively).Citation43
In a multicenter, randomized, double-masked, parallel controlled study in the People’s Republic of China, of 235 POAG patients, 67 (28.5%) experienced adverse events: 32 patients (26.5%) in the BTFC group and 35 patients (30.7%) in the unfixed-combination group. Most adverse events were mild or moderate in severity. Adverse events that the investigator considered to be treatment-related occurred in 20.7% (25/121) of patients in the BTFC group and in 23.7% (27/114) of individuals in the unfixed-combination group (). The most frequent treatment-related adverse event in both groups was conjunctival hyperemia: it occurred in 16.5% (20/121) of patients in the BTFC group and 18.4% (21/114) in the concurrent group, but none of these events was rated as severe. The investigator considered eye pain to be treatment-related in 1.7% (2/121) of patients in the BTFC group and 5.3% (6/114) of subjects in the unfixed-combination group. Numerically, there were fewer treatment-related adverse events in the BTFC group compared with those in the unfixed-combination group. No treatment-related systemic adverse events occurred during the study. In terms of adverse events, in general, there were no clinically meaningful differences between two treatment groups.Citation15
Table 3 The comparison of adverse events of BTFC and unfixed combinations in Chinese patients
A meta-analysis that reviewed the incidence of hyperemia was not significantly less with LTFC than with BTFC (relative risk =1.32; P>0.1).Citation43 Tolerability differences between the fixed combinations appear to be slight, probably because the addition of timolol to the PGA component lessens the associated hyperemia.
Advantages of BTFC for Chinese glaucoma patients
BTFC has been reported to have great efficacy and tolerability in Chinese patients and scores highly for patient-focused perspectives, such as quality of life, adherence, and acceptability. It also has some advantages that improve patient satisfaction.
First, compared with bimatoprost, BTFC reduces IOP more effectively and can help more glaucoma patients achieve their target IOP. Due to the severity of disease in many Chinese patients with glaucoma, most of individuals need more than one medication to control their IOP. The higher efficacy of BTFC will encourage more of those to control their IOP, which will avoid deterioration of the vision field and thus enable preservation of their quality of life.
Second, instillation of multiple IOP-lowering medications introduces the risk of drug washout and decreased the absorption of medications.Citation25 By combining two IOP-lowering agents in the same solution and suspension, fixed-combination eye drops eliminate the risk of drug washout of their components.Citation52,Citation53 BTFC can achieve a similar reduction in IOP to unfixed combinations and reduce the times of instillation. Therefore, it can avoid the washout of earlier eye drop and can keep a stable, effective concentration of eye drops.
Third, a reduction in the number of drops required will decrease the amount of preservative, such as benzalkonium chloride, that is deposited on the ocular surface. Agents such as these can damage the health of the conjunctiva and cornea, and even lead to dry eye and other ocular surface diseases.Citation54 A survey performed in 1999 with 4,107 patients showed that of a range of symptoms that included discomfort upon instillation, and symptoms between instillations, such as burning and stinging, foreign body sensation, dry-eye sensation, tearing, and eyelid itching, were more prevalent for eye drops that contained preservatives compared with preservative-free eye drops (P<0.001). The incidence of ocular signs was higher in patients using eye drops that contained preservatives, and the prevalence of the signs and symptoms increased as the number of drops instilled increased. In addition, a reduction in symptoms and signs was observed when patients changed from preservative-containing to preservative-free eye drops (P<0.001).Citation55
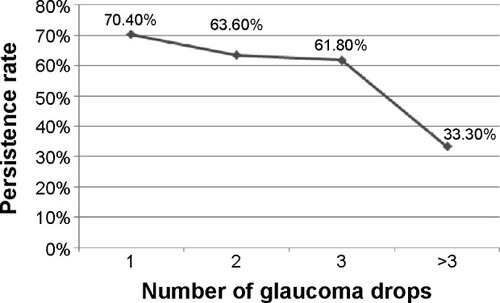
Fourth, a reduction in the number of eye drops has a beneficial effect on the patients’ adherence to the glaucoma treatment and to their persistence with treatment. We carried out a survey on the adherence and persistence rate of glaucoma patients with antiglaucoma eye drops in Shanghai and Beijing. The survey’s results showed with the increase of eye drop, from one to more than three, the persistence rate decreased from 70.4% to 33.3% () (Sun X et al, unpublished data, 2007).
