Abstract
Breast cancer (BC) is the most frequent tumor worldwide. Triple-negative BCs are characterized by the negative estrogen and progesterone receptors and negative HER2, and represent 15% of all BCs. In this review, data on the use of taxanes in triple-negative BCs are analyzed, concluding they are effective in any clinical setting (neoadjuvant, adjuvant, and metastatic). Further, the role of nab-paclitaxel (formulation of albumin-bound paclitaxel) in these tumors is also evaluated. The available data show the clinical potential of nab-paclitaxel based combinations in terms of long-duration response, increased survival, and better quality of life of patients with triple-negative metastatic BC. The ongoing trials will give further information on the better management of this type of tumor.
Introduction
Breast cancer (BC) is the most frequent tumor worldwide. In 2008, 1,380,000 new cases and 458,000 deaths for BC were reported worldwide, of which there were 332,000 new cases and 89,000 deaths in Europe. Although the improvement in early diagnosis and adjuvant therapy has reduced mortality, BC is still the main cause of death for cancer in women both in industrialized and in developing countries.Citation1
In Italy, BC is the most frequent tumor in women (29%), with about 48,000 new cases diagnosed in 2014. In 2011, BC represented the first cause of death for cancer in women, with approximately 11,959 deaths estimated. The 5-years relative survival, moderately but constantly increasing apart from other comorbidities, is 87% for women diagnosed between 2005 and 2007.Citation2
BC is a heterogeneous disease, and therefore, a “golden standard” treatment, suitable for all the molecular types of cancer, is not available.Citation3 The most important biological markers, not only for classification of BC but also for, the therapeutic strategy are the hormonal receptors (estrogen [ER] and progesterone [PgR] receptor) and the HER2 receptor status.
The triple-negative BCs (TNBCs)
Tumors that are ER-, PgR-, and HER2-negative are known as TNBC and account for about 15% of BCs.Citation4 These tumors develop earlier in life, especially in premenopausal women, and have a poorer prognosis than the other types of BC due to the higher aggressiveness. These factors may be a major reason for the high-risk relapse, and shorter progression-free survival (PFS) and overall survival (OS) reported for this disease.Citation3–Citation6
The main general characteristics of TNBC are summarized in .
TNBC is not a unique clinical entity. It comprises several types of cancers now characterized by molecular profiles, which represent different diseases with probably different treatment options and different response to chemotherapy, biological agents, and/or other therapeutic regimens.
After 2002, gene expression profiles have identified the different molecular subtypes of BC, in particular, in the neoadjuvant settingCitation7–Citation10 and in particular, regarding TNBC.Citation11 The PAM50 gene expression assayCitation12 classifies BCs into at least five groups, including luminal A, luminal B, HER2-enriched, basal-like (BL), and normal breast-like. More recent gene expression array analysis has identified six different groups of TNBC, including two BLs (BL1 and BL2), an immune-modulatory (IM), a mesenchymal (M), a mesenchymal stem-like (MSL), and a luminal androgen receptor (LAR) subtype.Citation13 Recently Tobin et al reported that with PAM50 intrinsic BC subtypes array, 25% of relapses were basal, 32% HER2, 10% luminal A, 28% luminal B, and 5% normal breast-like. Importantly, the intrinsic subtype at relapse was significantly associated with postrelapse survival (P=0.012).Citation14 At the 2015 American Society of Clinical Oncology (ASCO) Meeting, Dent et al presented interesting data from the Surveillance, Epidemiology, and End Results (SEER) database on 10,000 women diagnosed in 2010 and 2011 with TNBC in the USA.Citation15 This population reflects the current clinical practice in the USA at the time: 34% were at stage I, 42% at stage II, 15% at stage III, and 6% at stage IV, with a 24-month OS of 97%, 93%, 71%, and 27%, respectively. The median OS in metastatic disease was 13 months.
The treatment of TNBCs
A proportion of TNBC is highly sensitive to chemotherapy but with a short PFS and a lower OS.Citation4–Citation6,Citation13,Citation15 Current therapeutic strategies include chemotherapeutic drugs (anthracyclines, taxanes, platinum derivatives, and ixabepilone) and biological drugs.Citation5,Citation6 The efficacy of anthracyclines and taxanes in metastatic BC is higher in ER-negative tumors; for this reason, both classes are indicated as first-line treatment of TNBC, even if with a short-lasting benefit.Citation4 Another group of drugs with proven activity in TNBC are the platinum derivatives cisplatin and carboplatin.Citation3–Citation6 The biologic drugs already evaluated or under active research include angiogenesis inhibitors (bevacizumab), PARP1 and EGFR inhibitors, tyrosine kinase and ERK inhibitors, mTOR inhibitors, heat shock protein 90 inhibitors, and AR antagonists.Citation3–Citation6,Citation13
Guidelines for the treatment of TNBCs
There are no specific guidelines for the management of TNBC: the National Comprehensive Cancer Network (NCCN), European Society for Medical Oncology (ESMO), and Associazione Italiana di Oncologia Medica (AIOM) Guidelines recommend that TNBC be treated with chemotherapy (monotherapy or combination therapy) but do not specify the most appropriate drugs ().Citation1
Table 2 Taxanes and their combinations recommended by NCCN, ESMO, and AIOM guidelines for triple-negative BCCitation1,Citation16,Citation17
The ESMO Guidelines states that cytotoxic chemotherapy is the standard of care for the treatment of TNBC and that the choice of the regimen should be made after consideration of disease-related factors (disease-free survival [DFS], previous therapies and response, tumor burden, and need for rapid disease/symptom control) and patient-related factors (patient preferences, biological age, menopausal status, comorbidities and performance status, and socioeconomic and psychological factors). Combination chemotherapy is more often required because of frequent visceral involvement, aggressive course, and risk of rapid patient deterioration. Finally, there is no a standard approach for chemotherapy after first line.Citation1
The role of taxanes in TNBC
The role of taxanes in TNBC is well established after the many studies evaluating the efficacy of taxane-based regimens in neoadjuvant, adjuvant, and metastatic disease settings.
The neoadjuvant setting
Neoadjuvant therapy has been used for a long time for reducing the size and the extension of locally advanced tumors, but now it is extensively used also in early BC not suitable for primary conservative surgery, with an added predictive value for the long-term outcome of the disease. Actually the best efficacy target for neoadjuvant therapy is expressed as pathological complete response (pCR). The predictive value of pCR as a surrogate for long-term clinical benefit has been recently confirmed by the retrospective pooled analysis of Cortazar et al.Citation18 This meta-analysis was based on the pCR, overall response rate (ORR), and event-free survival (EFS) data of 12 international clinical trials on a total 11,550 patients. The analysis compared the three main definitions of pCR in order to establish their association with long-term efficacy: ypT0 ypN0 (no invasive and in situ tumor in the breast and auxiliary lymph nodes); ypT0/is ypN0 (no invasive tumor in the breast and auxiliary lymph nodes, independent of the presence of in situ ductal carcinoma); and ypT0/is ypN0/is (no invasive tumor in the breast, independent of the presence of in situ ductal carcinoma or lymph nodes involvement). The retrospective analysis showed that complete tumor eradication (breast and lymph nodes) (ypT0 ypN0 or ypT0/is ypN0 pCR) was strongly associated to the improvement of EFS and OS as compared with tumor eradication in the breast only (ypN0/is). The better combination between pCR and long-term effect was observed in patients with an aggressive tumor (TNBC; high-grade; ER/PgR-positive, HER2-negative; HER2-positive; and ER- and PgR-negative). Authors also stated in the paper that “pooled analysis could not validate pCR as a surrogate end point for improved EFS and OS” and that the potential explanation could be the heterogeneous BC subtypes in the examined trials.Citation19
These results were confirmed by a further analysis of pooled data showing that the association between pCR and long-term outcome is particularly evident in patients with aggressive BCs.Citation20,Citation21
Several studies on neoadjuvant therapy confirmed the sensitivity of TNBC to cytotoxic drugs, as well as the importance of taxane-based chemotherapy. Rouzier et alCitation22 evaluated the molecular-based chemosensitivity in 82 patients treated with anthracyclines and taxanes neoadjuvant therapy, and a pCR was observed in 45% of BL tumors and in 6% of luminal tumors (A and B).
An MD Anderson Cancer Center studyCitation23 evaluated 1,118 patients (23% with TNBC) treated with neoadjuvant therapy. The pCR rates were significantly higher in TNBC treated with anthracyclines-based regimens. Anthracycline- and taxane-based regimens were more active, but both PFS and 3-year OS were significantly worse in TNBC (hazard ratio [HR] 1.86, 95% confidence interval [CI] 1.39–2.50, P<0.0001; and HR 2.53, 95% CI 1.77–3.57, P<0.0001, respectively). Patients with TNBC with residual disease had a poorer outcome that did those with non-TNBC tumors (3-year DFS rate 68% vs 88%) (P=0.0001). In patients with pCR, both PFS and OS were no different between TNBC and other types of tumors ().
