Abstract
Background
HCP1004 is a newly developed fixed-dose combination of naproxen (500 mg) and esomeprazole strontium (20 mg) that is used in the treatment of rheumatic diseases and can reduce the risk of nonsteroidal anti-inflammatory drug-associated ulcers. The aim of this study was to evaluate the pharmacokinetics (PK) and safety of HCP1004 compared to VIMOVO® (a marketed fixed-dose combination of naproxen and esomeprazole magnesium).
Subjects and methods
An open-label, randomized, two-treatment, two-sequence crossover, single-dose clinical study was conducted in 70 healthy volunteers. In each period, a reference (VIMOVO®) or test (HCP1004) drug was administered orally, and serial blood samples for PK analysis were collected up to 72 hours after dosing. To evaluate the PK profiles, the maximum plasma concentration (Cmax) and the area under the concentration–time curve from 0 to the last measurable time (AUC0−t) were estimated using a noncompartmental method. Safety profiles were evaluated throughout the study.
Results
Sixty-six of the 70 subjects completed the study. The Cmax (mean ± standard deviation) and AUC0−t (mean ± standard deviation) for naproxen in HCP1004 were 61.67±15.16 µg/mL and 1,206.52±166.46 h·µg/mL, respectively; in VIMOVO®; these values were 61.85±14.54 µg/mL and 1,211.44±170.01 h·µg/mL, respectively. The Cmax and AUC0−t for esomeprazole in HCP1004 were 658.21±510.91 ng/mL and 1,109.11±1,111.59 h·ng/mL, respectively; for VIMOVO®, these values were 595.09±364.23 ng/mL and 1,015.12±952.98 h·ng/mL, respectively. The geometric mean ratios and 90% confidence intervals (CIs) (HCP1004 to VIMOVO®) of the Cmax and AUC0−t of naproxen were 0.99 (0.94–1.06) and 1.00 (0.98–1.01), respectively. For esomeprazole, the geometric mean ratios (90% CI) for the Cmax and AUC0−t were 0.99 (0.82–1.18) and 1.04 (0.91–1.18), respectively. The overall results of the safety assessment showed no clinically significant issues for either treatment.
Conclusion
The PK of HCP1004 500/20 mg was comparable to that of VIMOVO® 500/20 mg for both naproxen and esomeprazole after a single oral dose. Both drugs were well-tolerated without any safety issues.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been used to treat a wide variety of clinical conditions, including osteoarthritis, arthritis, and musculoskeletal disorders.Citation1 Many patients who have osteoarthritis use NSAIDs for pain reduction; however, 50% of chronic NSAID users are at risk of gastrointestinal (GI) ulcers.Citation2 In a 6-month treatment period, 5%–15% of patients with rheumatoid arthritis discontinued NSAID therapy due to GI side effects, including dyspepsia.Citation3 The results of a trial conducted by Lanas et alCitation4 demonstrated that cotreatment with an NSAID and a proton pump inhibitor (PPI) was associated with a reduction in the risk of upper GI ulcer bleeding.
The present study revealed a significant reduction in the frequency of GI ulcers in high-risk patients with arthritis. Several strategies and guidelines for NSAIDs have been proposed for patients taking NSAIDs who are at high risk of upper GI toxicity. The recommendation is that these patients should be prescribed an acid-reducing medication, such as a PPI, to reduce the risk of GI complications associated with NSAIDs.Citation5–Citation7 For the management of NSAID-related GI adverse events, the use of fixed-dose combinations (FDCs) can improve medication compliance and ease of use. According to a study of compliance with combination therapy compared with single-drug therapy in cardiovascular disease, treatment with the FDC resulted in better clinical outcomes.Citation8 In addition to its utility as an effective treatment for osteoarthritis, an FDC of naproxen and esomeprazole offers advantages with respect to adherence to treatment compared with a single-drug regimen.
