Abstract
Purpose
The aim of this retrospective study was to investigate the relationship between UGT1A1 polymorphisms and toxicities in Chinese patients with pancreatic or biliary tract cancer receiving irinotecan-containing regimens as the second- or third-line chemotherapy.
Patients and methods
A total of 36 patients with unresectable pancreatic cancer and 12 patients with unresectable biliary tract cancer were included. Approximately 33 patients were treated with FOLFIRI regimen, a chemotherapy regimen, where FOL stands for folinic acid, F for fluorouracil, and IRI for irinotecan (irinotecan 180 mg/m2 at day 1, CF 200 mg/m2 at day 1–2, 5-FU 400 mg/m2 at day 1–2, followed by continuous infusion of 5-FU 600 mg/m2 for 22 hours at day 1–2, every 2 weeks). The other 15 patients were treated with irinotecan monotherapy (180 mg/m2, every 2 weeks). UGT1A1*6/*28 polymorphisms were detected by direct sequencing.
Results
The frequencies of GG, GA, AA genotypes for UGT1A1*6 were 70.8% (n=34), 25.0% (n=12), and 4.2% (n=2), respectively. And those of TA6/TA6, TA6/TA7, TA7/TA7 for UGT1A1*28 were 79.2% (n=38), 18.8% (n=9), and 2.0% (n=1), respectively. A total of 22 patients (45.8%) had grade III–IV neutropenia, and six patients (12.5%) experienced grade III–IV diarrhea. The incidence of grade III–IV neutropenia in patients with UGT1A1*6 GA or AA genotype was 71.4%, which was significantly higher than that with GG genotype (35.3%, P=0.022). No relationship was found between grade III–IV neutropenia and UGT1A1*28 polymorphism. The statistical analysis between grade III–IV diarrhea and UGT1A1*6/*28 polymorphisms was not conducted in view of the limited number of patients.
Conclusion
In Chinese patients with pancreatic or biliary tract cancer administered irinotecan-containing regimens, those with UGT1A1*6 variant may have a high risk of severe neutropenia.
Introduction
Pancreatic cancer accounts for only approximately 3% of all malignancies, but is one of the most fatal malignancies worldwide.Citation1 Over 80% of patients presenting with advanced disease at diagnosis require chemotherapy.Citation2 Gemcitabine is recognized as the standard first-line regimen for metastatic pancreatic cancer.Citation3 Half of the metastatic pancreatic cancer patients remain in relatively good performance status even after the failure of gemcitabine treatment and may undergo subsequent line(s) of chemotherapy. A fluoropyrimidine-based regimen is commonly used for gemcitabine-refractory patients.Citation4 Irinotecan, an inhibitor of DNA topoisomerase I, demonstrated potent activity against pancreatic cancer.Citation5 A FOLFIRINOX regimen, containing leucovorin (FOL), 5-fluorouracil (F), irinotecan (IRIN), and oxaliplatin (OX), showed survival benefits compared with gemcitabine monotherapy in the first setting. However, FOLFIRINOX had increased toxicity and should be selectively used in cases with good performance status.Citation6 Both irinotecan monotherapy and the FOLFIRI regimen were moderately effective and tolerable in pancreatic patients as a second- or further-line treatment.Citation7–Citation9
Biliary tract cancer is an uncommon but highly fatal malignancy. In Western countries, only 10% of patients present with early resectable disease. However, the majority of even those patients who undergo radical operation ultimately experience recurrence. Chemotherapy is commonly used to postpone tumor progression and prolong survival.Citation10,Citation11 Gemcitabine plus cisplatin has become the standard regimen for advanced biliary tract cancer.Citation12 However, evidence regarding second-line chemotherapy remains limited. Irinotecan monotherapy had a modest antitumor effect in patients previously treated with gemcitabine, cisplatin, or fluoropyrimidine.Citation13 FOLFIRI was reported feasible and well tolerated in patients with biliary tract cancer.Citation14
Severe delayed-onset diarrhea and neutropenia were the major dose-limiting toxicities of irinotecan. SN-38, the active metabolite of irinotecan, is the main cause of irinotecan-related toxicities. SN-38 is inactivated by uridine diphosphate glucuronosyltransferases (UGTs), of which UGT1A1 plays an important role. UGT1A1 gene polymorphisms, especially UGT1A1*28 and UGT1A1*6, influence the protein expression and enzyme activity of UGT1A1. Controversial conclusions remain regarding the distinct frequency of UGT1A1 genotypes between Western and Asian populations.Citation15,Citation16 This study set out to investigate the relationship between UGT1A1*6/*28 polymorphisms and toxicities in Chinese patients with advanced pancreatic or biliary tract cancer to whom were administered irinotecan-containing regimens as the second- or third-line chemotherapy.