Figure 1 Effect of neoadjuvant chemotherapy in data of triple-negative BCs vs non-triple-negative BCs.Citation23
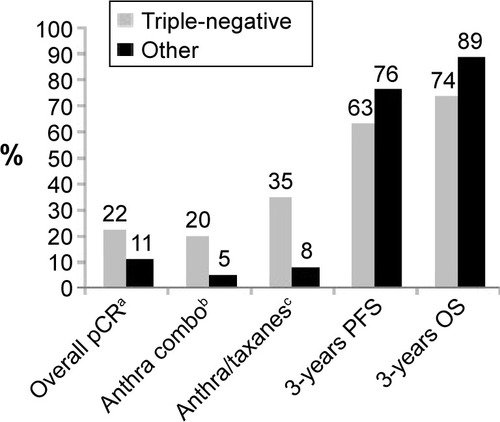
A similar studyCitation24 carried out in the same center on 1,731 patients treated with neoadjuvant anthracycline and taxane regimens reported an overall pCR rate of 13%. In the 317 TNBC patients, the pCR rate was 22.4%. In this group, pCR seemed to be a strong predictive factor of long-term survival, with 84% of patients still alive at 10 years vs 59% in the case of residual disease.
In a retrospective Japanese study, among 151 patients treated with anthracycline- and taxane-based neoadjuvant therapy, TNBC (14%) had a pCR rate higher than did the non-TNBC tumors (38% vs 12%).Citation25
A study by the Istituto Europeo di OncologiaCitation26 in 30 patients with TNBC, four cycles of epirubicin, cisplatin, and continuous-infusion fluorouracil followed by three cycles of weekly paclitaxel achieved an objective response in 26 cases (86%) and a pCR in 12 cases (40%). A total 26 patients (86%) underwent conservative surgery, and the 2-year DFS was 87.5%.
The GeparDuoCitation27,Citation28 study evaluated the pCR rate in 913 women randomized to neoadjuvant doxorubicin and docetaxel for four cycles or doxorubicin and cyclophosphamide (AC) for four cycles followed by docetaxel for four cycles. The overall pCR rate was 10.6% (7% with the two-drug regimen and 14.3% with the triplet one). It should be noted that GeparDuo also tested four vs eight cycles of chemotherapy. Either way, the probability of pCR was three times higher in the endocrine receptor-negative tumors vs the endocrine receptor-positive subgroup (22.8% vs 6.2%). The trial I-SPYCitation29 evaluated 190 patients treated with neoadjuvant anthracyclines and taxanes: in the subgroup of TNBCs (28%), the pCR rate was 33%, significantly higher than that observed in HER2-negative and ER/PgR-positive tumors (10%).
The efficacy of taxane-based neoadjuvant therapy was further confirmed by more recent trials. Wu et alCitation30 evaluated the efficacy of neoadjuvant therapy with docetaxel plus epirubicin, and the OS in 54 patients with TNBC and in 195 patients with non-TNBC. A pCR was observed in 25.9% of TNBCs, significantly higher than in the other subtypes (P=0.019). However, patients with TNBC with residual disease had a shorter 5-year DFS and OS vs patients with non-TNBC. In the subgroup with pCR, survival was equivalent between the two groups, similarly to data previously reported by the MD Anderson group.Citation23
Sakuma et alCitation31 evaluated 44 patients with TNBC treated with anthracycline- and taxane-based neoadjuvant therapy, and reported a pCR in 36% of cases with a long-term outcome significantly better that that with residual disease.
The use of angiogenesis inhibitors in TNBC is supported by the highly proliferative nature of this tumor and by the importance of VEGF for its microvascular growth,Citation32 and bevacizumab could play a role in the neoadjuvant setting.Citation33 The recent Phase II KCSG BR-0905 trialCitation34 evaluated the addition of bevacizumab to neoadjuvant docetaxel and carboplatin in 45 patients with TNBC. Also, in this study, the pCR rate was high (42%), with a clinical response rate of 96%. This allowed a conservative surgery in 35 cases (78%). The Phase III GeparQuinto trialCitation35 compared epirubicin and cyclophosphamide followed by docetaxel with or without bevacizumab in 1,948 HER2-negative BC patients, with an overall pCR rate (breast and nodes) of 18.4% with bevacizumab vs 19.9% for controls (P=0.04). Among the 663 patients with TNBC, pCR rates were 39.3% with bevacizumab vs 27.9% in controls, and the difference was highly significant (odds ratio [OR] 1.67, 95% CI 1.21–2.31, P=0.003). Results in the ER/PgR-positive population were not as good, with or without bevacizumab, with a pCR rate of 7.8% and 7.7%, respectively (OR 0.99, 95% CI 0.66–1.50, P=1.00). It should be noted that the test for interaction showed just a trend to significance.
The New England Journal of Medicine published a report by Bear et alCitation36 of another trial of neoadjuvant chemotherapy with or without bevacizumab, the NSABP B-40 study. This Phase III randomized trial assigned 1,206 patients with HER2-negative BC to receive docetaxel (100 mg/m2 every 21 days) or docetaxel (75 mg/m2 day 1) plus capecitabine (825 mg/m2 twice a day days 1 to 14) or docetaxel (75 mg/m2 day 1) plus gemcitabine (1,000 mg/m2 days 1 and 8) for four cycles. All regimens were followed by AC for a further four cycles. All patients were also randomized to receive bevacizumab (15 mg/kg) or not for the first six cycles of chemotherapy. Results showed first of all that the addition of capecitabine and gemcitabine did not improve the rate of pCR vs docetaxel alone and showed increased toxicity and that the toxicity of bevacizumab was manageable and as expected from previous trials and, significantly increased the overall pCR rate (34.5% vs 28.2%) (P=0.02). The multiple logistic regression model showed that TNBC subtype, high grade, and smaller tumor size were associated with higher rates of pCR in the breast, but when considering the pCR in breast and nodes, the addition of bevacizumab was significantly related to a better result in hormone receptor-positive tumors only.
In the randomized Phase II GeparSixto trial,Citation37 315 patients with TNBC were treated with weekly paclitaxel plus nonpegylated liposomal doxorubicin (once a week for 18 weeks) and bevacizumab every 3 weeks and were randomized to receive weekly carboplatin (area under the time–concentration curve [AUC] =2) or not. The pCR (ypT0ypN0) rate was 16% higher with the addition of carboplatin (53.2% vs 36.9%) (P=0.005). The toxicity was also significantly higher, with 53% discontinuation (41% with AUC reduced to 1.5). Data on the BRCA mutation are not yet available in order to assess the correct role of carboplatin.
At the ASCO 2015 meeting, further interesting data from the GeparSixto trial were presented,Citation38 showing that the addition of carboplatin to taxane and anthracycline increased the pCR rate from 45.2% to 64.9% in TNBC with homologous recombination deficiency. In tumors without deficiency, carboplatin did not improve the pCR rate.
Other data on the role of bevacizumab added to neoadjuvant chemotherapy were also recently reported from the Cancer and Leukemia Group B (CALGB) 40603 trial.Citation39 A standard chemotherapy plus carboplatin and bevacizumab obtained a pCR rate higher (60%) vs the same without bevacizumab (49%) or standard chemo alone (+/− bevacizumab: 43% vs 39%). The addition of carboplatin led to significant but small improvement in pCR rate, at the price of increased toxicity. At the ASCO 2015 meeting, an update of this trial reported a rate of conversion to the possibility of breast conservative surgery in favor of the bevacizumab arm of 42% in TNBC.Citation40
In June 2015, Earl et alCitation41 reported results of the multicenter British ARTemis Phase III trial. Between 2009 and 2013, 880 patients with HER2-negative early BC (tumor size >20 mm, clinically positive or negative Nodes) were randomized to three cycles of docetaxel (100 mg/m2 every 21 days) followed by three cycles of 5-Fluoruracile, Epirubicine at 100 mg/sqm, Cyclophosphamide regimen every 21 days, with or without four cycles of bevacizumab (15 mg/kg). The primary end point was pCR (tumor and nodes). Results showed a significant increase of pCR with the addition of bevacizumab (22% vs 17%) (P=0.03). The most important result of this trial is the great added value of bevacizumab in the TNBC population (pCR 45% vs 31%) (P<0.0001) as compared with the ER-positive HER2-neg population (pCR 7% vs 6%). ARTemis results are consistent with those of GeparQuintoCitation35 and CALGB 40603Citation39 and could also explain the different results for the HER2-negative ER-positive population in the NSABP B40 trial,Citation36 where the cutoff for the ER-positive population was very low (1% of positive cells) compared with both GeparQuinto and CALGB 40603 (cutoff 10%).
The role of pCR as a surrogate end point for DFS and OS has so far not been defined, and the answer will be possible from an extensive meta-analysis of long-term results of the Phase III randomized trials.
The adjuvant setting
Studies in adjuvant setting also confirmed the activity and relevance of taxanes in TNBCs. Hayes et al retrospectively analyzed the histological samples of 1,322 patients enrolled in the CALGB 9344 studyCitation42 in order to evaluate the role of HER2 status on clinical end points. Patients were divided in four groups: endocrine receptor- and HER2-negative (TNBC); endocrine receptor- and HER2-positive; endocrine receptor-positive and HER2-negative; endocrine receptor-negative and HER2-positive. Adding paclitaxel to anthracycline improved DFS both in HER2-positive patients, independently from endocrine receptor status, and in TNBC patients. No clinical benefit was observed in HER2-negative and endocrine receptor-positive tumors. This explorative analysis suggests that paclitaxel added to the adjuvant regimen significantly improves the outcome in TNBCs.
