Abstract
The aim of this work was to prepare a combined drug dosage form of famotidine (FAM) and quercetin (QRT) to augment treatment of gastric ulcer. FAM was prepared as freeze-dried floating alginate beads using ion gelation method and then coated with Eudragit RL100 to sustain FAM release. QRT was prepared as solid dispersion with polyvinyl pyrrolidone K30 to improve its solubility. Photo images and scanning electron microscope images of the prepared beads were carried out to detect floating behavior and to reveal surface and core shape of the prepared beads. Anti-ulcerogenic effect and histopathological examination of gastric tissues were carried out to investigate the effect of the combined drug formulation compared with commercial FAM tablets and FAM beads. Gastric glutathione (GSH), superoxide dismutase, catalase, tissue myeloperoxidase, and lipid peroxidation enzyme activities and levels in rat stomach tissues were also determined. Results revealed that spherical beads were formed with an average diameter of 1.64±0.33 mm. They floated immediately with no lag time before floating, and remained buoyant throughout the test period. Treatment with a combination of FAM beads plus QRT showed the absence of any signs of inflammation or hemorrhage, and significantly prevented the indomethacin-induced decrease in GSH levels (P<0.05) with regain of normal GSH gastric tissue levels. Also, there was a significant difference in the decrease of malondialdehyde level compared to FAM commercial tablets or beads alone (P<0.05). The combined formula significantly improved the myeloperoxidase level compared to both the disease control group and commercial FAM tablet-treated group (P<0.05). Formulation of FAM as floating beads in combination with solid dispersion of QRT improved the anti-ulcer activity compared to commercially available tablets, which reveals a promising application for treatment of peptic ulcer.
Introduction
Peptic ulcer, which includes both gastric and duodenal ulcer, is one of the most common disorders affecting the gastrointestinal system.Citation1 The pathophysiology of acid–peptic disease is attributed mainly to the imbalance between aggressive factors (such as acid, pepsin, Helicobacter pylori infection, smoking, stress, and excessive alcohol intakes) and cytoprotective action of the gastrointestinal mucosa, like secretion of bicarbonate, mucus, and prostaglandins.Citation2 Histamine is a major stimulant for acid secretion through the H2 receptors; therefore, blocking these receptors may lead to reduction in acid secretion. The inhibition of gastric acid secretion is still a key therapeutic target for the ulcer diseases of any cause, gastro-esophageal reflux disease, Zollinger–Ellison syndrome, and gastritis.Citation3 This goal is best achieved by blocking the acid secretory effect of histamine through the use of H2 receptor antagonists or the irreversible H+/K+ ATPase inhibitors, popularly referred to as proton pump inhibitors.Citation4 H2 receptor antagonist are specific antagonists that inhibit acid secretion by competitively and reversibly blocking the H2 receptors on the basolateral membrane of the parietal cell.Citation5
Famotidine (FAM) is the most potent antagonist available for clinical use.Citation6 FAM is used for treatment of gastroesophageal reflux, heart burn, and peptic ulcers without side effects.Citation7 Studies showed that the concomitant use of FAM could increase the cure rates of H. pylori infection by a triple therapy with lansoprazole, clarithromycin, and amoxicillin at the standard doses.Citation8 FAM is incompletely absorbed from the gastrointestinal tract. The low oral bioavailability (40%–45%) and short biological half-life (2.5–3.5 hours) of FAM favors development of a sustained release formulation.Citation9 FAM has pH-dependent solubility (basic drug, pKa 7.06); therefore, its gastric retention would allow adequate time for its dissolution, the rate limiting step in drug absorption.
Flavonoids are intensively studied because of their proposed potentially beneficial effects in preventing various diseases.Citation10–Citation12 The flavonoid quercetin (QRT) is reported to exhibit an antiradical property toward hydroxyl and peroxyl radicals and superoxides anions, a mechanism involved in peptic ulcer.Citation13 It was demonstrated that QRT is an effective cytotoxic agent in the case of gastric carcinoma cell lines.Citation14 QRT has been shown to have anti-ulcer and gastroprotective effects.Citation15,Citation16 QRT inhibits growth of H. pylori in a dose-dependent manner in vitro, which contributes to its anti-ulcer effect.Citation17
Floating drug delivery systems are among the mechanisms available for controlling the gastric retention of solid dosage forms. A gastro-retentive floating system made up of multiple-unit particulate (eg, beads) has relative merits compared with a single-unit preparation. Floating calcium alginate beads have been investigated as a possible gastro-retentive dosage form, and are designed to enhance the bioavailability of certain drugs from oral preparations.Citation18
This study is aimed at the preparation of a combined oral dosage form containing floating FAM alginate beads and a solid dispersion of QRT–polyvinyl pyrrolidone K30 (PVP k30) to augment their effect for treatment of peptic ulcer. The anti-ulcerogenic activity of FAM alginate floating beads in combination with QRT–PVP was evaluated using rats as an animal model. Histopathological examinations of gastric tissues were carried out to investigate the effect of the prepared formulation compared with commercial FAM product and FAM beads only. Gastric glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), tissue myeloperoxidase (MPO), and lipid peroxidation (LPO) enzyme activities and levels in rat stomach tissues were also determined.