Figure 1 Change in persistence rates according to number of antiglaucoma drops administrated in a Beijing and Shanghai survey (Sun X et al, unpublished data, 2007).
Fifth, the cost-effectiveness of BTFC in the People’s Republic of China is very high. As shown in , the daily cost of BTFC is cheaper than latanoprost, travoprost, and LTFC.Citation19 The daily cost of BTFC is approximately 0.55 USD, which is very close to bimatoprost. Therefore, Chinese patients would be satisfied with the great cost-effectiveness of BTFC.
Table 4 PGA and PGA-timolol fixed-combination monotherapy bottle contents and costs for the People’s Republic of China
Conclusion
The population of glaucoma patients in the People’s Republic of China is large. Antiglaucoma fixed combinations have the characteristics of reliable efficacy, low incidence of adverse events, and possibly improved treatment adherence and persistence with treatment, which suggest that they will become increasingly popular in the People’s Republic of China. As one of the available fixed combinations, BTFC might be a good choice for Chinese glaucoma patients. However, BTFC formally entered the People’s Republic of China only in June 2014, and the efficacy of long-term use, the comparative studies with other PGA-timolol fixed combinations, and the effects on Chinese patients’ adherence, acceptability, and satisfaction are unknown and will require further study.
Acknowledgments
Medical writing support was funded by National Natural Science Foundation of China (grant number NSFC81100667).
Disclosure
Xinghuai Sun is a consultant or speaker for Alcon, Allergan, Pfizer, and Santen. The authors report no other conflicts of interest in this work.
References
- ChoHKKeeCPopulation-based glaucoma prevalence studies in AsiansSurv Ophthalmol201459443444724837853
- YuanZLGlaucoma clinical trials in ChinaZhonghua Yan Ke Za Zhi2012486485487 Chinese22943800
- LiHZhangYYLiuSCPrevalence of open-angle glaucoma in southwestern China: the Yongchuan Glaucoma studyJ Huazhong Univ Sci Technolog Med Sci201434113714124496693
- HeJZouHTongXPrevalence of primary glaucoma among adults aged 50 years or above population in Huamu community: a cross-sectional survey in Shanghai, 2011Zhonghua Yan Ke Za Zhi2014505349354 Chinese25052804
- LiangYBFriedmanDSZhouQHandan Eye Study GroupPrevalence of primary open angle glaucoma in a rural adult Chinese population: the Handan eye studyInvest Ophthalmol Vis Sci201152118250825721896871
- ZhongHLiJLiCThe prevalence of glaucoma in adult rural Chinese populations of the Bai nationality in Dali: the Yunnan Minority Eye StudyInvest Ophthalmol Vis Sci20125363221322522511635
- SongWShanLChengFPrevalence of glaucoma in a rural northern China adult population: a population-based survey in Kailu county, inner MongoliaOphthalmology2011118101982198821684607
- YangYFYuMBProgress of anti-glaucoma fixed combination formulationZhonghua Yan Ke Za Zhi2011472176180 Chinese21426848
- European Glaucoma SocietyTerminology and Guidelines for Glaucoma3rd edSavonaEditrice Dogma2008
- LichterPRMuschDCGillespieBWCIGTS Study GroupInterim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgeryOphthalmology2001108111943195311713061
- HeijlALeskeMCBengtssonBHymanLBengtssonBHusseinMEarly Manifest Glaucoma Trial GroupReduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma TrialArch Ophthalmol2002120101268127912365904
- Collaborative Normal-Tension Glaucoma Study GroupComparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressuresAm J Ophthalmol199812644874979780093