The study of Sparano et alCitation43 conducted on 4,950 patients, evaluated in the adjuvant setting the efficacy of AC followed by weekly or 3-weekly (q3w) docetaxel or paclitaxel. The results showed an improvement both in DFS and 5-year OS with weekly paclitaxel with respect to q3w paclitaxel. In TNBCs, the benefit of conventional weekly paclitaxel in term of DFS was 37% higher than the q3w regimen.
Other studies also evaluated anthracycline- and taxane-based adjuvant therapy in TNBC. The Breast Cancer International Research Group (BCIRG) 001 studyCitation44 compared docetaxel plus doxorubicin plus cyclophosphamide vs fluorouracil plus doxorubicin plus cyclophosphamide, showing that taxane was able to increase the efficacy in the TNBC cohort. Similar results were reported in a multicenter randomized Phase III study by Loesch et alCitation45 comparing AC followed by paclitaxel with doxorubicin and paclitaxel followed by weekly paclitaxel in high-risk BC patients.
Finally, a recent meta-analysis of 14 randomized Phase III studies on 25,067 patientsCitation46 evaluated the impact of a docetaxel-based adjuvant therapy on DFS and OS in early BC. The improvement in survival obtained with docetaxel-based regimens with respect to docetaxel-free regimens was observed, not only in the general population but also, in several subgroups, TNBC included.
All the above cited data confirm the high activity of taxanes in TNBC; however, at the ASCO 2015 meeting, interesting data from the adjuvant Phase III TITAN trial were reported.Citation47 In this trial, 614 early TNBC patients were randomized to adjuvant AC for four cycles followed by ixabepilone q3w for four cycles or weekly paclitaxel for 12 cycles. At a median follow up of 48 months, no difference was found between the two arms in 5-year DFS (87% vs 85.4%) or OS (92.3% vs 90.2%). Both regimens performed well, with different toxicity profile: ixabepilone had lower rate of neurotoxicity and fewer dose reductions.
The open-label, randomized Phase III BEATRICE study assessed the addition of bevacizumab to chemotherapy in the adjuvant setting for 2,591 women with TNBC. The primary analysis showed that invasive DFS (IDFS) events were observed in 16% of patients treated with chemotherapy alone compared with 14% of those treated with chemotherapy plus bevacizumab (P=0.18); the 3-year IDFS was 82.7% and 83.7%, respectively. After 200 deaths, no difference in OS was noted between the groups (P=0.23). The addition of bevacizumab was associated with increased incidences of Grade 3 or worse hypertension, severe cardiac events, and treatment discontinuation. For these reasons, the authors stated that bevacizumab cannot be recommended as adjuvant treatment in unselected patients with TNBC.Citation48
The metastatic setting
Conventional taxanes have a central role in the treatment of metastatic BC, based on several evidences of their benefits on clinical outcomes, such as OS, time to progression (TTP), and ORR.Citation49
Even if conventional taxanes demonstrated to be more active in endocrine receptor-negative tumors and are indicated in the first-line treatment of TNBC (although a specific benefit in this setting was not observed), it should be considered that they are commonly used in adjuvant therapy and cannot be rechallenged in case of short disease-free interval (<12 months).Citation4,Citation50
The duration of response to chemotherapy of TNBC is usually short, as demonstrated by a retrospective analysis of 111 cases treated with monotherapy or combinations.Citation51 The mean duration of the response was 12 weeks after first-line treatment, 9 weeks after second-line, and 4 weeks after third-line treatment. For this reason, some recent studies evaluated new first-line therapeutic regimens, combining taxanes with other cytotoxic drugs or new molecules.
The role of bevacizumab in metastatic disease was also explored in several Phase III trials. A meta-analysis of the three main Phase III studies of bevacizumab combined with first-line chemotherapy, showed an increase of PFS vs chemotherapy alone (8.1 vs 5.4 months) in 621 patients with TNBC.Citation52 In the RIBBON-2 study,Citation53 patients progressed after first-line chemotherapy and treated with second-line bevacizumab with or without chemotherapy were enrolled. Recently, a subanalysis of 159 (23%) cases of TNBCs in the RIBBON-2 study, most treated with taxanes, reported a median PFS of 6 months with bevacizumab plus chemotherapy vs 2.7 months with chemotherapy alone (P=0.0006); the median OS was 17.9 vs 12.6 months (P=0.0534), and the ORR was 41% vs 18% (P=0.0078). The Phase III IMELDA Trial was published by Gligorov et al Citation54 in late 2014, mainly based on the meta-analysis of Gennari et alCitation55 which found a first-line chemotherapy until progression led to a longer PFS and a small but appreciable increase in OS.Citation55 In the AVADOCitation52 trial where the response rate was very high, but a prolonged treatment with docetaxel was unrealistic because of cumulative toxicity. The open-label Phase III IMELDA trial investigated the combination of capecitabine and bevacizumab after initiation of docetaxel and bevacizumab. Previously untreated HER2-negative metastatic BC patients were treated with three to six cycles of bevacizumab (15 mg/kg) and docetaxel (75–100 mg/m2) every 21 days. Patients with progressive disease were excluded, and responders (complete or partial response [CR or PR]) or patients with stable disease were randomized to maintenance with bevacizumab or bevacizumab plus capecitabine (1,000 mg/m2 twice daily days 1–14 q3w, until disease progression (PD), unacceptable toxicity, or consent withdrawal. Bevacizumab and capecit-abine significantly improved overall median PFS (11.9 vs 4.3 months) (HR 0.38, P<0.001) and OS (39 vs 23.7 months) (HR 0.43, P<0.001), without unexpected safety problems. In the TNBC population, the median PFS was 7.6 months with the combination and 3.6 months with bevacizumab alone (HR 0.48). At the 2014 San Antonio Symposium, the OS in prespecified subgroups was presented, confirming the very good result in the TNBC population, with a death risk reduction of 53% (HR 0.47) compared with 57% in the overall population and with a 2-years OS of 62%.Citation54
Fan et alCitation56 evaluated the efficacy of docetaxel combined with cisplatin or capecitabine in the first-line treatment of patients with metastatic TNBC. The ORR was significantly higher in patients treated with docetaxel plus cisplatin than with docetaxel plus capecitabine (63% vs 15.4%) (P=0.001), as were the median PFS (10.9 vs 4.8 months) (P<0.001) and median OS (32.8 vs 21.5 months) (P=0.027), confirming the role of platinum in TNBC.
At the 2014 San Antonio Symposium, Tutt et alCitation57 presented results from the randomized Phase III TNT trial comparing carboplatin (AUC =6) with docetaxel (100 mg/m2), both every 21 days for six cycles, as first-line treatment in 376 patients with advanced TNBC or BRCA1/2-positive BC. The primary end point was the objective response rate in the intent-to-treat population. Of note, nearly all cancers with BRCA1 mutations are triple negative, whereas tumors with BRCA2 mutations can be either ER positive or triple negative. So, in the BRCA2 population of this study, there were also some ER-positive patients.
The TNT trial was based on the hypothesis that because BRCA1/2 germline mutations produce BCs that have defects in homologous recombination DNA repair, carboplatin would be lethal to cells with germline and somatic mutations in BRCA1/2. In other words, carboplatin might be an especially good therapy in terms of exploiting the defect in homologous recombination DNA repair, and this is why patients with BRCA1/2 mutations were included with TNBC patients. The results showed no significant difference in response rates between carboplatin and docetaxel in the overall patient group or in patients who received either agent as first-line therapy and then crossed over to the other agent as second-line treatment.
The only significant difference was in patients with BRCA1/2 germline mutations (response rate with carboplatin 68% vs 33% with docetaxel) (P=0.03). Similarly, PFS was 6.8 months vs 4.5. This is the outstanding result of this study, showing that platinum compounds could be more active than taxanes in patients with BRCA1/2 germline mutations. In patients with wild-type BRCA1/2, there was a nonsignificant trend for a higher response rate with docetaxel. No difference was found in PFS or OS.
A Japanese study,Citation58 interesting despite the low number of patients, evaluated the efficacy of the combination gemcitabine plus paclitaxel in 56 patients with metastatic BC, including 14 cases of TNBC. In the general population, the ORR was 44.6%, median TTP was 8.6 months, and median OS was 27.1 months, whereas in the TNBC population, the ORR was 35.7% and median TTP was 6 months.
An interesting recent Phase I studyCitation59 evaluated the efficacy and safety of olaparib, an oral PARP inhibitor, associated to paclitaxel in first- or second-line treatment in 19 patients with TNBC. Despite a good global efficacy (37% of confirmed partial responses), the combination of olaparib plus weekly paclitaxel had an unexpected higher neutropenia rate, even after secondary prophylaxis.