Patients and methods
Patients
A total of 36 patients with unresectable pancreatic cancer and 12 patients with unresectable biliary tract cancer, who were treated in the Department of Oncology of Shanghai Ruijin Hospital from January 2012 to December 2014, were retrospectively included in this study. All patients received irinotecan-containing regimens as the second- or third-line chemotherapy. The main inclusion criteria were histologically or cytologically confirmed adenocarcinoma, relatively good performance status (Eastern Cooperative Oncology Group performance status, 0–1), life expectancy of at least 3 months, and adequate organ function (bone marrow: leukocytes ≥3,000/mm3, neutrophils ≥1,500/mm3, hemoglobin ≥9.0 g/dL, and platelets ≥100,000/mm3; liver function: total bilirubin <1.5 times the upper limit of normal, aspartate/alanine aminotransferase <1.5 times the upper limit of normal; renal function: serum creatinine <1.2 mg/dL). All patients gave their written informed consents, approved by the institutional review board of Shanghai Ruijin Hospital.
Treatment and evaluation
Approximately 33 patients were treated with the FOLFIRI regimen (irinotecan 180 mg/m2 at day 1, CF 200 mg/m2 at day 1–2, 5-FU 400 mg/m2 at day 1–2, followed by continuous infusion of 5-FU 600 mg/m2 for 22 hours at day 1–2, every 2 weeks). A total of 15 patients were treated with irinotecan monotherapy (180 mg/m2, every 2 weeks). When grade IV neutropenia or delayed-onset diarrhea occurred, the dose of irinotecan was reduced to 80% in subsequent cycles. In the case of liver dysfunction due to biliary tract obstruction, the therapy was reintroduced without dose reduction after biliary tract drainage. The treatment was terminated in case of disease progression or intolerable toxicities. Objective response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST 1.0). Adverse events were evaluated by the Common Terminology Criteria for Adverse Events (CTCAE 3.0). Grade III–IV neutropenia and diarrhea were defined as severe toxicities.
Genotyping of UGT1A1
Peripheral blood samples were collected before chemotherapy. Genomic DNA was extracted by the QIAamp Blood Kit (Qiagen, Hilden, Germany). DNA fragments containing UGT1A1*6 (211 G > A) and UGT1A1*28 (−53 TA6 > TA7) were both amplified by PCR (polymerase chain reaction) using primers (5′-CCTGCTACCTTTGTGGACTGAC-3′ [forward primer] and 5′-TGCCCGAGACTAACAAAAGACT-3′ [reverse primer]). Steps were taken strictly according to the working protocols to prevent contamination, such as wearing gloves, using aerosol tips (tips with a wad of cotton at the top), and not spitting in the tubes. The reaction conditions were 94°C for 5 minutes, 40 cycles of 94°C for 15 seconds, 60°C for 25 seconds, 72°C for 30 seconds, and final extension at 72°C for 7 minutes. PCR products were preserved in −20°C. All polymorphisms were confirmed by direct sequencing.
Statistical analysis
Hardy–Weinberg equilibrium was tested in all polymorphisms. The association between UGT1A1 polymorphisms and irinotecan-related toxicities was analyzed by the χ2 test or Fisher’s exact test. Data were analyzed by SPSS 16.0 (SPSS Inc, Chicago, IL, USA), and two-sided P<0.05 was considered a significant difference.
Results
Patients’ characteristics
The clinical characteristics of the patients included in this study are summarized in . The patients consisted of 25 males and 23 females with a median age of 56.2 years (range: 24–72 years). A total of 20 patients (41.6%) underwent biliary drainage due to obstructive jaundice. All patients had previously received gemcitabine-based monotherapy or combination chemotherapy as the first-line treatment. Approximately 12 patients received S-1-containing regimens as the second-line treatment. A total of 288 cycles of irinotecan-containing chemotherapy were administered in this study. The median number of cycles of administration was six (range: 2–12).