Materials and methods
FAM was a kind gift from Medical Union Pharmaceutical (MUP, Abou Sultan – Ismailia, Egypt) Company, Egypt. Eudragit RL100 was a kind gift from Evonik Rohme, Germany. Sodium alginate, medium viscosity (200 cPs for 1% aqueous solution at 20°C) was purchased from Alpha Laboratories Ltd., Eastleigh, Hampshire, UK. PVP k30, anhydrous calcium chloride, sodium dihydrogen phosphate, sodium hydrogen phosphate dibasic, and indomethacin were from Sigma-Aldrich Co., St Louis, MO, USA.
Methods
Preparation and characterization of coated FAM floating beads
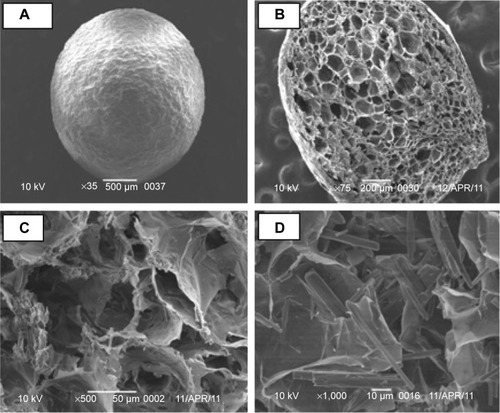
Floating freeze-dried calcium alginate beads were prepared by the ionotropic gelation method. Sodium alginate was dissolved in deionized water to prepare solutions of 2% (w/v). FAM was dispersed in sodium alginate solution at a drug:polymer ratio of 2:1 w/w, and the mixture was homogenized (Ultra Turrax T25, IKA®-Werke GmbH & Co. KG, Staufen, Germany) for 15 minutes. The bubble-free drug-loaded mixture was extruded through a 22G syringe needle, at a constant rate, into 250 mL of 0.5 M calcium chloride solution, maintained under gentle agitation at a dropping distance of 5 cm from the surface of calcium chloride solution. The formed beads were cured for 30 minutes at room temperature, separated, washed three times with 500 mL of distilled water, then collected and freeze-dried (Christ Beta 1–8 LD Freeze Dryer; Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). The prepared beads were then coated by immersion in Eudragit RL100 (5% w/v) in absolute ethanol followed by solvent evaporation using a rotary evaporator (Rotavapor II; BÜCHI Labortechnik AG, Flawil, Switzerland). The coating process continued until coating weight was obtained (5% w/w). The coated beads were then dried at room temperature for 24 hours. The dried beads were stored in desiccator containing anhydrous calcium chloride. Photo images and scanning electron microscope images of the prepared beads were carried out to detect floating behavior and to reveal the surface and core shape of the prepared beads.
Preparation of QRT solid dispersion
Weighed amounts of QRT and PVP K30, to prepare a 1:1 (w/w) ratio, were dissolved in absolute ethanol and coprecipitated by slow evaporation of the solvent at 60°C using rotary evaporator. The produced solid mass was further dried in an oven at 70°C for 24 hours. The dried solid mass was pulverized and sieved to obtain a particle size range of 125–250 μm and kept in a desiccator.
It is worth mentioning that the detailed investigation of the factors affecting characteristics of the prepared beads using different methods, in addition to various parameters that influence the behavior of the beads in vitro and QRT preparation, are to be submitted as a separate article.
In vivo studies
Animals
Male albino rats (150–250 g) were used. Animals were supplied from the Animal House of Faculty of Medicine, Assiut University, Assiut, Egypt. Animals were kept for 1 week before the study as a pre-breeding period for acclimatization to accommodate the test conditions. Animal use was approved by the Institutional Review Board for Animal Research/Studies who ensured that the care and use of animals conformed to the EU Directive 2010/63/EU on the protection of animals used for scientific purposes.
Anti-ulcerogenic effect
Animals were fasted for 24 hours with free access to water. Gastric ulceration was induced by oral administration of aqueous suspension of indomethacin in 1% sodium carboxy-methylcellulose (CMC) as 50 mg/kg of body weight, 2 mL orally, for all groups except animals in group 1 for 2 consecutive days. The animals were further fasted for 12 hours, and then randomly divided into five experimental groups of eight rats each. The first and second groups received 1% sodium CMC solution (2 mL, oral) once daily for 15 consecutive days and represent control and disease control, respectively. The third group (standard) received crushed commercial FAM tablets. Group 4 (test 1 group), received the prepared FAM alginate beads. Group 5 (test 2 group) received FAM alginate beads and QRT–PVP coprecipitate.
FAM and QRT doses were 12 mg/kg and 50 mg/kg, respectively. They were administered as a suspension in 1% sodium CMC (2 mL, oral) once daily for 15 consecutive days. On the 16th day, excised stomachs were examined macroscopically for hemorrhagic lesions. The number of ulcers per stomach was noted and the severity of the ulcers was observed microscopically. Ulcer index of each animal was calculated by the following scoring system: 0 for normal-colored stomach, 0.5 for red coloration, 1 for spot ulceration, 1.5 for hemorrhagic streaks, 2 for ulcers >3 mm but <5 mm, 3 for ulcer >5 mm, and 4 for perforations.