HCP1004 is an incrementally modified drug of VIMOVO® (AstraZeneca PLC, London, UK) that was developed by Hanmi Pharmaceutical Co., Ltd. (Seoul, Republic of Korea) as a tablet combining esomeprazole strontium (20 mg), which is immediately released in the stomach, and enteric-coated naproxen (500 mg), which is released later in the small intestine.Citation9 HCP1004 and VIMOVO® both contain naproxen and esomeprazole; however, the salt of esomeprazole differs. HCP1004 contains esomeprazole strontium, which is a new formulation of esomeprazole that incorporates a strontium salt rather than a magnesium salt. VIMOVO® contains esomeprazole magnesium.Citation9 Esomeprazole strontium is a new crystalline salt that improves the optical purity and thermo stability of esomeprazole.Citation10 The efficacy of esomeprazole strontium has been established in a previous study, as the bioequivalence of the proposed product to the reference product (esomeprazole magnesium) has been demonstrated.Citation10 The toxicity profile of esomeprazole strontium was comparable to that of esomeprazole magnesium in a preclinical study, and no developmental toxicity was observed in a dose-ascending study examining its effects on embryo–fetal development.Citation11
As a result, esomeprazole strontium is expected to be used as an osteoarthritis treatment to improve patient compliance and to enable the effective management of GI symptoms.Citation12 We therefore compared the tolerability and pharmacokinetics (PK) of HCP1004 and VIMOVO® 500/20 mg in healthy Korean male volunteers to assess the bioequivalence of the two drugs.
Subjects and methods
Subjects
Based on a previous study of naproxen and esomeprazole in healthy volunteers, the intrasubject variability was assumed to be 45.5% for the area under the curve (AUC) of esomeprazole.Citation13 Based on the estimated intrasubject variability, a total sample size of 68 subjects was chosen to account for possible dropouts. This sample size can achieve a power of at least 80% at a 5% significance level, and the bioequivalence criteria of the mean ratio are 0.80 and 1.25, assuming no difference between the test drug and the reference drug.
Healthy male volunteers aged 20–50 years were eligible for enrollment in this study. All subjects provided written informed consent before the screening procedure. Subjects who had a history of GI disease that could significantly alter the PK and tolerability of the study drugs were excluded. Participants with a history of known allergy, hypersensitivity, or intolerance to any NSAID or PPI were also excluded. Smoking and consumption of grapefruit, caffeine-containing foods, and alcohol were not permitted during the hospitalization periods. After approval from the Institutional Review Board of Seoul National University Hospital (SNUH), the study was performed at the clinical trials center of SNUH, Republic of Korea in accordance with the Declaration of Helsinki and good clinical practice. This trial is registered at ClinicalTrials.gov (NLM identifier: NCT00938132).
Study design
This was an open-label, randomized, two-treatment, two-sequence crossover, single-dose study. Subjects were randomly allocated to one of two sequence groups. The subjects were given the reference drug (VIMOVO®) or the test drug (HCP1004) in period 1 and the other drug in period 2 (7 days after the first drug). In each period, the oral treatments were given under fasting conditions at approximately 9 am on day 1 and day 8. During each treatment period, the subjects were hospitalized the evening before the day of drug administration (−1 day, 7 days). The last follow-up visit was performed on day 15, 16, or 17 after dosing.
Blood samples for the PK assessment were taken at 0 hours, 0.17 hours (esomeprazole only), 0.33 hours (esomeprazole only), 0.5 hours, 0.75 hours (esomeprazole only), 1 hour, 1.5 hours (esomeprazole only), 2 hours, 2.5 hours (esomeprazole only), 3 hours, 3.5 hours, 4 hours, 5 hours, 6 hours, 7 hours (naproxen only), 8 hours, 10 hours, 12 hours, 16 hours (naproxen only), 24 hours (naproxen only), 36 hours (naproxen only), 48 hours (naproxen only), and 72 hours (naproxen only) after drug administration. Blood samples were collected into sodium heparin vacutainer tubes. The tubes were inverted several times and centrifuged at 3,000 rpm for 10 minutes at 4°C. The plasma supernatant from each participant was divided into three equal aliquots, placed in storage tubes, and frozen at −70°C until analysis.