Table 1 Patient characteristics
Status of UGT1A1*6/*28 polymorphisms
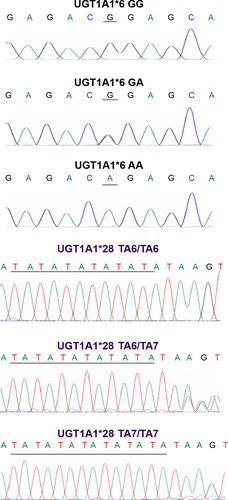
The frequencies of GG, GA, AA genotypes for UGT1A1*6 and TA6/TA6, TA6/TA7, and TA7/TA7 for UGT1A1*28 were 70.8% (n=34), 25.0% (n=12), 4.2% (n=2) and 79.2% (n=38), 18.8% (n=9), 2.0% (n=1), respectively (). Both polymorphisms fit Hardy–Weinberg equilibrium (P=0.488 and 0.598, respectively). According to the numbers of variants, the patients were classified into three groups: double wild-type (patients with GG and TA6/TA6 genotype, n=24, 50%), heterozygous variant type (patients with GA and TA6/TA6 genotype or GG and TA6/TA7 genotype, n=21, 43.8%), and homozygous variant type (patients with GG and TA7/TA7 genotype or AA and TA6/TA6 genotype, n=3, 6.2%). Examples of the sequencing chromatograms are shown in .
Table 2 Genotyping of UGT1A1*6/*28 in 48 patients
Analysis of irinotecan-related severe toxicities
Grade III–IV neutropenia occurred in 22 patients (45.8%), and six patients (12.5%) experienced grade III–IV diarrhea. No relationship was found between grade III–IV toxicities and clinical parameters, including age, sex, performance status, and line of treatment. Compared with those treated with irinotecan monotherapy, patients receiving the FOLFIRI regimen had a trend for higher risk of severe neutropenia (37.5% vs 8.3%, P=0.072; ).
Table 3 Relationship between clinical characteristics and irinotecan-related toxicities
Relationship between UGT1A1*6/*28 polymorphisms and irinotecan-related toxicities
The incidence of grade III–IV neutropenia in patients with UGT1A1*6 GA or AA genotype was 71.4%, significantly higher than that with GG genotype (35.3%, P=0.022). However, no relationship was found between grade III–IV neutropenia and UGT1A1*28 polymorphism. Moreover, compared with those with double wild-type, patients with heterozygous variant type (GG and TA6/TA7 genotype or GA and TA6/TA6 genotype) or homozygous variant type (GG and TA7/TA7 genotype or AA and TA6/TA6 genotype) showed a significantly higher incidence of grade III–IV neutropenia (P=0.004, ).
Table 4 Relationship between UGT1A1*6/*28 polymorphisms and neutropenia
However, the statistical analysis between grade III–IV diarrhea and UGT1A1*6/*28 polymorphisms was not conducted, because only six patients developed grade III–IV diarrhea. Among them, one was double wild-type, three were heterozygous variant type (two carrying GG and TA6/TA7 genotype, and one carrying GA and TA6/TA6 genotype), and the other two were homozygous variant type (one carrying GG and TA7/TA7 genotype, and one carrying AA and TA6/TA6 genotype).
Discussion
The role of UGT1A1 polymorphisms in the occurrence of irinotecan-related toxicities has been substantially documented, especially in colorectal cancer.Citation15 Recently, Sharma et alCitation17 investigated dose modification of FOLIRINOX based on UGT1A1*28 genotyping in previously untreated patients with advanced pancreatic, biliary tract, and gastric cancer. In this study, we analyzed the relationship between UGT1A1*6/*28 polymorphisms and the development of toxicities in Chinese patients with pancreatic or biliary tract cancer, who were administered irinotecan-containing regimens as the second- or third-line chemotherapy. Patients with UGT1A1*6 GA or AA genotype had a significantly higher risk of developing severe neutropenia than those with GG genotype.