Histopathological examination of gastric tissues
Specimens of the isolated gastric tissues were fixed in 10% formalin solution for 24 hours. Sections of 4–5 μm thickness were stained with hematoxylin and eosin. The sections were mounted and observed under light microscope.
Biochemical studies
GSH, SOD, CAT, MPO, and LPO enzyme activities and levels in rat stomach tissues were determined. For biochemical estimations, 0.5 g of the whole gastric tissue samples were ground with liquid nitrogen and then mixed with the appropriate buffer. Mixtures were homogenized on ice. Homogenates were used for determination of the enzymatic activities.Citation19
GSH determination was carried out using a modified Sedlak and Lindsay method.Citation20 Briefly, the mucosal surface of the stomach was collected by scraping, weighed, and properly homogenized in 2 mL of 50 mM Tris-HCl buffer containing 20 mM ethylenediaminetetraacetic acid and 0.2 mM sucrose, pH 7.5. The homogenate was immediately precipitated with 0.1 mL of ice-cold 25% trichloroacetic acid and centrifuged at 4,000 rpm for 40 minutes at 4°C. The supernatant was measured at wavelength of maximum absorption (λmax) 412 nm. LPO content was estimated by measuring the formation of malondialdehyde (MDA) – an end product of LPO – using the thiobarbituric acid test. The corpus mucosa was scraped, weighed, and homogenized in 10 mL of 100 g/L KCl. The homogenate (0.5 mL) was added to a solution containing 0.2 mL of 80 g/L sodium lauryl sulfate, 1.5 mL of 200 g/L acetic acid, 1.5 mL of 8 g/L 2-thiobarbiturate, and 0.3 mL of distilled water. The mixture was incubated at 98°C for 1 hour. Upon cooling, 5 mL of n-butanol:pyridine (15:1 v/v) was added. The mixture was vortexed for 1 minute and centrifuged at 4,000 rpm for 30 minutes. The supernatant was measured spectroscopically at λmax of 532 nm for MDA.Citation21 MPO activity was determined using Bradley procedure.Citation22 SOD activity was measured using the method of Sun et al.Citation23 CAT measurement was carried out by estimation of H2O2 decomposition in the presence of CAT at λmax 240 nm.Citation22
Statistical analysis
The experimental data were expressed as mean ± standard deviation. Statistical analysis was carried out using GraphPad Prism version 3.0 (GraphPad Software, Inc., La Jolla, CA, USA). Groups of data were compared with one-way analysis of variance, followed by the Tukey’s test. The values were considered statistically significant when P<0.05.
Results and discussion
Preparation of FAM floating beads
Sodium alginate can form gel by ionotropic gelation with divalent cations such as Ca2+ and Ba2+. Cross-links are formed between the divalent ions and the negatively charged carboxyl groups of the alginate molecules.Citation24 Alginate beads are one of the particulate delivery systems that protect drugs from the acidic environment, and improve bioavailability of the target drug at a specific site.Citation25 Spherical beads were formed with an average diameter of 1.64±0.33 mm. They floated immediately with no lag time float, and remained buoyant throughout the test period (). This may be due to their highly porous internal structure as shown in scanning electron microscope images (). This might be as a result of water sublimation leaving pores and cavities in the alginate matrix.
Preparation of QRT solid dispersion
Despite the biological activities of QRT, its low aqueous solubility hampers its use as a therapeutic agent.Citation26 The solubility of QRT determined at room temperature was 0.441±0.0487 μg/mL.Citation27 Therefore, any attempt to enhance the dissolution rate would improve absorption and bioavailability of QRT.Citation28 Different methods and techniques had been utilized to improve the solubility of QRT.Citation29–Citation33 Complexation of QRT with PVP could be an applicable approach to improve QRT dissolution, and hence bioavailability. PVP improves markedly the aqueous dissolution of QRT when incorporated with the later as coprecipitate. Solid dispersions of QRT containing high proportions of PVP were most effective in enhancing the drug dissolution rate (full detailed data will be published elsewhere).