Determination of plasma naproxen and esomeprazole concentrations
Plasma concentrations of naproxen and esomeprazole were determined using a validated high-performance liquid chromatography tandem mass spectrometry method. The plasma sample preparation involved a simple protein precipitation step with acetonitrile. For the quantitation of naproxen, ketoprofen was used as an internal standard (IS). Naproxen and IS were separated on a Sunfire C18 column (5 µm; 2.1×150 mm2) and detected via ion-spray ionization in multiple reaction monitoring (MRM) mode. The MRM was based on the transition of m/z 229.0>169.8 for naproxen and 253.0>208.9 for ketoprofen (IS). The lower limit of quantification was 0.3 µg/mL for naproxen. The intraday and interday accuracies ranged from 99.9% to 106.3% and from 98.8% to 105.5%, respectively, and the intraday and interday precisions ranged from 1.9% to 2.8% and from 4.0% to 6.6%, respectively. The mobile phase for naproxen was composed of an ammonium acetate–acetonitrile mixture (30:70 v/v) containing 1,200 mL of 2 mM ammonium acetate and 2,800 mL of acetonitrile. Using a SevenEasy™ pH meter (Mettler Toledo, Urdorf, Switzerland), the pH was adjusted to 6.3 with distilled acetic acid. Acetonitrile and the IS solution containing ketoprofen were added to the plasma sample. For the quantitation of esomeprazole, esomeprazole-d3 was used as an IS. Esomeprazole and IS were separated on a Capcell Pak C18 (3 µm; 2.0×50 mm2) and detected via ion-spray ionization in MRM mode. The MRM was based on the transition of m/z 346.2>198.0 for esomeprazole and 349.2>198.1 for esomeprazole-d3 (IS). The lower limit of quantification was 5 ng/mL for esomeprazole. The intraday and interday accuracy values were 89.0%–98.5% and 98.9%–101.3%, respectively, and the intraday and interday precision values were 0.7%–2.6% and 2.7%–5.4%, respectively. The mobile phase for esomeprazole consisted of 10 mM of ammonium formate–acetonitrile (30:70 v/v) with a mixture of 600 mL of 10 mM ammonium formate and 1,400 mL of acetonitrile. Acetonitrile and the IS solution, including esomeprazole-d3, were added to the plasma sample.
PK analysis
The individual PK parameters for naproxen and esomeprazole were calculated with the noncompartmental method using Phoenix WinNonlin (version 6.4; Certara, L.P., Princeton, NJ, USA). The area under the plasma concentration versus time curve was calculated according to a linear-log trapezoidal method from time zero to the last quantifiable concentration (AUC0−t). The parameters used for the PK assessment included the maximum plasma concentration (Cmax), the time to reach Cmax (Tmax), AUC0−t, the area under the plasma concentration versus time curve extrapolated to infinity (AUCinf), and the apparent elimination half-life (t1/2).
Safety and tolerability assessments
For the tolerability assessment, vital signs, physical examinations, 12-lead electrocardiograms, and clinical laboratory tests (hematology, urinalysis, and blood chemistry) were performed. A safety assessment was conducted for all subjects who received the drug, and the subjects were monitored by the investigators. The investigators confirmed the medical status of the subjects through questions and examinations during the study. All the adverse events, including status changes after enrollment, were recorded and categorized as mild, moderate, or severe, and their relationship to the study drug was determined.
Statistical analyses
Statistical analysis was performed with SPSS 21.0™ ((SPSS Inc., Chicago, IL, USA). The results of the PK analyses of the treatments with naproxen and esomeprazole are presented using the mean and standard deviation values for the treatment groups. The plasma concentrations of naproxen and esomeprazole were subjected to analysis of variance for statistical analysis of the parameters. The PK treatment comparisons are described as the geometric mean ratios of Cmax and AUC0−t of the test drug and the reference drug with associated 90% confidence intervals (CIs).
Results
Demographic characteristics
A total of 70 subjects participated in this study, and they were randomized into two sequence groups. Two subjects who withdrew consent before drug administration were replaced, and four additional subjects dropped out during the study. In total, 66 subjects completed the study and were eligible for PK assessments. The average age of the subjects was 29.8±5.9 years (range: 22–51 years), the average body weight was 69.0±7.0 kg (55.2–84.1 kg), the average height was 174.2±5.1 cm (161–189 cm), and the average body mass index was 22.7±2.0 kg/m2 (18.7–26.7 kg/m2). The demographic characteristics of the subjects were not significantly different between the two groups.