- VanVeldhuisenPCEdererFGaasterlandDEThe AGIS InvestigatorsThe Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deteriorationAm J Ophthalmol2000130442944011024415
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol20021206701713 discussion 829–83012049574
- LingZZhangMHuYSafety and efficacy of bimatoprost/timolol fixed combination in Chinese patients with open-angle glaucoma or ocular hypertensionChin Med J (Engl)2014127590591024571886
- XuLChenJHLiJJThe prevalence and its screening methods of primary open angle glaucoma in defined population-based study of rural and urban in BeijingZhonghua Yan Ke Za Zhi20044011726732 Chinese15634477
- Chinese Glaucoma Society [Glaucoma treatment guidelines]Zhonghua Yan Ke Za Zhi2014505382383 Chinese
- FiscellaRGGreenAPatuszynskiDHWilenskyJMedical therapy cost considerations for glaucomaAm J Ophthalmol20031361182512834665
- GaoYWuLLiADaily cost of glaucoma medications in ChinaJ Glaucoma200716759459718091176
- LiangYWoodwardDFGuzmanVMIdentification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexesBr J Pharmacol200815451079109318587449
- TorisCBGabeltBTKaufmanPLUpdate on the mechanism of action of topical prostaglandins for intraocular pressure reductionSurv Ophthalmol200853Suppl 1S107S12019038618
- ChenJHuangHZhangSChenXSunXExpansion of Schlemm’s canal by travoprost in healthy subjects determined by Fourier-domain optical coherence tomographyInvest Ophthalmol Vis Sci20135421127113423322574
- WatsonPGBarnettMFParkerVHaybittleJA 7 year prospective comparative study of three topical beta blockers in the management of primary open angle glaucomaBr J Ophthalmol200185896296811466256
- ChraiSSMakoidMCEriksenSPRobinsonJRDrop size and initial dosing frequency problems of topically applied ophthalmic drugsJ Pharm Sci19746333333384820359
- AptelFDenisPBalancing efficacy and tolerability of prostaglandin analogues and prostaglandin-timolol fixed combinations in primary open-angle glaucomaCurr Med Res Opin201127101949195821878000
- KhouriASRealiniTFechtnerRDUse of fixed-dose combination drugs for the treatment of glaucomaDrugs Aging200724121007101618020533
- HolmstromSBuchholzPWaltJWickstrømJAagrenMAnalytic review of bimatoprost, latanoprost and travoprost in primary open angle glaucomaCurr Med Res Opin200521111875188316307709
- WangKXuLYuanZIntraocular pressure-lowering efficacy and safety of bimatoprost 0.03% therapy for primary open-angle glaucoma and ocular hypertension patients in ChinaBMC Ophthalmol2014142124568617
- JinXHQianSHSunXH0.03% bimatoprost ophthalmic solution with 0.005% latanoprost eye drops IOP lowering efficacy and safety comparisonChinese Journal of Practical Ophthalmology2005237712714 Chinese
- KongXMSunXHMengFRA comparison of the ocular hypotensive efficacy of three prostaglandin analogsChinese J Opt and Ophthalmology200684228230 Chinese
- HuangHLSunXHXiaoMComparison of intraocular pressure reducing effects of three prostaglandin eyedrops in open-angle glaucomaZhonghua Yan Ke Za Zhi2011472109113 Chinese21426839
- QuarantaLBiagioliERivaIProstaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysisJ Ocul Pharmacol Ther201329438238923231442
- LequeuITheuwisKAbegāo PintoLVandewalleEStalmansILong term IOP lowering efficacy of bimatoprost/timolol fixed combination: a 12 month prospective studyBull Soc Belge Ophtalmol201332210511024923090
- KatsanosADastiridouAIFanariotisMKotoulaMTsironiEEBimatoprost and bimatoprost/timolol fixed combination in patients with open-angle glaucoma and ocular hypertensionJ Ocul Pharmacol Ther2011271677121214361