In vivo studies
Nonsteroidal anti-inflammatory drugs (NSAIDs) may damage the gastrointestinal mucosa by two distinct mechanisms: 1) suppression of prostaglandin synthesis and 2) direct irritant action causing alterations of mucosal permeability.Citation34 In the stomach, prostaglandins play a vital protective role by stimulating secretion of HCO3− and mucous, maintaining mucosal blood flow, and regulating mucosal cell turnover and repair. Thus, the suppression of prostaglandin synthesis by NSAIDs like indomethacin results in increased susceptibility to mucosal injury and gastroduodenal ulceration.Citation35 Previous results have shown that reactive oxygen species (ROS) plays an important role in pathogenesis of mucosal damage caused by indomethacin besides inhibition of cyclooxygenase enzymes.Citation36 Indomethacin induces higher gastric damage in rats when compared with other NSAIDs.Citation37 Additionally, It is reported that FAM prevents indomethacin-induced gastric ulcers in rats.Citation38
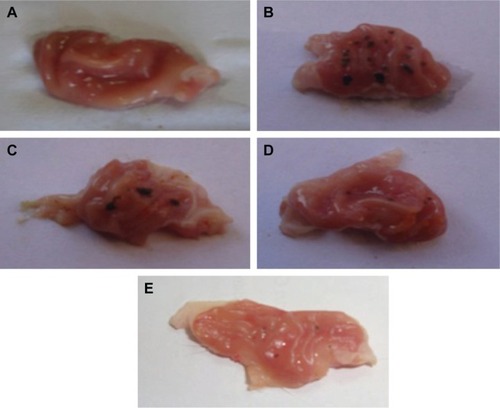
Macroscopic examination of the investigated rat stomachs is shown in . The normal control group (1) stomachs () revealed normal mucosa, no inflammation, and no hemorrhage or patches of hyperemia. On the other hand, the diseased rats group (2) showed marked severe injuries in the gastric mucosa (). Oral administration of indomethacin (50 mg/kg) produced severe hemorrhagic lesions predominantly on glandular mucosa segment of the stomach and few or none in the antrum. Dark reddish patches of ulcers covered the majority of the stomach body, and there were numerous ulcer patches with different forms and sizes within the stomach mucosa. There was remarkable hyperemia in the stomach mucosa. Upon treating the animals with crushed commercial FAM tablets (), the stomachs showed significantly lower numbers of ulcers. They showed moderate mucosal injuries compared to those of ulcerated control rats, and ulcers appeared as discontinuous lesions in the gastric mucosa; no perforation was noticed in all treated animal groups. While treatment with FAM beads () showed milder ulceration reaction, few small lesions appeared in the gastric mucosa and produced more prominent inhibition effect when compared with the commercial FAM tablets treatment group. On the other hand, treatment with a combination of beads formula plus QRT–PVP coprecipitate () showed a nearly normal stomach appearance, as minor ulceration was observed in addition to the absence of any sign of inflammation or hemorrhage. Animals of treated groups were healthy and showed nearly normal activity during the experiment.
Figure 3 Gross appearance of the gastric mucosa in stomachs of indomethacin-induced models.
Abbreviations: FAM, famotidine; PVP, polyvinyl pyrrolidone; QRT, quercetin.
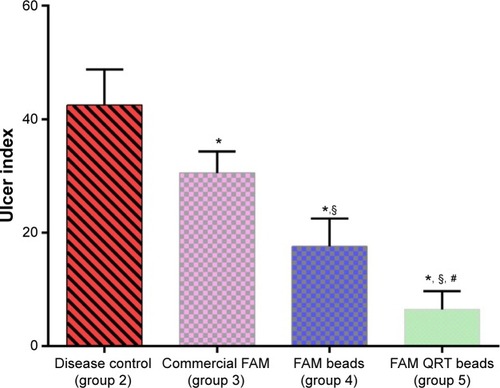
illustrates ulcer indices for animals in the indomethacin-induced ulcerated model. Ulcer index in group 2 was 42.50±0.83, which confirms ulcer formation. The ulcer index for the rats treated with FAM tablets was 30.53±0.86; a value significantly lower than that of group 2 (P<0.05). On the other hand, the ulcer index for rats treated with FAM beads was 17.58±0.57 which is significantly reduced compared to the disease control group (2) and the animals treated with commercial FAM tablets. However, rats treated with formulated FAM beads in combination with QRT–PVP showed an ulcer index of 6.46±0.64, which was significantly the lowest compared with all other ulcer indices of investigated groups (P<0.05) as shown in .
Figure 4 Effects of different treatments on gastric ulcer in indomethacin-induced ulcers in rats.
Abbreviations: FAM, famotidine; QRT, quercetin.