Pharmacokinetics
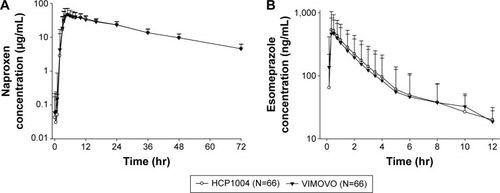
The mean and the standard deviation values for the PK parameters for naproxen and esomeprazole are shown in and . The Cmax and AUC0−t values of the test drug were comparable to those of the reference drug. The mean concentration–time curves of naproxen and esomeprazole after a single dose of HCP1004 or VIMOVO® were similar, as shown in . The median Tmax value for naproxen, which reflects the rate of absorption, was approximately 5 hours for both HCP1004 and VIMOVO®. Esomeprazole was rapidly absorbed, and the mean plasma concentrations of HCP1004 and VIMOVO® were 658.21 ng/mL and 595.09 ng/mL within 0.5 hours of dosing, respectively. There were no significant differences between the Cmax and AUC0−t values of HCP1004 and those of VIMOVO®.
Table 1 Summary of pharmacokinetic results for naproxen and esomeprazole
Figure 1 Mean plasma concentration–time profiles of naproxen and esomeprazole.
Abbreviations: N, number of subjects; hr, hours.
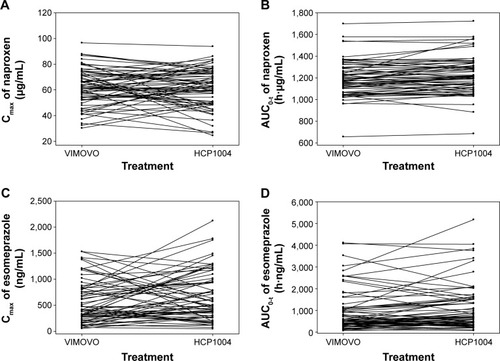
The individual differences in the AUC0−t and the Cmax of naproxen and esomeprazole are depicted in . In 36 patients, an increase in the Cmax of naproxen was observed after a single administration of HCP1004 or VIMOVO®, whereas in the other 30 patients, the Cmax decreased (P=0.539) (). Treatment with HCP1004 or VIMOVO® did not significantly change the AUC0−t of naproxen (P=0.902) (). After a single dose of HCP1004 or VIMOVO®, the Cmax of esomeprazole increased in 35 patients and decreased in 31 patients (P=0.712) (), and the AUC0−t values of esomeprazole increased in 38 patients (P=0.268) ().
Figure 2 Individual changes in pharmacokinetic parameters.
Abbreviations: Cmax, maximum plasma concentration; AUC0−t, area under the concentration–time curve from time 0 to the last measurable time point.
Estimates of the PK parameters (with 90% CIs) for the assessment of bioequivalence of naproxen and esomeprazole and the ratios of the parameters (with 90% CIs) are shown in .
Table 2 Pharmacokinetic comparison of naproxen and esomeprazole by treatment
Safety assessment
Subjects who received at least one dose of the study medication were included in the safety assessment. Among the 67 subjects who received the test drug, HCP1004, 12 experienced a total of 15 adverse events (AEs). HCP1004-related AEs included two cases of dyspepsia and a single case each of headache, feeling hot, and diarrhea. One case of headache and one case of diarrhea were determined to be related to the reference drug, VIMOVO®. In the treatment comparison, a total of ten AEs occurred in eight of the 67 subjects receiving the test drug, HCP1004. Only five of the events were considered to be drug related. A total of five AEs occurred in four of the 66 subjects receiving VIMOVO®. Two events were considered to be drug related. There were no significant differences in the number of subjects with AEs (P=0.365; ), the total number of AEs (P=0.302; ), or the treatment effects in the two groups. None of the subjects discontinued due to AEs. No clinically significant changes were observed in the clinical laboratory results, electrocardiography findings, vital signs, or results of physical examinations that were carried out in all subjects after administration of the study drug.