- BrandtJDCantorLBKatzLJBatoosinghALChouCBossowska I; Ganfort Investigators GroupIIBimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertensionJ Glaucoma200817321121618414107
- Gutierrez-DiazESilva CottaJMuñoz-NegreteFJGutierrez-OrtizCMorgan-WarrenRJMaltmanJBimatoprost/timolol fixed combination versus latanoprost in treatment-naïve glaucoma patients at high risk of progression: a pilot studyClin Ophthalmol2014872573224748767
- CentofantiMOddoneFVetrugnoMEfficacy of the fixed combinations of bimatoprost or latanoprost plus timolol in patients uncontrolled with prostaglandin monotherapy: a multicenter, ran-domized, investigator-masked, clinical studyEur J Ophthalmol2009191667119123151
- MartinezASanchezMA comparison of the safety and intraocular pressure lowering of bimatoprost/timolol fixed combination versus latanoprost/timolol fixed combination in patients with open-angle glaucomaCurr Med Res Opin20072351025103217519068
- MartinezASanchezMBimatoprost/timolol fixed combination vs latanoprost/timolol fixed combination in open-angle glaucoma patientsEye (Lond)200923481081818535605
- MackyTABimatoprost/timolol versus travoprost/timolol fixed combinations in an Egyptian population: a hospital-based prospective randomized studyJ Glaucoma201423856156623429621
- CentofantiMOddoneFGandolfiSComparison of Travoprost and Bimatoprost plus timolol fixed combinations in open-angle glaucoma patients previously treated with latanoprost plus timolol fixed combinationAm J Ophthalmol2010150457558020688314
- RigolletJPOndateguiJAPastoALopLRandomized trial comparing three fixed combinations of prostaglandins/prostamide with timolol maleateClin Ophthalmol2011518719121383947
- AptelFCucheratMDenisPEfficacy and tolerability of prostaglandin-timolol fixed combinations: a meta-analysis of randomized clinical trialsEur J Ophthalmol201222151822167538
- ChengJWChengSWGaoLDLuGCWeiRLIntraocular pressure-lowering effects of commonly used fixed-combination drugs with timolol: a systematic review and meta-analysisPLoS One201279e4507923028770
- HollóGThe side effects of the prostaglandin analoguesExpert Opin Drug Saf200761455217181451
- HonrubiaFGarcía-SánchezJPoloVde la CasaJMSotoJConjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogues in patients with ocular hypertension or glaucoma: a meta-analysis of randomised clinical trialsBr J Ophthalmol200993331632119019922
- AptelFCucheratMDenisPEfficacy and tolerability of prostaglan-din analogs: a meta-analysis of randomized controlled clinical trialsJ Glaucoma200817866767319092464
- InoueKShiokawaMHigaRAdverse periocular reactions to five types of prostaglandin analogsEye (Lond)201226111465147223037910
- PeplinskiLSAlbiani SmithKDeepening of lid sulcus from topical bimatoprost therapyOptom Vis Sci200481857457715300114
- ParkJChoHKMoonJIChanges to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprostJpn J Ophthalmol2011551222721331688
- InoueKShiokawaMWakakuraMTomitaGDeepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogsJ Glaucoma201322862663122936280
- HollóGTopouzisFFechtnerRDFixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practiceExpert Opin Pharmacother201415121737174724998246
- MartinezASanchezMEfficacy and safety of bimatoprost/timolol fixed combination in the treatment of glaucoma or ocular hypertensionExpert Opin Pharmacother20089113714318076345
- Management and Therapy Subcommittee of the International Dry Eye WorkShopManagement and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf20075216317817508120
- PisellaPJPouliquenPBaudouinCPrevalence of ocular symptoms and signs with preserved and preservative free glaucoma medicationBr J Ophthalmol200286441842311914211