It is clear from the obtained results that treatment of animals with the formulated beads produced more effective protection against gastric ulcers when compared with that of the commercial tablets. This may be explained on the basis of the extended duration of drug release and providing effective drug concentration over a prolonged time. Upon using combination treatment of FAM beads plus QRT–PVP, the data showed a significantly improved protection against gastric ulceration when compared with FAM beads alone. This could be attributed to the gastroprotective effect of QRT as a result of its antioxidation and free radical-scavenging property, which inhibits lipid peroxide level in gastric mucosa.Citation39
Histological examination
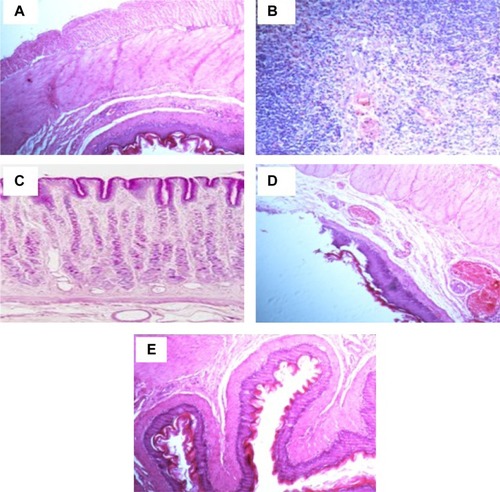
Histopathological examination was carried out to confirm the morphological changes in stomach tissues in the studied animal groups. shows the histopathological effect of different treatments on indomethacin-induced ulcer model. Stomach histology of control normal rats shows normal microscopic architecture with normal glands, without any signs of abnormalities. The parietal cells located in the upper half of gastric glands with eccentric nuclei and pale eosinophilic vacuoles in the cytoplasm (). On the other hand, the figure reveals a severe inflammatory reaction, manifested by submucosal edema with local mononuclear leucocytic infiltration (mainly lymphocytes and few eosinophils) in the lamina propria, muscularis mucosa, and submucosal layers of the stomach tissue for the indomethacin-treated control group (2). The submucosal blood vessels were congested and the muscular coat was hypertrophied and partially hyalinized. These observations revealed the acute phase of ulcer and a severe inflammatory reaction (). The stomach tissue of group 3, receiving the commercial FAM tablets, showed moderate inflammatory reaction (). The reaction severity was milder in case of animals receiving the beads (), who showed moderate amounts of leucocytic inflammatory cell infiltration in the submucosal layer and muscularis mucosa. Animals in group 5, receiving the beads formula plus the QRT–PVP (), were the best-preserved group in terms of morphological integrity. It showed a uniform epithelial and glandular structure and arrangement, and good tissue architecture. Only minor leucocytic inflammatory cells were encountered in tissue sections.
Figure 5 Histological evaluation of stomach tissues from indomethacin-induced ulcer model in rats of hematoxylin and eosin staining of gastric mucosa (magnification ×100).
Abbreviations: FAM, famotidine; PVP, polyvinyl pyrrolidone; QRT, quercetin.
Biochemical studies
Gastric ulcer production by indomethacin is mainly attributed to the inhibition of prostaglandin synthesis in the stomach tissue. It has also been shown that ROS plays an important role in the pathogenesis of mucosal damage caused by indomethacin.Citation40,Citation41 The results revealed elevated levels of MPO and MDA in the gastric tissue of the animals that were given indomethacin. On the other hand, enzymatic and nonenzymatic antioxidant parameters, such as GSH, SOD, and CAT, were reduced.Citation42 These antioxidants are protective factors in the control of indomethacin-induced damage. GSH, SOD, and CAT prevent the tissue damage by maintaining ROS at low levels in the cells. ROS-mediated degradation of cell membrane initiates mucosal lesions, increased vascular permeability, and depletion of the mucus layer.Citation43
SOD is the antioxidant enzyme which catalyzes the conversion of superoxide free radical (O2−) to hydrogen peroxide (H2O2) and to molecular oxygen (O2). SOD and endogenous antioxidant enzymes render the free radicals harmless and protect the tissues from the harmful effects of free radicals and active oxygen species. When these antioxidants’ defense mechanisms are inadequate, free radicals lead to serious damage in the tissues, with LPO being the most important and most harmful effect that the free radicals trigger in the cell.Citation44
MDA is the end product of LPO which leads to further damage in the cells, whereas MPO is the enzyme that catalyzes the production of toxic hypochlorous acid from H2O2. It is present in the phagocytic cells. Excessive production of MPO and other reactive radicals causes oxidative damage.Citation45 A previous report demonstrated that MPO activity increases in NSAID-damaged stomach tissue.Citation37 CAT is a highly reactive enzyme that detoxifies hydrogen peroxide and disrupts free radical synthesis. CAT reacts with H2O2 to form water and molecular oxygen.Citation46 Indomethacin-induced gastric ulcers may be eliminated with antioxidant effect. It was demonstrated that the increase of mucosal oxidants and the decrease of enzymatic and nonenzymatic antioxidants, induced by indomethacin, may be reversed with the use of FAM.Citation47 Within the flavonoid family, QRT is the most potent scavenger of ROS.Citation48 Therefore, a combination of QRT and FAM could improve anti-ulcer activity. Effects of different treatments on the levels of GSH and various enzymes are shown in .
Table 1 Effects of different treatments on the level of GSH and various enzymes in different experimental groups
Gastric GSH levels
The elevated levels of GSH in gastric mucosa are essential to maintain mucosal integrity, and depletion of GSH could induce mucosal ulceration. In addition, depletion of GSH could limit the activity of GSH-dependent enzymes.Citation49 shows that the GSH level in the gastric tissues of rats given indomethacin was significantly lower than that of the normal control group rats (P<0.05). Treatment of animals with FAM increased the total GSH level significantly when it was compared with diseased control group (group 2) (P<0.05). Treatment with FAM beads (group 4) significantly improved the GSH level in the stomach tissue when it was compared with group 3 (P<0.05). Treatment with a combination of FAM beads plus QRT significantly prevented the indomethacin-induced decrease in GSH levels (P<0.05) and the gastric tissue regained its normal GSH levels.