Discussion
The aim of this study was to evaluate the PK and tolerability of HCP1004 and VIMOVO® 500/20 mg in healthy Korean male volunteers. Our study showed that there were no significant differences in the mean plasma concentration–time curves of HCP1004 and VIMOVO® in 66 healthy volunteers. The FDC of naproxen and esomeprazole in HCP1004 was comparable to that in VIMOVO®. In our study, the PK evaluation demonstrated that HCP1004 and VIMOVO® are pharmacokinetically equivalent; the 90% CIs for the geometric mean ratios of Cmax and AUC0−t satisfied the commonly accepted bioequivalence criteria (0.8–1.25) proposed by the United States Food and Drug Administration.Citation14,Citation15
A similar bioequivalence study of an FDC of naproxen and esomeprazole was conducted previously in healthy subjects.Citation13 The same reference drug was used in that study and in the present study. For naproxen, the PK parameters obtained in the present study were similar to those reported previously; the mean Cmax was 66.9 µg/mL, and the mean AUC0−t was 1,226 h·µg/mL. In contrast, the mean Cmax and AUC0−t values for esomeprazole in the present study were higher than those described in the previous study, which reported that the mean Cmax was 425 ng/mL and the AUC0−t was 465 h·ng/mL for esomeprazole.Citation13 In the present study, the esomeprazole Cmax was 658.21 ng/mL, and the AUC0−t was 1,109.11 h·ng/mL.
These different PK results for esomeprazole may be explained by the different enzyme activities of cytochrome P450 (CYP) in different ethnic groups. The contribution of ethnic differences in the activity of CYP enzymes to variability in drug PK has been extensively reported.Citation16,Citation17 Individuals who are poor metabolizers (PMs) are deficient in CYP2C19, which is the enzyme primarily responsible for the metabolism of esomeprazole, and they have higher plasma concentrations of PPIs compared to extensive metabolizers. The PK of esomeprazole could be affected by variations in the CYP2C19 genotypes. A previous study indicated that the mean AUC values for esomeprazole in PMs were approximately 100% higher than those in extensive metabolizers (EMs), and the mean Cmax values for esomeprazole in PMs were approximately 60% higher than those in EMs.Citation9 PMs constitute approximately 13%–20% of Asian populations and 2%–6% of American populations.Citation18 Our study recruited only healthy Korean volunteers, whereas the previous study enrolled subjects from different ethnic groups, including White (85%), Black/African American (13%), and Asian (3%).Citation13 Although we did not perform genotyping of CYP2C19, the subject group in our study may have had a relatively high proportion of PMs compared to the subject groups in previous studies, which likely contributed to the increased exposure to esomeprazole. Moreover, the individual changes in the AUC0−t and the Cmax of esomeprazole showed high intrasubject variability (), which is consistent with earlier reports.Citation13 In addition to its high intrasubject variability, esomeprazole PK also exhibits substantial intersubject variability.Citation9 Indeed, the present study revealed a high level of interindividual variability in esomeprazole PK. Based on the results of our study, the coefficient of variation (%CV) for the esomeprazole Cmax of the test drug and the reference drug were 77.62% and 61.21%, respectively, and the %CVs for the AUC0−t were 100.22% and 93.88%, respectively. This high intersubject variability in the esomeprazole PK values is similar to that reported in a previous study of VIMOVO®, which reported values of 81% for the Cmax and 91% for the AUC0−t.Citation13
For naproxen, the intraindividual %CV was estimated to be 13.80%–24.59% for the PK parameters (Cmax and AUC0−t) of the test drug and the reference drug; these values are similar to those reported previously.Citation13 It is known that individuals with the genetic polymorphism CYP2C9 can metabolize many NSAIDs, including naproxen. A previous study examined the effect of the CYP2C9 polymorphism on the PK of naproxen in Korean subjects.Citation19 These authors reported that the CYP2C9 polymorphism did not significantly affect the PK of naproxen. Moreover, the PK parameters of naproxen in our study are similar to those of a previous study, and the variability in these values was less than 25%.Citation13
The administration of naproxen and esomeprazole, either as HCP1004 or VIMOVO®, was well-tolerated by all of the subjects (). Adverse events were reported by 17.9% of the subjects, and all of the reported AEs were mild. The most common AEs included diarrhea, dyspepsia, headache, and feeling hot. In our study, 9.0% of subjects experienced at least one drug-related AE. In the previous study, dyspepsia, dizziness, and headache were reported but were not considered to be clinically significant.Citation13 The safety profile of naproxen and esomeprazole in the current study was comparable to the safety results in another study of an FDC product containing naproxen and esomeprazole.Citation13 These results suggest that the study products are not associated with any notable safety concerns.
Table 3 Incidence of AEs after a single dose of HCP1004 or VIMOVO®
The volunteers for this study were not representative of the general patient population, and further studies may be required to confirm the efficacy and safety of HCP1004 in other patient groups.