Many studies reported that the frequencies of UGT1A1 variants varied distinctly by different ethnic populations. Regarding UGT1A1*28, ~50% were TA6/TA6, 40% were TA6/TA7, and 10% were TA7/TA7 genotypes in the white population. The proportion of TA7/TA7 genotype was also ~10% in individuals of African origin, but less than half of that in Asians.Citation15,Citation16 In contrast, the frequency of the UGT1A1*6 allele (211 A) in Eastern Asians was much higher than that in white Americans.Citation18 However, the distribution of UGT1A1*6/*28 was similar in Chinese and Japanese.Citation19 In the Chinese population, no significant difference in the distribution of UGT1A1*6/*28 polymorphisms was observed among primary tumor sites of pancreatic or biliary tract cancer, gastric cancer, esophageal cancer, and colorectal cancer, according to the findings in this study and the genotyping results of 42 gastric cancer patients, 91 esophageal cancer patients, and 276 colorectal cancer patients in the other studies.Citation20,Citation21
A series of TA repeats in the proximal promoter of UGT1A1 vary from five to eight in length; the lower the number of repeats, the more efficient the transcriptional activity of the gene. The commonest alleles are those with six and seven repeats. It was reported that the patients carrying the UGT1A1*28 TA7/TA7 genotype in Western countries had an increased risk of severe irinotecan-related toxicities, especially delayed-onset diarrhea.Citation22 In this study, severe diarrhea occurred only in six patients. The statistical analysis between severe diarrhea and UGT1A1*28 polymorphism was not conducted because of the limited number of patients. It was controversial that UGT1A1*28 polymorphism was associated with severe diarrhea in Chinese patients with gastrointestinal cancer.Citation19–Citation21 The low frequency of the TA7/TA7 genotype in Chinese might not completely contribute to the low incidence of severe diarrhea. The results need validation in the larger samples.
It had been reported that the UGT1A1*28 polymorphism could predict irinotecan-related severe neutropenia,Citation21,Citation22 but the association between UGT1A1*28 and severe neutropenia was not found in this study. The overall incidence of severe neutropenia was 45.8%, while it was 42.1% in patients carrying the TA6/TA6 genotype. Thus, the role of UGT1A1*28 in predicting severe neutropenia needs further investigation in the Chinese population. The administration of additional chemotherapy agents was an additional significant risk factor.Citation15 In our study, the patients receiving the FOLFIRI regimen had a trend for higher incidence of severe neutropenia. To diminish the risk of severe neutropenia, a reduced irinotecan dose of 150 mg/m2 is recommended in the FOLFIRI regimen in Japan.Citation23
Particularly important to Eastern Asians, UGT1A1*6 (211 G > A) reduced catalytic function by 60%.Citation24 The results of several studies indicated that the UGT1A1*6 allele could predict severe neutropenia in Eastern Asians.Citation20,Citation21,Citation25 However, the relationship between the UGT1A1*6 allele and diarrhea was rare. Consistent with those results, it was observed that the UGT1A1*6 allele was related to severe neutropenia, but not severe diarrhea. Such patients carrying GA or AA genotype should be monitored carefully when receiving irinotecan-containing regimens.
A subset of patients without variants of UGT1A1*6/*28 experienced severe toxicity, indicating that some other factors besides UGT1A1 polymorphisms could impact the occurrence of irinotecan-related toxicities, such as prior chemotherapy, dose of irinotecan, total bilirubin, other polymorphisms in UGT1A gene family members, and other participators in irinotecan metabolism.Citation26–Citation29 Until now, no clear dosing strategy for the variants has been defined. Concerns about efficacy interference are appropriate and need to be addressed prospectively. Further large sample studies aiming to guide the irinotecan dose modification based on UGT1A1 polymorphisms are needed.