Gastric LPO levels
From the data of , the MDA level of indomethacin-treated (group 2) increased significantly when compared with the normal control rat group. MDA level was not significantly (P>0.05) decreased compared to the diseased control group in rats treated with commercial FAM tablets, whereas the level of MDA decreased significantly with the use of FAM beads (P<0.05). Upon using combination treatment, there was a significant difference in the decrease of MDA level compared to FAM commercial tablets or beads alone (P<0.05). LPO was almost completely inhibited by FAM beads plus QRT; the difference between the normal control and QRT group was not significant (P>0.05) as the value of the LPO may be normalized. It is clear from the results that there was a significant positive correlation between the ulcer index values and LPO, indicating that LPO may be one of the main causes of mucosal damage.
Gastric MPO activity
The MPO activity is an index of neutrophil-dependent inflammatory response and neutrophil infiltration in various gastric injuries.Citation50 MPO activity is a sensitive and specific marker of acute inflammation and reflects polymorphonuclear cell infiltration into the parenchyma.Citation46 MPO is an essential marker for normal neutrophil function. shows the effect of different treatments on the MPO activity. From , it is clear that indomethacin significantly increased MPO levels in rat stomach tissue compared to the normal control rats (P<0.05).
The interaction of MPO with radicals such as O2−, H2O2, and OH− released as a result neutrophil activation produces hypochlorous acid and N-chloramine that lead to tissue damage.Citation51 Indomethacin exerts its effects via inhibition of the MPO pathways.Citation52 Treatment of rats with commercial FAM tablets produced a nonsignificant increase in the MPO level (P>0.05), while treatment of rats with FAM beads significantly improved the MPO level compared to both the disease control group and commercial FAM tablet-treated group (P<0.05). Treatment of rats with a combination of FAM beads plus QRT revealed significant improvement in the MPO level, as compared to beads alone (P<0.05). In addition, treatment of rats with the combination of beads and QRT normalized the MPO level as there was nonsignificant difference between group 5 and the normal control group 1 (P>0.05).
Gastric SOD activity
SOD activity reflects the enzymatic antioxidative properties of tissues as it converts the highly reactive radicals (O2−) into the less reactive H2O2 that can be destroyed by the CAT reaction.Citation53 From , it can be noticed that treatment of rats with indomethacin significantly decreased SOD activity compared to the normal control rats (P<0.05). Treatment with commercial FAM tablets significantly increased the SOD activity compared to the indomethacin-treated rat group (P<0.05). On the other hand, treatment with the FAM beads significantly improved the SOD activity compared with group 2 (P<0.05). Using a combination of FAM beads plus QRT for treatment of rats resulted in a significant increase in the SOD level compared with group 4 (P<0.05). There was significant difference between the rats in this group and the rats in groups treated with either commercial FAM tablets or with FAM beads alone (P<0.05). Again, it was noticed that there was nonsignificant difference between this group and rats of the normal control group (P>0.05). This finding indicated the ability of the combination to renormalize SOD activity following indomethacin administration.
Gastric CAT activity
Inhibition of CAT activity enhances the generation of hydroxyl radicals as a result of prevention of antioxidant activity that leads to lipid peroxide formation.Citation54 From , it could be noticed that the CAT activity was significantly decreased by indomethacin treatment when compared to its activity in the normal control group (P<0.05). Treatment of animals with commercial FAM tablets significantly increased the CAT activity compared to the indomethacin-treated group (P<0.05). Treatment of animals with the FAM beads significantly increased the CAT activity compared to the indomethacin-treated (group 2) or commercial FAM tablets-treated groups (group 3) (P<0.05). Treatment of rats with a combination of beads plus QRT (group 5) significantly increased the CAT activity compared to the group treated with beads alone (group 4) (P<0.05). The activity of CAT was found to be almost normalized upon treating animals with combination of FAM beads plus QRT. There was nonsignificant difference between CAT activities in rats of this group compared with that of the normal control group (P>0.05). SOD and CAT have a major role in gastric oxidative/antioxidative balance. Reduction of both enzymes’ activities in gastric mucosa as a result of ulcerogenic exposure leads to elevation in ROS levels. Accordingly, MDA levels increase.Citation55 In our study, indomethacin induced inhibition of SOD and CAT activities with increase in the MDA concentration, which is in agreement with this finding.
From these results, the antioxidant activity of the prepared formula in gastric mucosal homogenates observed from decrease in LPO may be due to increase in SOD and CAT activities. It is well known that antioxidant activity is commonly related with gastroprotection and cytoprotection.Citation56 Generally, the results obtained from this study showed the decrease of enzymatic and nonenzymatic oxidant parameters (compared to the control group that received indomethacin). The increase of antioxidant parameters observed in the gastric tissue of the animals that received FAM are in agreement with results of another study.Citation47 Our study demonstrated that oral treatment of indomethacin-induced gastric ulcer with the prepared FAM beads plus QRT for 15 consecutive days accelerates significantly the healing of chronic gastric ulcer in rats compared to either FAM commercial tablets or FAM beads alone.