Approximately 10% to 15% of TNBC express androgen receptor (AR).Citation13 The LAR subtype of TNBC actually is a Luminal one, rich in AR,Citation13 and this is the rationale for an antiandrogen therapy. Enzalutamide is a potent AR inhibitor, but AR expression does not necessarily mean sensitivity to endocrine treatment. At the ASCO 2015 meeting, Parker et alCitation60 reported results of a randomized study in which a new gene profile was able to predict the benefit from treatment with enzalutamide in metastatic TNBC. Actually, 50% of patients with the positive diagnostic profile obtained 39% of clinical benefit rates at 16 months and 36% at 24 months, versus 11% and 6% respectively of patients with the negative diagnostic profile. This interesting result may be useful for a more targeted selection of TNBC patients suitable for an antiandrogen treatment in future trials.
In conclusion, the main reason of failure in metastatic BC is resistance to the standard drugs, which can be intrinsic or acquired. Patients with disease progression or resistance could not have a cross-resistance with other drugs, such as capecitabine, gemcitabine, vinorelbine, eribulin, or nab-paclitaxel, which demonstrated their efficacy in patients with advanced BC pretreated with anthracyclines and/or taxanes.Citation32
Nab-paclitaxel
Nab-paclitaxel is a nanoparticle with median size 130 nm, solvent-free.Citation61,Citation62 Nanotechnology utilizes the natural properties of albumin to potentiate the selective uptake of paclitaxel in tumors and for targeting the drug directly into the cancer cells. Preclinical studies showed that nab-paclitaxel achieves a 33% higher tumor uptake vs conventional paclitaxel but lower uptake in normal tissue and plasma; furthermore, these studies demonstrated a lower toxicity in animals, a higher activity in animal models with xenograft tumors (breast, lung, ovarian, prostate, and colon), and that nab-paclitaxel is four times more efficient in crossing layers of endothelial cells.Citation63–Citation65
This unique and innovative mechanism of transport of nab-paclitaxel allows a higher concentration of the active drug in the tumor, better efficacy, and safety vs those for both conventional paclitaxel and docetaxel observed in clinical trials in metastatic BC.
Nab-paclitaxel in the metastatic setting
The Phase III pivotal study by Gradishar et alCitation66 evaluated the efficacy of nab-paclitaxel (260 mg/m2 intravenously [IV] q3w without premedication) vs conventional paclitaxel (175 mg/m2 IV with premedication) in 454 patients with metastatic BC. Nab-paclitaxel resulted significantly superior in ORR (33% vs 19%) (P=0.001) and in TTP (23 vs 16.9 weeks) (P=0.006) vs conventional paclitaxel; OS was significantly higher in patients treated with nab-paclitaxel beyond the first line than in patients treated with conventional paclitaxel (56.4 vs 46.7 weeks) (P=0.024). As far as safety is concerned, the grade 3/4 neutropenia rate was significantly lower with nab-paclitaxel (34% vs 54%) (P<0.001), and Grade 4 neutropenia alone was even better (9% vs 22%) (P<0.001), despite an almost double dose of paclitaxel (49% higher); the grade 3 sensitive neuropathy rate was higher (10% vs 2%) (P<0.001) but rapidly reversed to a ≤2 grade than did conventional paclitaxel. This study shows an important benefit of q3w nab-paclitaxel over q3w conventional paclitaxel, with an improved therapeutic index and the lack of premedication with steroids.
About the optimal schedule of conventional paclitaxel
It is widely accepted that weekly paclitaxel is a “different treatment” compared with the q3w schedule, in any setting.Citation67 In the neoadjuvant setting, the weekly schedule was superior, with a 28.2% pCR vs 15.7% (P=0.02);Citation68 in the adjuvant setting, by the already cited trial of Sparano et al,Citation43 the comparison of weekly to q3w paclitaxel (and docetaxel) after four cycles of AC showed a significant advantage in DFS in favor of weekly paclitaxel (HR 1.27, P=0.006), in particular in TNBC. In the metastatic setting, weekly paclitaxel was also significantly superior to the q3w scheduleCitation69 in response rate (42% vs 29%) (OR 1.75, P=0.0004), median TTP (9 vs 5 months) (HR 1.43, P<0.0001), and median OS (24 vs 12 months) (HR 1.28, P=0.0092), at an expected price of a significant increase in Grade 3 neurotoxicity (824% vs 12%) (P=0.0003), which was defined as the treatment-limiting toxicity.
Nowadays the q3w conventional paclitaxel schedule is rarely used, and the preferred schedules for conventional taxanes are weekly paclitaxel or q3w docetaxel.
An open-label, multicenter, randomized Phase II studyCitation70 evaluated the efficacy and safety of three nab-paclitaxel-based regimens (300 mg/m2 q3w, 100 mg/m2 weekly, or 150 mg/m2 weekly) vs docetaxel 100 mg/m2 q3w in the first-line treatment of 302 patients with metastatic BC. The nab-paclitaxel 150 mg/m2 weekly schedule obtained significantly longer PFS than did docetaxel by both independent (12.9 vs 7.5 months) (P=0.0065) and investigator (14.6 vs 7.8 months) (P=0.012) assessment. According to the independent radiologist review, both schedules of 150 mg/m2 (49%) and 100 mg/m2 (45%) weekly of nab-paclitaxel demonstrated a higher ORR vs docetaxel (35%), but this did not reach statistical significance. The evaluation of ORR by investigators showed a statistically significant difference between the weekly schedules of nab-paclitaxel and docetaxel (74% with 150 mg/m2 and 62% with 100 mg/m2 vs 39%) (P<0.05). The final analysis of OS of this study, published by Gradishar et al in 2012,Citation71 showed a median OS of 33.8 months with weekly nab-paclitaxel 150 mg/m2 vs 22.2, 27.7, and 26.6 months, respectively, with weekly nab-paclitaxel 100 mg/m2 and 300 mg/m2 q3w, and docetaxel 100 mg/m2 q3w (P=0.047).
A trend toward a longer OS was noted in all the patients subgroups, independent from age (<65 vs ≥65 years), type of metastatic site (visceral vs not), number of visceral lesions (<5 vs ≥5), and menopausal status. The best clinical response was already observed after two cycles of treatment with weekly nab-paclitaxel 150 or 100 mg/m2 vs five cycles of docetaxel (P<0.001), highlighting the quick response of this new drug. Further, the median number of cycles administrated with weekly nab-paclitaxel 150 mg/m2 was higher than with docetaxel (eight vs ten). The weekly dose of 150 mg/m2 of nab-paclitaxel could be the most effective dosage, from a clinical point of view, for previously untreated and fit patients.Citation71
A retrospective analysis of previous studies (CA012 and CA024)Citation72 evaluated the efficacy of nab-paclitaxel in patients with poor prognostic factors: dominant visceral metastasis and short DFS. In the first study (CA012), the ORR was higher in patients treated with nab-paclitaxel vs those treated with conventional paclitaxel both in dominant visceral metastasis (42% vs 23%) (P=0.022) and in short DFS (43% vs 33%) (P=0.417). Also, in the second study (CA024), patients treated with weekly nab-paclitaxel showed a better ORR than did patients treated with docetaxel, significantly higher in the cases of dominant visceral metastases.
PFS and OS showed a similar trend, but a statistically significant difference was observed only in the second study, which showed PFS in dominant visceral metastasis (13.1 months for nab-paclitaxel 150 mg/m2 vs 7.8 months for docetaxel [P=0.019] and 7.5 months for nab-paclitaxel 100 mg/m2 [P=0.010]). The results of this analysis suggest that nab-paclitaxel is a therapeutic option also for patients with very aggressive disease.
The study of Blum et alCitation73 demonstrated the efficacy of monotherapy with weekly nab-paclitaxel at 100 mg/m2 (n=106) or 125 mg/m2 (n=75) in patients with metastatic BC heavily pretreated with conventional taxanes. The ORR was 14% and 16% with 100 mg/m2 and 125 mg/m2, respectively; stable disease for more than 16 weeks was observed in 12% and in 21% of cases, respectively. Median PFS and OS were 3 and 9.2 months with 100 mg/m2, and 3.5 and 9.1 months with 125 mg/m2.
Nab-paclitaxel, administered to patients with metastatic BC pretreated with conventional taxanes, has a significant antitumor activity and is well tolerated. Further evidence of efficacy of nab-paclitaxel in patients previously treated with conventional taxanes is the recently published prospective, multicenter Italian study that aimed to evaluate the efficacy and safety of nab-paclitaxel 260 mg/m2 q3w in second-line treatment of 52 HER2-negative, taxane-pretreated metastatic BC patients. The ORR was 48% (13.5% of complete response), the overall clinical benefit rate was 77%, and the median PFS was 8.9 months. Adverse events were expected and manageable, with good patient compliance and quality of life even after long-term treatment.Citation74
Some clinical trials evaluated the combination of nab-paclitaxel with other chemotherapy drugs commonly used in first-line treatment for metastatic BC (ie, capecitabine and gemcitabine). Schwartzberg et alCitation75 analyzed the efficacy of weekly nab-paclitaxel at 125 mg/m2 plus capecitabine 825 mg/m2 twice daily orally for 15 days per cycle in 46 patients with metastatic BC. The ORR was 61% (complete response [clinical or radiological] 4% and partial response 57%), seven patients had a stable disease (≥24 weeks), with an overall clinical benefit of 76.1%. The median PFS was 10.6 months and median OS was 19.9 months. Another open-label Phase II studyCitation76 evaluated the combination of weekly nab-paclitaxel (125 mg/m2) and gemcitabine (1,000 mg/m2) in 50 nonpre-treated patients. Findings were that 8% and 42% of patients showed a complete and partial response, respectively. The median duration of the response was 6.9 months, the median PFS 7.9 months, and the median OS was not yet reached. The treatment was well-tolerated. An unplanned analysis of subgroups showed a clinical response in ten out of 13 patients (77%), with TNBC vs 16 of other patients (44%).