Conclusion
In summary, this study revealed that the PK values for the test and reference products, which were FDCs of naproxen and esomeprazole, were within the commonly accepted bioequivalence range of 0.80–1.25. In addition, the safety profiles of the two products were comparable.
Acknowledgments
This study was sponsored by Hanmi Pharm. Co., Ltd, Seoul, Republic of Korea.
Supplementary material
Table S1 Comparison of AEs by treatment group
Disclosure
The authors report no conflicts of interest in this work.
References
- HaroutiunianSDrennanDALipmanAGTopical NSAID therapy for musculoskeletal painPain Med201011453554920210866
- American College of Gastroenterology [webpage on the Internet]Understanding ulcers, NSAIDs and GI bleedingBethesda, MDAmerican College of Gastroenterology Available from: http://s3.gi.org/patients/pdfs/UnderstandGIBleednew.pdfAccessed April 30, 2014
- SinghGTriadafilopoulosGEpidemiology of NSAID induced gastrointestinal complicationsJ Rheumatol Suppl199956182410225536
- LanasAGarcía-RodríguezLAArroyoMTInvestigators of the Asociación Española de Gastroenterología (AEG)Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulantsAm J Gastroenterol2007102350751517338735
- LanzaFLChanFKQuigleyEMPractice Parameters Committee of the American College of GastroenterologyGuidelines for prevention of NSAID-related ulcer complicationsAm J Gastroenterol2009104372873819240698
- CryerBLSostekMBFortJGSvenssonOHwangCHochbergMCA fixed-dose combination of naproxen and esomeprazole magnesium has comparable upper gastrointestinal tolerability to celecoxib in patients with osteoarthritis of the knee: results from two randomized, parallel-group, placebo-controlled trialsAnn Med201143859460522017620
- GoldsteinJLHochbergMCFortJGZhangYHwangCSostekMClinical trial: the incidence of NSAID-associated endoscopic gastric ulcers in patients treated with PN 400 (naproxen plus esomeprazole magnesium) vs enteric-coated naproxen aloneAliment Pharmacol Ther201032340141320497139
- BangaloreSKamalakkannanGParkarSMesserliFHFixed-dose combinations improve medication compliance: a meta-analysisAm J Med2007120871371917679131
- VIMOVO®Product MonographMississauga, ONAstraZeneca Canada Inc2011
- U.S. Food and Drug Administration [webpage on the Internet]Esomeprazole strontium delayed-release capsulesSilver Spring, MDU.S. Food and Drug Administration2013 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202342Orig1s000TOC.cfmAccessed April 30, 2014
- ParekhPJOldfieldEC4thJohnsonDATreatment of gastroesophageal reflux disease: two new oral formulations dexlansoprazole MR and esomezol (esomeprazole strontium)Expert Opin Pharmacother20141591215122224749891
- DhillonSNaproxen/esomeprazole fixed-dose combination: for the treatment of arthritic symptoms and to reduce the risk of gastric ulcersDrugs Aging201128323724821329403
- Wang-SmithLFortJZhangYSostekMPharmacokinetics and relative bioavailability of a fixed-dose combination of enteric-c oated naproxen and non-enteric-coated esomeprazole magnesiumJ Clin Pharmacol201252567068021628602
- U.S. Food and Drug Administration [webpage in the Internet]. Guidance for IndustryBioavailability and Bioequivalence Studies for Orally Administered Drug Products – General Considerations Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM154838.pdfAccessed June 28, 2014
- U.S. Food and Drug Administration [webpage on the Internet]Orange Book: Approved drug products with therapeutic equivalence evaluationsSilver Spring, MDU.S. Food and Drug Administration2015 Available from: http://www.fda.gov/cder/ob/default.htmAccessed June 28, 2014
- MaQLuAYPharmacogenetics, pharmacogenomics, and individualized medicinePharmacol Rev201163243745921436344
- Ingelman-SundbergMPharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and futureTrends Pharmacol Sci200425419320015063083
- YangJCLinCJCYP2C19 genotypes in the pharmacokinetics/pharmacodynamics of proton pump inhibitor-based therapy of Helicobacter pylori infectionExpert Opin Drug Metab Toxicol201061294119968574
- BaeJWKimJHChoiCIEffect of CYP2C9*3 allele on the pharmacokinetics of naproxen in Korean subjectsArch Pharm Res200932226927319280158