Conclusion
Our study demonstrated that oral treatment of indomethacin-induced gastric ulcer with the prepared FAM floating beads in combination with QRT–PVP for 15 consecutive days accelerates significantly the healing of chronic gastric ulcer in rats compared with either FAM commercial tablets or FAM beads alone. The combined drug formula lowered the enzymatic and nonenzymatic oxidant parameters, compared with the control group that received indomethacin. Biochemical assay and histopathological studies confirmed the anti-ulcer effect for both the formulated FAM beads and the combination treatment. Combination of the formulated FAM beads plus QRT–PVP promisingly revealed improved effectiveness in treatment of gastric ulcer compared with the commercial FAM tablets.
Disclosure
The authors report no conflicts of interest in this work.
References
- MalfertheinerPChanFKMcCollKEPeptic ulcer diseaseLancet20093471449146119683340
- WuYFassihiRStability of metronidazole, tetracycline HCl and famotidine alone and in combinationInt J Pharm200529011315664125
- PilottoAFranceschiMMaggiSAddanteFSancarloDOptimal management of peptic ulcer disease in the elderlyDrugs Aging20102754555820583849
- FranceschiMDi MarioFLeandroGMaggiSPilottoAAcid-related disorders in the elderlyBest Pract Res Clin Gastroenterol20092383984819942162
- HuangJQHuntRHPharmacological and Pharmacodynamic essentials of H(2)-receptor antagonists and proton pump inhibitors for the practising physicianBest Pract Res Clin Gastroenterol20011535537011403532
- LinJHPharmacokinetic and pharmacodynamics properties of histamine H2-receptor antagonists. Relationship between intrinsic potency and effective plasma concentrationsClin Pharmacokinet1991202182361673880
- MyersDH2 Receptor AntagonistsJ Exot Pet Med200615150152
- OkudairaKFurutaTShiraiNSugimotoMMiuraSConcomitant dosing of famotidine with a triple therapy increases the cure rates of Helicobacter pylori infections in patients with the homozygous extensive metabolizer genotype of CYP2C19Aliment Pharmacol Ther20052149149715710002
- RazaviMNyamathullaSKarimianHNoordinMINovel swellable polymer of orchidaceae family for gastroretentive drug delivery of famotidineDrug Des Devel Ther2014813151329
- MurotaKHottaAIdoHAntioxidant capacity of albumin-bound quercetin metabolites after onion consumption in humansJ Med Invest20075437037417878690
- Perez-VizcainoFDuarteJFlavonols and cardiovascular diseaseMol Aspects Med20103147849420837053
- AhmedOABadr-EldinSMTawfikMKAhmedTAEl-SayKMBadrJMDesign and optimization of self-nanoemulsifying delivery system to enhance quercetin hepatoprotective activity in paracetamol-induced hepatotoxicityJ Pharm Sci201410360261224395640
- SantosMRRodríguez-GómezMJJustinoGCCharroNFlorencioMHMiraLInfluence of the metabolic profile on the in vivo antioxidant activity of quercetin under a low dosage oral regimen in ratsBr J Pharmacol20081531750176118311191
- BorskaSChmielewskaMWysockaTDrag-ZalesinskaMZabelMDziegielPIn vitro effect of quercetin on human gastric carcinoma: targeting cancer cells death and MDRFood Chem Toxicol2012503375338322750388
- ManachCTexierOMorandCComparison of the bioavailability of quercetin and catechin in ratsFree Radic Biol Med1999271259126610641719
- Sánchez de MedinaFGálvezJGonzálezMZarzueloABarrettKEEffects of quercetin on epithelial chloride secretionLife Sci199761204920559366512
- SumbulSAhmadMAMohdAMohdARole of phenolic compounds in peptic ulcer: An overviewJ Pharm Bioallied Sci2011336136721966156
- AhmedOABadr-EldinSMAhmedTAKinetic study of the in vitro release and stability of theophylline floating beadsInt J Pharm Pharm Sci20135179184
- FreitasFFFernandesHBPiauilinoCAGastroprotective activity of Zanthoxylumrhoifolium Lam. In animal modelsJ Ethnopharmacol201113770070821723384
- SedlakJLindsayRHEstimation of total, protein-bound, and nonprotein sulfhydryl groups in tissues with Ellmann’s reagentAnal Biochem1968251922054973948
- PandaVSonkambleMAnti-ulcer activity of Ipomoea batatas tubers (sweet potato)Journal of Functional Foods in Health and Disease201224861
- PolatBAlbayrakYSuleymanBAntiulcerative effect of dexmedetomidine on indomethacin-induced gastric ulcer in ratsPharmacol Rep20116351852621602607
- SunYOberleyLWLiYA simple method for clinical assay of superoxide dismutaseClin Chem1988344975003349599
- SriamornsakPThirawongNPuttipipatkhachornSEmulsion gel beads of calcium pectinate capable of floating on the gastric fluid: effect of some additives, hardening agent or coating on release behavior of metronidazoleEur J Pharm Sci20052436337315734303
- RangarajGKishoreNDhanalekshmiUMRajaMDSenthil kumarCReddyPNDesign and study of formulation variables affecting drug loading and its release from Alginate beadsJournal of Pharmaceutical Sciences and Research201027781