Even if it is not possible to draw conclusive consideration in this small subgroup of patients, these data suggest the possibility that TNBC could be particularly responsive to nab-paclitaxel-based regimens.
Nab-paclitaxel and bevacizumab in the metastatic setting
The combination of nab-paclitaxel and bevacizumab was investigated by several authors in the first-line treatment of TNBC. In 2010, Lobo et alCitation77 published an open-label Phase II study of first-line treatment with weekly nab-paclitaxel (150 mg/m2), bevacizumab (10 mg/kg), and gemcitabine (1,500 mg/m2) in 30 patients with metastatic HER2-negative BC. The median PFS was 10.4 months and ORR was 75.9%, including eight complete responses (27.6%). In this trial, 13 patients (44.8%) had TNBC. The results showed a good clinical response in this subgroup, with a complete response in five cases (38.4%), a partial response in four cases (30.7%), and stable disease in a further two cases (6.9%). Finally, the 18-month OS rate was 77.2% in the overall population and 82.5% (95% CI 46.1%–95.3%) in TNBC patients. Eight patients (27.6%) experienced a grade 3/4 toxicity. Since first-line treatment with a triplet chemotherapy was demonstrated to be very active, with an acceptable toxicity, the authors suggested further evaluation of this combination.
Hamilton et alCitation78 published, in 2013, the results of a multicenter Phase II study of the combination of nab-paclitaxel plus bevacizumab plus carboplatin in first-line treatment for TNBC. Patients received weekly nab-paclitaxel (100 mg/m2) plus carboplatin (AUC 2) for three times in a cycle of 28 days and bevacizumab (10 mg/kg) at days 1 and 15 of the cycle. The treatment was continued until disease progression, unacceptable toxicity, or voluntary withdrawal from protocol. The primary end points were safety and tolerability; secondary end points were PFS, ORR, and clinical benefit. A total 34 patients, with median age 50 years (range: 30–76 years) were enrolled; of these, 26 (77%) were treated in the adjuvant setting with anthracyclines and taxanes, and 88% had visceral metastases.
Despite the limitation of the low number of patients, this study is very interesting because the triple combination was able to obtain encouraging results, with a median PFS of 9.2 months (95% CI 7.8–25.1 months), and a 6-month and 9-month progression-free rate of 88% and 64% respectively. The ORR was 85%, with 17.7% having complete response and a very high rate of clinical benefit (94%) (). The randomized Phase III CALGB 40502 study compared weekly nab-paclitaxel 150 mg/m2 or weekly ixabepilone 16 mg/m2 to weekly paclitaxel 90 mg/m2, all of them for 3 of 4 weeks and associated with bevacizumab, as first-line therapy for 783 patients with advanced BC. The median PFS was 11 months for paclitaxel, 7.4 months for ixabepilone (P<0.001), and 9.3 months for nab-paclitaxel (P=0.054). In an explorative unplanned subgroup analysis of the TNBC population, no significant differences between the nab-paclitaxel plus bevacizumab and paclitaxel plus bevacizumab groups were observed (median PFS 7.4 and 6.5 months, respectively).Citation79 It seems that the lack of superiority of nab-paclitaxel over paclitaxel in the CALGB study could be attributable to a suboptimal drug dose and imperfect knowledge of nab-paclitaxel pharmacokinetics in association with bevacizumabCitation80 – the dose reductions and treatment interruptions were much higher in the nab-paclitaxel plus bevacizumab arm vs conventional paclitaxel plus bevacizumab.
Figure 2 Efficacy parameters in patients with triple-negative BC treated with nab-paclitaxel/bevacizumab/carboplatin.Citation70
Abbreviations: BC, breast cancer; CBR, clinical benefit rate; CI, confidence interval; CR, complete response; ORR, overall response rate; PR, partial response; SD, stable disease.
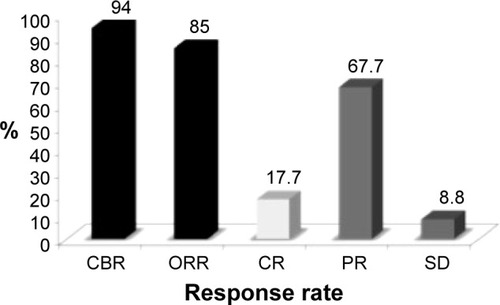
The safety profile was very good: neutropenia and thrombocytopenia were the most frequent grade 3/4 adverse events (53% and 18% respectively). According to the authors, first-line treatment with the combination of nab-paclitaxel, bevacizumab, and carboplatin seems to be effective and well-tolerated in patients with metastatic TNBC, with PFS, ORR, clinical benefit, and safety comparable with that observed with other standard first-line treatments.
Nab-paclitaxel in the neoadjuvant setting
The neoadjuvant setting is very important as stated before, in particular, for the still discussed but widely accepted value of pCR as a surrogate point for long-term outcome.Citation18–Citation21,Citation81 In the past few years, many studies with nab-paclitaxel-based neoadjuvant regimens have been published.
Nab-paclitaxel and bevacizumab in the neoadjuvant setting
The efficacy and safety profile of the combination of nab-paclitaxel and bevacizumab was evaluated also in neoadjuvant therapy in the Phase II SWOG S0800 study.Citation82 This study enrolled 215 patients with inflammatory BC (IBC) or locally advanced BC, comparing bevacizumab (10 mg/kg IV every 14 days for 12 weeks) plus weekly nab-paclitaxel 100 mg/m2 for 12 weeks followed by AC (doxorubicin 69 mg/m2 IV for six cycles every 14 days and pegfilgrastim 6 mg subcutaneously) with nab-paclitaxel alone after or before AC.Citation82 Based on its several clinical benefits in terms of efficacy and safety, the authors used nab-paclitaxel as the backbone of the study. The results showed that the combination of nab-paclitaxel plus AC was able to obtain a pCR rate higher than that of other nab-paclitaxel-free regimens (21% vs 10%–11%). Nab-paclitaxel plus bevacizumab significantly increased the pCR in the intention-to-treat population (36%), with the higher rate in hormone-negative patients (pCR 59% vs 28% of controls). It is noteworthy that the combination nab-paclitaxel and bevacizumab was not associated with increased grade 3/4 toxicity.Citation82
Another interesting Phase II study of neoadjuvant treatmentCitation83 involved 42 patients with TNBCs >2 cm treated with nab-paclitaxel at 100 mg/m2 was given on days 1, 8, and 15, and carboplatin (AUC =6) day 1, every 4 weeks for four cycles, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2, “dose-dense” every 14 days. Bevacizumab 10 mg/kg was administered every two weeks with chemotherapy, and continued postoperatively for a total of 1 year. The pCR rate was very high: 53% in breast and lymph nodes; the only severe toxicity was Grade 3 (56%) and Grade 4 (24%) neutropenia, preventable with granulocyte growth factors. These results are very important considering the triple-negative setting.
Neoadjuvant nab-paclitaxel with other combinations
One recent Phase II studyCitation84 in the neoadjuvant setting matched 30 patients with weekly nab-paclitaxel (125 mg) and 90 patients with conventional paclitaxel, both combined with carboplatin (AUC =2) and trastuzumab, in the HER2-positive population. The results were similar for the two taxanes with respect to pCR (nab-paclitaxel 26.7% vs paclitaxel 25.6%, and 43.6% vs 39.6% with trastuzumab, respectively). Grade 4 neutropenia was higher with nab-paclitaxel. This study is small, as stated by the authors, with just two TNBC cases, but it confirms the study by Snider et alCitation83 regarding the need of growth factors use when combining a taxane with carboplatin. The strength of this combination is the absence of anthracycline, which is interesting for cardiopathic patients. The authors cited, in the concluding remarks, several “ongoing large Phase III trials able to provide a definitive answer on the role of nab-paclitaxel in the neoadjuvant setting”. We believe this recently happened with the reporting of the GeparSeptoCitation85 trial, in which there were 275 TNBCs.
The GeparSepto study
The GeparSepto (NCT01583426)Citation85 neoadjuvant study compared weekly nab-paclitaxel (at the initial dose of 150 mg/m2/week in the first 400 patients, amended for toxicity to 125 mg/m2/week in the next 800), or conventional paclitaxel (80 mg/m2/week) for 12 weeks, both followed by four cycles of epirubicin plus cyclophosphamide, in more than 1,200 patients with early BC (275 with TNBC). The results were presented at the 2014 San Antonio Breast Cancer Symposium. In this study, HER2-positive patients with a planned treatment with pertuzumab (loading dose 840 mg followed by 420 mg every 4 weeks) and trastuzumab (loading dose 8 mg/kg, followed by 6 mg/kg every 4 weeks) were also included. The primary end point was pCR (defined as ypT0 ypN0 or N-positive); the secondary end points were pCR defined as ypT0/is ypN0 and ypT0 ypN0 0/+, toxicity, compliance, and pCR associated to secreted protein acidic and rich in cysteine (SPARC) expression. Some subgroup analyses were preplanned as per protocol. A total 23% of patients in both arms had a TNBC. The pCR rate (ypT0 ypN0) was 29% in patients treated with paclitaxel and 38% in patients treated with nab-paclitaxel (OR 1.5, P<0.01) ().