Acknowledgments
This study was supported by the National Science Foundation of China (81372645), the Shanghai Natural Science Foundation from municipal government (13ZR1425900), Shanghai Jiao Tong University School of Medicine Science and Technology Foundation (13XJ10035), and Fong Shu Fook Tong Foundation and National Key Clinical Discipline (Oncology).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- HidalgoMPancreatic cancerN Engl J Med2010362171605161720427809
- BurrisHA3rdMooreMJAndersenJImprovements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trialJ Clin Oncol1997156240324139196156
- OettleHRiessHStielerJMSecond-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trialJ Clin Oncol201432232423242924982456
- UenoHOkusakaTFunakoshiAA phase II study of weekly irinotecan as first-line therapy for patients with metastatic pancreatic cancerCancer Chemother Pharmacol200759444745416855842
- ConroyTDesseigneFYchouMFOLFIRINOX versus gemcitabine for metastatic pancreatic cancerN Engl J Med2011364191817182521561347
- ZaniboniAAitiniEBarniSFOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II studyCancer Chemother Pharmacol20126961641164522576338
- NeuzilletCHenticORousseauBFOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-saltsWorld J Gastroenterol201218334533454122969226
- TakaharaNNakaiYIsayamaHUridine diphosphate glucuronosyl transferase 1 family polypeptide A1 gene (UGT1A1) polymorphisms are associated with toxicity and efficacy in irinotecan monotherapy for refractory pancreatic cancerCancer Chemother Pharmacol2013711859223053265
- SasakiTIsayamaHNakaiYCurrent status of chemotherapy for the treatment of advanced biliary tract cancerKorean J Intern Med201328551552424009445
- CeredaSBelliCRognoneASecond-line therapy in advanced biliary tract cancer: what should be the standard?Crit Rev Oncol Hematol201388236837423786845
- ValleJWasanHPalmerDHCisplatin plus gemcitabine versus gemcitabine for biliary tract cancerN Engl J Med2010362141273128120375404
- SasakiTIsayamaHNakaiYA pilot study of salvage irinotecan monotherapy for advanced biliary tract cancerAnticancer Res20133362619262223749917
- MorettoRRaimondoLDe StefanoAFOLFIRI in patients with locally advanced or metastatic pancreatic or biliary tract carcinoma: a monoinstitutional experienceAnticancer Drugs201324998098523928570
- O’DwyerPJCatalanoRBUridine diphosphate glucuronosyltransferase (UGT) 1A1 and irinotecan: practical pharmacogenomics arrives in cancer therapyJ Clin Oncol200624284534453817008691
- DesaiAAInnocentiFRatainMJPharmacogenomics: road to anticancer therapeutics nirvana?Oncogene200322426621662814528287
- SharmaMCatenacciDVTKarrisonTA UGT1A1 genotype-guided dosing study of modified FOLFIRINOX (mFOLFIRINOX) in previously untreated patients (pts) with advanced gastrointestinal malignanciesJ Clin Oncol201432Suppl abstr 4125
- InnocentiFKroetzDLSchuetzEComprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokineticsJ Clin Oncol200927162604261419349540
- ZhouCFMaTSuYUGT1A1 gene polymorphisms and the toxicities of FOLFIRI in Chinese Han patients with gastrointestinal cancerAnticancer Agents Med Chem201313223524122934695
- GaoJZhouJLiYAssociations between UGT1A1*6/*28 polymorphisms and irinotecan-induced severe toxicity in Chinese gastric or esophageal cancer patientsMed Oncol201330363023783485
- GaoJZhouJLiYUGT1A1 6/28 polymorphisms could predict irinotecan-induced severe neutropenia not diarrhea in Chinese colorectal cancer patientsMed Oncol201330360423686699
- NagarSBlanchardRLPharmacogenetics of uridine diphosphoglucuronosyl transferase (UGT) 1A family members and its role in patient response to irinotecanDrug Metab Rev200638339340916877259
- OkuyamaYHazamaSNozawaHProspective phase II study of FOLFIRI for mCRC in Japan, including the analysis of UGT1A1 28/6 polymorphismsJpn J Clin Oncol201141447748221303789
- PremawardhenaAFisherCALiuYTThe global distribution of length polymorphisms of the promoters of the glucuronosyltransferase 1 gene (UGT1A1): hematologic and evolutionary implicationsBlood Cells Mol Dis20033119810112850492
- JadaSRLimRWongCIRole of UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan-induced neutropenia in Asian cancer patientsCancer Sci20079891461146717627617
- HuZYYuQZhaoYSDose-dependent association between UGT1A1*28 polymorphism and irinotecan-induced diarrhoea: a meta-analysisEur J Cancer201046101856186520335017
- InnocentiFUndeviaSDIyerLGenetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia ofirinotecanJ Clin Oncol20042281382138815007088
- HanJYLimHSShinESComprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatinJ Clin Oncol200624152237224416636344
- MarshSHoskinsJMIrinotecan pharmacogenomicsPharmacogenomics20101171003101020602618