Abstract
The correlation between hypoxia and pancreatic cancer has long been discussed. Hao’s research team made many efforts on revealing the oncogenic function of hypoxic inducible factor-1 (HIF-1) in pancreatic cancer progression and development in recent years. Based on their research, they linked micro-environmental regulation of pancreatic cancer and its clinical significance. Hao’s research team suggests it is a promising approach to target HIF-1 for the management of pancreatic cancer progression and invasion.
Keywords:
Introduction
Despite continuous progress in combinational treatment and radical surgery techniques, pancreatic ductal adenocarcinoma (PDAC) remains the most lethal tumor, with an average 5-year survival rate below 6%. Two prominent biological characteristics of PDAC, hypoperfusion and desmoplasia, play leading roles in the formation of a hypoxic microenvironment. Hypoxic inducible factor-1 (HIF-1) is a master regulator of cell adaption to hypoxia, and is highly expressed in 88% of pancreatic cancer tissues. Previous studies showed the overexpression of HIF-1 is correlated with poor prognosis, yet the underlying mechanism remains elusive.Citation1
During the past several years, Hao’s research teamCitation2–Citation7 have focused on understanding the role of HIF-1 in pancreatic cancer and have published much innovative work in this field. They found that HIF-1 G1790A and C1772T single nucleotide polymorphisms appeared more frequently in PDAC, and predicted higher risk for the development of pancreatic cancer. Furthermore, the G1790A single nucleotide polymorphism was associated with expression of HIF-1 protein and tumor progression.Citation2 Through direct upregulation of its target factors, HIF-1 promoted cell proliferation through cyclophilin A (CypA).Citation3 In addition, HIF-1 played a crucial role in the perineural invasion and metastasis of pancreatic cancer.Citation3,Citation4 Fascin is an actin-bundling protein and is overexpressed in pancreatic cancer. HIF-1 directly activated the expression of fascin and mediated PDAC invasion through matrix metalloproteinase-2 (MMP-2).Citation4 Another actin-bundling protein, LASP-1 (LIM and SH3 protein 1), was also tightly regulated by HIF-1 and promoted metastasis in orthotopic xenograft and immunocompetent mouse models of PDAC.Citation5 Interestingly, HIF-1 regulated the expression of chemokine (C-X3-C motif) receptor 1 (CX3CR1), and CX3CR1 activated HIF-1 through the phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways. The crosstalk between HIF-1 and CX3CR1 mediated perineural invasionCitation6 and the Warburg effect of PDAC.Citation7
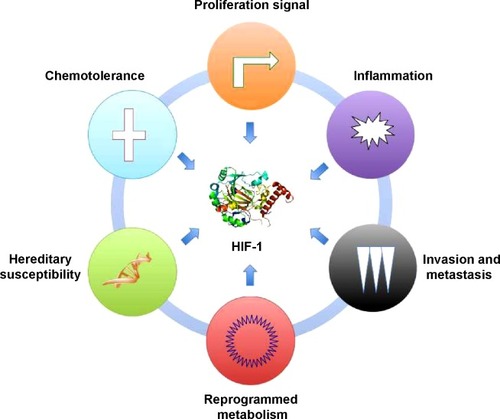
The outstanding work of Hao et al has indicated that HIF-1 represents a critical mediator connecting the hypoxic microenvironment and pancreatic cancer cells (). These findings suggest a new therapeutic strategy to inhibit pancreatic cancer growth by reprogramming the stroma to alleviate hypoxia, as recently shown in a study of vitamin D.Citation8 A growing number of reagents have been developed to inhibit HIF-1 activity, including those agents intended for use in clinical trials and US Food and Drug Administration (FDA)-approved drugs. It is reasonable to introduce HIF-1 inhibitors as new candidates for the treatment of pancreatic cancer.
Figure 1 HIF-1 is a central mediator of tumor-related biocharacteristics in pancreatic cancer.
Abbreviation: HIF-1, hypoxic inducible factor-1.
Although HIF-1 is well known to serve as a crucial oncogene, further details of how this transcription factor performs tumor mitogenic and migratory functions remain unknown. Hao’s recent work highlighted the experimental and clinical significance of HIF-1 in PDAC, which provides important “bench-to-bedside” clues for utilizing this key transcription factor in the early diagnosis and personalized therapy for PDAC. By further understanding the downstream target genes of HIF-1 and how these are related to the tumorigenic and metastatic phenotype of PDAC, Hao et al take the leading role in functional and mechanistic studies of microenvironmental regulation of hypoxia. Their research findings regarding HIF-1 indicate that the monitoring and management of the hypoxic microenvironment is a promising approach to exploring the aggressive biological nature of PDAC and improving the prognosis of this devastating malignancy.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShibajiTNagaoMIkedaNPrognostic significance of HIF-1 alpha overexpression in human pancreatic cancerAnticancer Research2003236C4721472714981919
- WangXLiuYRenHPolymorphisms in the hypoxia-inducible factor-1α gene confer susceptibility to pancreatic cancerCancer Biol Ther201112538338721709439
- ZhangHChenJLiuFCypA, a gene downstream of HIF-1α, promotes the development of PDACPLoS One201493e9282424662981
- ZhaoXGaoSRenHHypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein fascinCancer Res20147492455246424599125
- ZhaoTRenHLiJLASP1 Is a HIF1α target gene critical for metastasis of pancreatic cancerCancer Res201575111111925385028
- ZhaoTSGaoSWangXCHypoxia-inducible factor-1α regulates chemotactic migration of pancreatic ductal adenocarcinoma cells through directly transactivating the CX3CR1 genePLoS One201278
- RenHZhaoTSunJThe CX3CL1/CX3CR1 reprograms glucose metabolism through HIF-1 pathway in pancreatic adenocarcinomaJ Cell Biochem2013114112603261123857671
- ShermanMHYuRTEngleDDVitamin d receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapyCell20141591809325259922