Figure 3 Pathological Response rate in patients treated with conventional paclitaxel or nab-paclitaxel.Citation84
Abbreviation: pCR, pathological complete response.
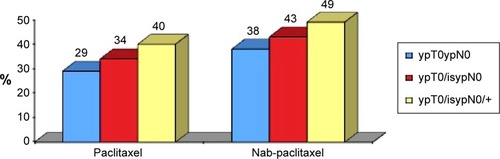
The primary end point of pCR was reached with statistical significance. Actually, the odds ratio was 1.53, indicating a 53% higher likelihood of achieving a pCR with albumin-bound paclitaxel than with conventional paclitaxel.
The benefit observed with nab-paclitaxel was confirmed in all the subgroups of patients; in particular, it is noteworthy that in the TNBC population, the pCR was almost doubled (48.2% vs 25.7%) (P<0.001). Globally, due to this very high increase of response in TNBC, nab-paclitaxel can be considered a valid therapeutic approach for the management of a disease characterized by a very poor prognosis.
Safety profile
Other important information from this study is the planned schedule, ie, weekly nab-paclitaxel 150 mg/m2, already used by Gradishar et al.Citation69 After the enrolment of the first 400 patients, the protocol was amended, reducing the dose of weekly nab-paclitaxel to 125 mg/m2 for the further 800 patients. The safety profile was correctly reported (intent-to-treat) for all the study population, showing the higher efficacy but also the higher toxicity of nab-paclitaxel in terms of grade 3/4 sensitive neuropathy (10.2% vs 2.7%). However, when considering (“per treatment given”) only the patients treated with the dose of 125 mg/m2 of nab-paclitaxel (800/1,200), the efficacy is the same but the toxicity is lower, without any difference in Grade 3 and Grade 4 toxicity (5.3% vs 5.7%) ().
Table 3 Sensorial neuropathy (nab-paclitaxel at 125 mg/m2)Citation84
An issue in the comparison between nab-paclitaxel and conventional paclitaxel in terms of neurotoxicity was also recently discussed at the ASCO 2015 meeting. In a Phase II trial (NCT0163710) of first-line treatment of metastatic HER2-negative BC by Ciruelos et alCitation86 weekly conventional paclitaxel (80 mg/m2) was compared with weekly nab-paclitaxel at the dose of 100 mgs/sqm days 1, 8, 15; 150 mgs/sqm days 1, 8, 15 or 150 mgs/sqm days 1, 15; any dose level any 28 days; neuropathy was the primary end point and neutropenia one of the secondary end points. The authors reported, as expected, an increased rate of Grade 3 neutropenia with weekly nab-paclitaxel at 150 mg, but no difference was reported between arms in terms of neurotoxicity or overall neutropenia.
Another trial – the randomized Phase II “ADAPT TN” trial – was reported at the ASCO 2015 meeting by Gluz et al.Citation87 Gemcitabine and carboplatin are interesting partners for taxane combinations, and the trial randomized 336 TNBC patients to 12 weeks of neoadjuvant nab-paclitaxel (125 mgs/sqm) and gemcitabine (1000 mgs/sqm) days 1 and 8 q3w or carboplatin (AUC =2). The end point was to identify early response markers for pCR (ypT0 ypN0) (drop of Ki-67 after 3 weeks from treatment start). The preplanned interim analysis with the first 130 patients was presented. pCR was reported in 25% of patients treated with nab-paclitaxel plus gemcitabine and in 49.2% of cases with nab-paclitaxel plus carboplatin (P=0.006).
One more interesting paper on nab-paclitaxel was presented at the ASCO 2015 meeting, by Matsuda et al from the MD Anderson Cancer Center,Citation88 based on the observations that EGFR overexpression is an independent poor prognostic factor in IBC and that in animal models an anti-EGFR treatment was able to inhibit IBC growth. In this single-arm Phase II study, 25 IBC patients were treated with the anti-EGFR monoclonal antibody panitumumab (2.5 mg/kg), nab-paclitaxel (100 mg/m2), and carboplatin (AUC =2) weekly, for four cycles, followed by four cycles of 5-Fluoruracile, Epirubicine at 100 mg/sqm, Cyclophosphamide regimen, surgery, radiation, and endocrine adjuvant treatment in ER-positive cases. The overall pCR rate (primary end point) was 36% and was 60% in TNBC and 20% in ER-positive patients. The association of pCR with subtype showed just a trend to significance, essentially due to the small sample size. This excellent result was not “for free” because Grade 3 and 4 hematological events occurred in 72% and other Grade 3 events in a further 36% though the median age of patients was 57 years and patients were fit. The historical pCR rate in IBC is around 15% and the prognosis poor. These data have no precedent, and a randomized Phase III trial is now starting.
Two clinical case reports
Case report 1
A 52-year-old woman with primary TNBC metastasized to bones, locoregional and mediastinal nodes, and lung, was treated with nab-paclitaxel, gemcitabine, and bevacizumab. Pain improved within a few weeks, (complete withdrawal of analgesic therapy). After 7 months, a complete radiological response was observed and maintained for 2 years, with a dose reduction due to asthenia but without other adverse events. The patient maintained normal daily activities, including work; the disease progression occurred after 24 months. Subsequent treatments stabilized the disease; however, death occurred after another 2 years. This case is indicative of how a treatment with nab-paclitaxel, bevacizumab, and gemcitabine can be very effective with minimal toxicity in selected patients with metastatic TNBC.Citation89
Case report 2
A 51-year-old women with TNBC, BRCA1-mutated, was treated with four cycles of a neoadjuvant carboplatin and etoposide regimen with a complete clinical/radiological response. A local progression after 19 months was treated with dose-dense AC with growth factor support, followed by paclitaxel q3w for four cycles, with an optimal disease control. After a further 3 years, diffuse brain metastases were discovered, treated with whole-brain radiation, in a rapidly progressive disease (with massive liver and retroperitoneal nodes, and multiple, bilateral pulmonary involvement). The patient started a carboplatin (AUC =5) plus weekly nab-paclitaxel 100 mg/m2 regimen. Despite growth factor support, carboplatin was suspended after four cycles, and nab-paclitaxel monotherapy, at 90 mg/m2 weekly for 3 out 4 weeks, was continued. After 4 months, a partial response with clinical improvement was observed and maintained for another 4 months until asymptomatic cerebral disease progression, followed by a rapid deterioration in the patient’s condition and exitus after 2 months.
This case is very interesting because it shows that in a patient heavily treated with carboplatin, etoposide, conventional paclitaxel, and anthracycline, third-line nab-paclitaxel, first combined with carboplatin for four cycles and then as monotherapy, was able to obtain a partial clinical/radiological response lasting for 8 months, with concomitant resolution of symptoms (pain, dyspnea, and severe asthenia) and virtual lack of toxicity.Citation90
New clinical trials of nab-paclitaxel in TNBC
Some interesting trials with nab-paclitaxel in TNBC are currently ongoing.
Tn Acity (ClinicalTrials.gov identifier NCT01881230)Citation91
Randomized Phase II/III study of weekly nab-paclitaxel associated to gemcitabine or carboplatin vs gemcitabine plus carboplatin, in the first-line treatment of TNBC
Phase II end points: efficacy (PFS) and safety of nab-paclitaxel 125 mg/m2 associated to gemcitabine or carboplatin vs their combination (randomized 1:1:1) (n=240) – in order to identify the best nab-paclitaxel-based regimen to compare in the Phase III with the standard regimen, gemcitabine plus carboplatin (randomized 1:1) (n=550)
Phase III end point: PFS
Phase III stratification factors: DFS < or >12 months, pretreatment with taxanes in the adjuvant or neoadjuvant setting
In both the study phases, treatment will continue until disease progression or unacceptable toxicity.
As of May 31, 2015, 176 patients were enrolled in the Phase II trial.
ETNA (ClinicalTrials.gov identifier NCT01822314)
Randomized Phase III study on neoadjuvant therapy with weekly nab-paclitaxel
632 patients with early HER2-negative BC at high risk of relapse, Eastern Cooperative Oncology Group (ECOG) performance status 0–1, to be randomized to weekly nab-paclitaxel 125 mg/m2 (three doses per cycle) for four cycles vs conventional weekly paclitaxel 90 mg/m2 (three doses per cycle) for four cycles, followed by four cycles of anthracycline-based regimens, surgery, and a 10-year follow up
Primary end point: pCR (ypT0/ypTis, ypN0)
Secondary end points:
○ pCR in endocrine receptor-positive vs triple-negative tumors
○ ORR after four cycles of both taxanes and before surgery
○ EFS (local, regional, and distant) and OS
○ Safety and tolerability; clinical and molecular tests able to identify markers predictive of clinical benefit.