- KhonkarnRMankhetkornSHenninkWEOkonogiSPEG-OCL micelles for quercetin solubilisation and inhibition of cancer cell growthEur J Pharm Biopharm20117926827521596135
- ZhengYHaworthISZuoZChowMSChowAHPhysicochemical and structural characterization of quercetin-beta-cyclodextrin complexesJ Pharm Sci2005941079108915793810
- SriKVKondaiahARatnaJVAnnapurnaAPreparation and characterization of quercetin and rutin cyclodextrin inclusion complexesDrug Dev Ind Pharm20073324525317454057
- SahooNGKakranMShaalLAPreparation and characterization of quercetin nanocrystalsJ Pharm Sci20111002379239021491450
- KakranMSahooNGLiLJudehZFabrication of quercetin nanoparticles by anti-solvent precipitation method for enhanced dissolutionPowder Technol20122235964
- VicentiniFTVazMMFonsecaYMBentleyMVFonsecaMJCharacterization and stability study of a water-in-oil microemulsion incorporating quercetinDrug Dev Ind Pharm201137475520550424
- ZhengYChowAHProduction and characterization of a spray-dried hydroxypropyl-beta-cyclodextrin/quercetin complexDrug Dev Ind Pharm20093572773419514988
- KhaledKAMahrousGMComparative Study of the Dissolution and Physicochemical Characteristics of the Binary Systems of Quercetin with Polyethylene Glycol, Polyvinyl pyrrolidone, and Hydroxypropyl-β-cyclodextrinSaudi Pharm J200193442
- TakeuchiKPathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotilityWorld J Gastroenterol2012182147216022611307
- BandyopadhyaySKPakrashiSCPakrashiAThe role of antioxidant activity of Phyllanthus emblica fruits on prevention from indomethacin induced gastric ulcerJ Ethnopharmacol20007017117610771207
- SharmaVRajaniGPEvaluation of Caesalpinia Linn. For anti-inflammatory and antiulcer activitiesIndian J Pharmacol20114316817121572651
- BiliciMOzturkCDursnHProtective effect of mirtazapine on indomethacin-induced ulcer in rats and its relationship with oxidant and antioxidant parametersDig Dis Sci2009541868187519034656
- SuleymanBHaliciZOdabasogluFGocerFThe Effects of Lacidipine on Indomethacin Induced Ulcers in RatsInt J Pharmacol201282115121
- de GrootHRauenUTissue injury by reactive oxygen species and the protective effects of flavonoidsFundam Clin Pharmacol1998122492559646056
- MiuraTMuraokaSFujimotoYLipid peroxidation induced by indomethacin with horseradish peroxidase and hydrogen peroxide: involvement of indomethacin radicalsBiochem Pharmacol2002632069207412093485
- SuleymanHAlbayrakABiliciMCadirciEHaliciZDifferent mechanisms in formation and prevention of indomethacin-induced gastric ulcersInflammation20103322423420084447
- NaitoYYoshikawaTYoshidaNKondoMRole of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injuryDig Dis Sci19984330S34S9753223
- El-AbharHSCoenzyme Q10: a novel gastroprotective effect via modulation of vascular permeability, prostaglandin E2, nitric oxide and redox status in indomethacin-induced gastric ulcer modelEur J Pharmacol201064931431920858483
- KelkelMJacobCDicatoMDiederichMReview: Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignanciesMolecules2010157035707420944521
- HalliwellBWhitemanMMeasuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean?Br J Pharmacol200414223125515155533
- KocMImikHOdabasogluFGastroprotective and anti-oxidative properties of ascorbic acid on indomethacin-induced gastric injuries in ratsBiol Trace Elem Res200812622223618726076
- KisaogluAOzogluBCetynNSuleymanBThe role of Alpha-2 Adrenergic Receptors in the Anti-ulcerative Activity of Famotidine and Omeprazole in Rats and its Relationship with Oxidant-antioxidant ParametersInt J Pharmacol201176682689
- CushnieTPLambAJAntimicrobial activity of flavonoidsInt J Antimicrob Agents20052634335616323269
- DeviRSNarayanSVaniGShyamala DeviCSGastroprotective effect of Terminalia arjuna bark on diclofenac sodium induced gastric ulcerChem Biol Interact2007167718317327128
- BayirYOdabasogluFCakirAThe inhibition of gastric mucosal lesion, oxidative stress and neutrophil-infiltration in rats by the lichen constituent diffractaic acidPhytomedicine20061358459016920514
- BombardierCAn evidence-based evaluation of the gastrointestinal safety of coxibsAm J Cardiol2002893D9D
- OdabasogluFCakirASuleymanHGastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in ratsJ Ethnopharmacol2006103596516169175
- KwiecienSBrzozowskiTKonturekSJEffects of reactive oxygen species action on gastric mucosa in various models of mucosal injuriesJ Physiol Pharmacol200253395011939718
- Alvarez-SuarezJMDekanskiDRistićSStrawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increasePLoS One20116e2587822016781
- GuptaSKatariaMGuptaPKMurganandanSYashroyRCProtective role of extracts of neem seeds in diabetes caused by streptozotocin in ratsJ Ethnopharmacol20049018518915013179
- ZahorodnyĭMIEffect of quercetin on sodium diclofenac-induced ulcerationLik Sprava200319699 Ukrainian12712624