Conclusion
TNBC is characterized by the absence of ER-, PgR-, and HER2-negativity: for this reason the only therapeutic option is chemotherapy. Even if these tumors are chemosensitive, as showed by the high pCR obtained with neoadjuvant therapy, metastatic patients have a short PFS; thus the chemosensitivity does not translate in an improvement of PFS or OS, and the overall prognosis for these tumors is poor.
The studies performed with taxane-based chemotherapy demonstrated their efficacy in the treatment of TNBC in any setting (neoadjuvant, adjuvant, and metastatic): international and national guidelines recommend the taxanes as possible active first-line therapeutic options for TNBC.
Nab-paclitaxel, a nanoparticle of albumin-bound paclitaxel, allows achievement of higher intratumoral concentrations of active drug and is demonstrated to be more effective and less toxic that conventional taxanes in metastatic BC; even in the case of aggressive and visceral disease and in neoadjuvant setting, the pCR is superior vs conventional taxanes.
Author contributions
Giorgio Mustacchi and Michelino De Laurentiis equally contributed to the conception, analysis, and interpretation of the literature, and drafting and revision of the paper, and both approved the final version. Both authors agree to be accountable for all aspects of the work.
Disclosure
This work was supported by an unconditional grant from Editree editors, Italy, and both authors received a fee. Giorgio Mustacchi receives honoraria from Roche, Celgene, and EISAI. Michelino De Laurentiis receives honoraria from Roche, Celgene, EISAI, Novartis, and AstraZeneca. The authors report no other conflicts of interest in this work.
References
- CardosoFHarbeckNFallowfieldLKyriakidesSSenkusEESMO Guidelines Working GroupLocally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201223Suppl 7vii11vii1922997442
- AIOM-AIRTUMI Numeri del Cancro in Italia. 2014 [The numbers of cancer in Italy. 2014]AIOM-AIRTUMMilanAssociazione Italiana di Oncologia Medica2014 Available from: http://www.aiom.it/area+pubblica/area+medica/pubblicazioni/1,332,1Accessed July 4, 2015 Italian
- de RujtersTCVeeckJde HoonJPJvan EngelandMTjan-HeijnenVCCharacteristics of triple-negative breast cancerJ Cancer Res Clin Oncol201113718319221069385
- ChacónRDCostanzoMVTriple-negative breast cancerBreast Cancer Res201012Suppl 2S321050424
- HudisCAGianniLTriple-negative breast cancer: an unmet medical needOncologist201116Suppl 1S1S11
- RapoportBLNaylerSDemetriouGSMoodleySDBennCATriple-negative breast cancer pathologic diagnosis and current chemotherapy treatment optionsOncol Hematol Rev20141012532
- SørlieTPerouCMTibshiraniRGene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implicationsProc Natl Acad Sci U S A20019819108691087411553815
- van’t VeerLJDaiHvan de VijverMJGene expression profiling predicts clinical outcome of breast cancerNature200241568753053611823860
- HortobagyiGNHayesDPusztaiLIntegrating newer science into breast cancer prognosis and treatment: Molecular predictors and profilesJohnsonDHAmerican Society of Clinical Oncology 2002 Annual Meeting Summaries, Orlando, FL, May 18–21 2002Alexandria, VAAmerican Society of Clinical Oncology200291202
- AyersMSymmansWFStecJGene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancerJ Clin Oncol200422122284229315136595
- DentRTrudeauMPritchardKITriple-negative breast cancer: clinical features and patterns of recurrenceClin Cancer Res20071315 Pt 14429443417671126
- LehmannBDBauerJAChenXIdentification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapiesJ Clin Invest201112172750276721633166
- LehmannBDPietenpolJATanARTriple-negative breast cancer: molecular subtypes and new targets for therapyAm Soc Clin Oncol Educ Book201535e31e3925993190
- TobinNPHarrellJCEgyhazi BrageSMolecular subtype of breast cancer metastases significantly influences patient post-relapse survivalAnn Oncol201425Suppl 1i5i7 Abstract
- DentRAMainwaringPNTanTJYSurvival in triple-negative breast cancer (TNBC): Evidence from the SEER database 2010–2011J Clin Oncol201533Suppl 15e12075 Abstract
- National Comprehensive Cancer Network (NCCN)Clinical Practice Guidelines in Oncology Breast Cancer version 2.2014Washington, PANational Comprehensive Cancer Network2014
- Associazione Italiana di Oncologia Medica (AIOM)Linee Guida: Neoplasie della Mammella [Guidelines: Neoplasms of the Breast]MilanAssociazione Italiana di Oncologia Medica2013 Italian
- CortazarPZhangLUntchMPathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysisLancet2014384993816417224529560
- CortazarPGeyerCEJrGianniLCameronDvon MinckwitzGPathological complete response in breast cancer – Authors’ replyLancet201538511411525706464
- von MinckwitzGUntchMNüeschEImpact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trialsBreast Cancer Res Treat2011125114515621042932
- von MinckwitzGUntchMBlohmerJUDefinition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypesJ Clin Oncol201230151796180422508812
- RouzierRPerouCMSymmansWFBreast cancer molecular subtypes respond differently to preoperative chemotherapyClin Cancer Res200511165678568516115903
- LiedtkeCMazouniCHessKRResponse to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancerJ Clin Oncol20082681275128118250347
- GuarneriVBroglioKKauSWPrognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factorsJ Clin Oncol20062471037104416505422
- WangSYangHTongFResponse to neoadjuvant therapy and disease free survival in patients with triple-negative breast cancerGan To Kagaku Ryoho200936225525819223741
- TorrisiRBalduzziAGhisiniRTailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxelCancer Chemother Pharmacol200862466767218064460
- von MinckwitzGRaabGCaputoADoxorubicin with cyclo-phosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast GroupJ Clin Oncol200523122676268515837982
- Darb-EsfahaniSLoiblSMüllerBMIdentification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapyBreast Cancer Res2009115R6919758440
- EssermanLJPerouCCheangMI-SPY InvestigatorsBreast cancer molecular profiles and tumor response of neoadjuvant doxorubicin and paclitaxel: The I-SPY TRIAL (CALGB 150007/150012, ACRIN 6657)J Clin Oncol200927Suppl 15SLBA515 Abstract
- WuJLiSJiaWSuFResponse and prognosis of taxanes and anthracyclines neoadjuvant chemotherapy in patients with triple-negative breast cancerJ Cancer Res Clin Oncol2011137101505151021830158
- SakumaKKurosumiMObaHPathological tumor response to neoadjuvant chemotherapy using anthracycline and taxanes in patients with triple-negative breast cancerExp Ther Med20112225726422977494
- AndréFZielinskiCCOptimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agentsAnn Oncol201223Suppl 6vi46vi5123012302
- AndreFDelucheEBonnefoiHBevacizumab: the phoenix of breast oncology?Lancet Oncol201516660060125975636
- KimHRJungKHImSAMulticentre phase II trial of bevacizumab combined with docetaxel-carboplatin for the neoadjuvant treatment of triple-negative breast cancer (KCSG BR-0905)Ann Oncol20132461485149023380385
- von MinckwitzGEidtmannHRezaiMGerman Breast GroupArbeitsgemeinschaft Gynäkologische Onkologie–Breast Study GroupsNeoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancerN Engl J Med2012366429930922276820
- BearHDTangGRastogiPBevacizumab added to neoadjuvant chemotherapy for breast cancerN Engl J Med2012366431032022276821
- von MinckwitzGSchneeweissALoiblSNeoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trialLancet Oncol201415774775624794243
- Von MinckwitzGTimmsKUntchMPrediction of pathological complete response (pCR) by Homologous Recombination Deficiency (HRD) after carboplatin-containing neoadjuvant chemotherapy in patients with TNBC: Results from GeparSixtoJ Clin Oncol201533Suppl 151004 Abstract
- SikovWMBerryDAPerouCMImpact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance)J Clin Oncol2015331132125092775
- GolshanMCirrincioneCTCareyLAImpact of neoadjuvant therapy on breast conservation rates in triple-negative and HER2-positive breast cancer: Combined results of CALGB 40603 and 40601 (Alliance)J Clin Oncol201533Suppl 151007 Abstract
- EarlHMHillerLDunnJAARTemis InvestigatorsEfficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): an open-label, randomised, phase 3 trialLancet Oncol201516665666625975632
- HayesDFThorADDresslerLGCancer and Leukemia Group B (CALGB) InvestigatorsHER2 and response to paclitaxel in node-positive breast cancerN Engl J Med2007357151496150617928597
- SparanoJAZhaoFMartinoSLong-term follow-up of the E1199 Phase III trial evaluating the role of taxane and schedule in operable breast cancerJ Clin Oncol Epub2015615
- HughJHansonJCheangMCBreast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohis-tochemical definition in the BCIRG 001 trialJ Clin Oncol20092781168117619204205
- LoeschDGrecoFO’ShaughnessyJA randomized, multicenter, phase III trial comparing doxorubicin + cyclophosphamide followed by paclitaxel or doxorubicin + paclitaxel followed by weekly pacli-taxel as adjuvant therapy for high-risk breast cancerJ Clin Oncol200725Suppl 18517 Abstract17290060
- JacquinJPJonesSMagnéNDocetaxel-containing adjuvant chemotherapy in patients with early stage breast cancer. Consistency of effect independent of nodal and biomarker status: a meta-analysis of 14 randomized clinical trialsBreast Cancer Res Treat2012134390391322270929
- YardleyDABossermanLDKeatonMRTITAN: Phase III study of doxorubicin/cyclophosphamide (AC) followed by ixabepilone (Ixa) or paclitaxel (Pac) in early-stage, triple-negative breast cancer (TNBC)J Clin Oncol20153315 Suppl1000 Abstract25667274
- CameronDBrownJDentRAdjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trialLancet Oncol2013141093394223932548
- GhersiDWillsonMLChanMMSimesJDonoghueEWilckenNTaxane-containing regimens for metastatic breast cancerCochrane Database Syst Rev20156CD00336626058962
- IsakoffSJTriple-negative breast cancer: role of specific chemo- therapy agentsCancer J2010161536120164691
- KassamFEnrightKDentRSurvival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial designClin Breast Cancer200991293319299237
- O’ShaughnessyJRomieuGDiérasVMeta-analysis of patients with triple negative breast cancer (TNBC) from three randomized trials of first-line bevacizumab (BV) and chemotherapy treatment for metastatic breast cancer (MBC)Programs and abstracts of the 33rd Annual CTRC-AACR San Antonio Breast Cancer SymposiumDecember 8–12 2010San Antonio, TX Abstract P6-12-03
- BrufskyAValeroVTiangcoBSecond-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trialBreast Cancer Res Treat201213331067107522415477
- GligorovJDovalDBinesJMaintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trialLancet Oncol201415121351136025273343
- GennariAStocklerMPuntoniMDuration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trialsJ Clin Oncol201129162144214921464403
- FanYXuBHYuanPDocetaxel-cisplatin might be superior to docetaxel-capecitabine in the first-line treatment of metastatic triple-negative breast cancerAnn Oncol20132451219122523223332
- TuttAEllisPKilburnLTNT: A randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012)Programs and abstracts of the 37th Annual CTRC-AACR San Antonio Breast Cancer SymposiumDecember 9–13 2014San Antonio, TX Abstract S3-01
- AogiKYoshidaMSagaraYThe efficacy and safety of gemcit-abine plus paclitaxel combination first-line therapy for Japanese patients with metastatic breast cancer including triple-negative phenotypeCancer Chemother Pharmacol20116751007101520628744
- DentRALindemanGJClemonsMPhase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancerBreast Cancer Res2013155R8824063698
- ParkerJSPetersonACTudorICHoffmanJUppalHA novel biomarker to predict sensitivity to enzalutamide (ENZA) in TNBCJ Clin Oncol20153315 Suppl1083 Abstract
- PetrelliFBorgonovoKBarniSTargeted delivery for breast cancer therapy: the history of nanoparticle-albumin-bound paclitaxelExpert Opin Pharmacother20101181413143220446855
- PiccartMNab™-paclitaxel: a targeted chemotherapy to improve outcomes in metastatic breast cancerAPJOH200911512
- DesaiNTrieuVYaoZIncreased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI–007, compared with cremophor-based paclitaxelClin Cancer Res20061241317132416489089
- ScheffRJBreast cancer and the new taxanes: focus on nab-paclitaxelCommunity Oncol200857 Suppl 8S7S13
- YardleyDANab-Paclitaxel mechanisms of action and deliveryJ Control Release2013170336537223770008
- GradisharWJTjulandinSDavidsonNPhase III trial of nano-particle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancerJ Clin Oncol200523317794780316172456
- Gonzalez-AnguloAMHortobagyiGNOptimal schedule of paclitaxel: weekly is betterJ Clin Oncol200826101585158718375889
- GreenMCBuzdarAUSmithTWeekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeksJ Clin Oncol200523255983599216087943
- SeidmanADBerryDCirrincioneCRandomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840J Clin Oncol200826101642164918375893
- GradisharWJKrasnojonDCheporovSSignificantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancerJ Clin Oncol200927223611361919470941
- GradisharWJKrasnojonDCheporovSPhase II trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: final analysis of overall survivalClin Breast Cancer201212531332122728026
- O’ShaughnessyJGradisharWJBharPIglesiasJNab-paclitaxel for first-line treatment of patients with metastatic breast cancer and poor prognostic factors: a retrospective analysisBreast Cancer Res Treat2013138382983723563958
- BlumJLSavinMAEdelmanGPhase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanesClin Breast Cancer200771185085618269774
- PalumboRSottotettiFTrifiròGNanoparticle albumin-bound paclitaxel (nab-paclitaxel) as second-line chemotherapy in HER2-negative, taxane-pretreated metastatic breast cancer patients: prospective evaluation of activity, safety, and quality of lifeDrug Des Devel Ther2015921892199
- SchwartzbergLSArenaFPMintzerDMEppersonALWalkerMSPhase II multicenter trial of albumin-bound paclitaxel and capecitabine in first-line treatment of patients with metastatic breast cancerClin Breast Cancer2012122879322154117
- RoyVLaPlantBRGrossGGBaneCLPalmieriFMNorth Central Cancer Treatment GroupPhase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531)Ann Oncol200920344945319087987
- LoboCLopesGBaezOFinal results of a phase II study of nab-paclitaxel, bevacizumab, and gemcitabine as first-line therapy for patients with HER2-negative metastatic breast cancerBreast Cancer Res Treat2010123242743520585851
- HamiltonEKimmickGHopkinsJNab-paclitaxel/bevacizumab/carboplatin chemotherapy in first-line triple negative metastatic breast cancerClin Breast Cancer201313641642024099649
- RugoHSBarryWTMoreno-AspitiaARandomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance)J Clin Oncol Epub201568
- TonissiFLattanzioLMerlanoMCInfanteLLo NigroCGarroneOThe effect of paclitaxel and nab-paclitaxel in combination with anti-angiogenic therapy in breast cancer cell linesInvest New Drugs Epub201557
- LowJABermanAWSteinbergSMDanforthDNLippmanMESwainSMLong-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapyJ Clin Oncol200422204067407415483018
- NahlehZABarlowWEHayesDFS0800: Nab-paclitaxel, doxorubicin, cyclophosphamide, and pegfilgrastim with or without bevacizumab in treating women with inflammatory or locally advanced breast cancer (NCI CDR0000636131)Programs and abstracts of the 37th Annual CTRC-AACR San Antonio Breast Cancer SymposiumDecember 9–13 2014San Antonio, TX Abstract P3-11-16
- SniderJNSachdevJCAllenJWPathologic complete response (pCR) with weekly nanoparticle albumin bound (Nab-P) plus carbo-platin (C) followed by doxorubicin plus cyclophosphamide (AC) with concurrent bevacizumab (B) for triple-negative breast cancer (TNBC)J Clin Oncol20133115 Suppl1068 Abstract
- HuangLChenSYaoLLiuGWuJShaoZPhase II trial of weekly nab-paclitaxel and carboplatin treatment with or without trastuzumab as nonanthracycline neoadjuvant chemotherapy for locally advanced breast cancerInt J Nanomedicine2015101969197525792830
- UntchMJackischCSchneeweißAA randomized phase III trial comparing neoadjuvant chemotherapy with weekly nanoparticle-based paclitaxel with solvent-based paclitaxel followed by anthracyline/cyclophosphamide for patients with early breast cancer (GeparSepto); GBG 69Program and abstracts of the 35th Annual CTRC-AACR San Antonio Breast Cancer SymposiumDecember 4–8 2012San Antonio, TX Abstract OT3-3-PD2-D6
- CiruelosEMartinezNCantosBPhase II randomized study of nab-paclitaxel versus conventional paclitaxel as first-line therapy of metastatic HER2-negative breast cancer for neurotoxicity char-acterization: An Oncosur Study Group studyJ Clin Oncol20153315 Suppl1029 Abstract
- GluzONitzUChristgenMEfficacy of 12 weeks neoadjuvant nab-paclitaxel combined with carboplatinum vs gemcitabine in triple-negative breast cancer: WSG-ADAPT TN randomized phase II trialJ Clin Oncol20153315 Suppl1032 Abstract
- MatsudaNAlvarezRHKrishnamurthySPhase II study of pani-tumumab, nab-paclitaxel, and carboplatin followed by FEC neoadjuvant chemotherapy for patients with primary HER-2 negative inflammatory breast cancerJ Clin Oncol20153315 Suppl1065 Abstract
- MonteroAGlückSLong-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancerCase Rep Oncol20125368769223341813
- ShakirARStrong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancerCase Rep Oncol20147125225924847251
- YardleyDACortesJColemanREWeekly nab-paclitaxel (nab-P) plus gemcitabine (gem) or carboplatin (carbo) vs gem/carbo as first-line treatment for metastatic triple-negative breast cancer (mTNBC) in a phase 2/3 trial (tnAcity)J Clin Oncol20153315 SupplTPS1106 Abstract
