Abstract
Hepatic ischemia/reperfusion (ISCH/REP) is a major clinical problem that is considered to be the most common cause of postoperative liver failure. Recently, mast cells have been proposed to play an important role in the pathophysiology of ISCH/REP in many organs. In contrast, the role played by mast cells during ISCH/REP-induced liver damage has remained an issue of debate. This study aimed to investigate the protective role of mast cells in order to search for an effective therapeutic agent that could protect against fatal ISCH/REP-induced liver damage. A model of warm ISCH/REP was induced in the liver of rats. Four groups of rats were used in this study: Group I: SHAM (normal saline, intravenously [iv]); Group II: ISCH/REP; Group III: sodium cromoglycate + ISCH/REP (CROM + ISCH/REP), and Group IV: ketotifen (KET) + ISCH/REP (KET + ISCH/REP). Liver damage was assessed both histopathologically and biochemically. Mast cell degranulation was assessed histochemically. Lipid peroxidation (malondialdehyde [MDA]) as well as the levels of glutathione (GSH), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α), the formation of nitric oxide (NO), and the expression of inducible NO synthase (iNOS) were determined. The results of this study revealed increased mast cell degranulation in the liver during the acute phase of ISCH/REP. Moreover, CROM, but not KET, decreased the activity of alanine aminotransferase, aspartate aminotransferase, and lactic dehydrogenase and maintained normal liver tissue histology. Both CROM and KET protected against mast cell degranulation in the liver. In addition, both CROM and KET decreased IL-6 and TNF-α. However, CROM, but not KET, decreased MDA formation and increased GSH. Furthermore, KET, but not CROM, increased both NO formation and iNOS expression. In conclusion, this study clearly demonstrated mast cell degranulation in warm ISCH/REP in the liver of rats. More importantly, CROM, but not KET, ameliorated the effect of ISCH/REP-induced injury in rat liver. CROM may protect the liver through mast cell stabilization, inhibition of TNF-α, IL-6, MDA, and iNOS and increased GSH. KET may maintain ISCH/REP-induced liver injury through the NO/iNOS pathway.
Introduction
Hepatic ischemia reperfusion injury (ISCH/REP) is a major clinical problem that arises during liver transplantation and liver partial resection surgery. It is one of the most common causes of postoperative liver dysfunction, graft rejection, and chronic liver diseases.Citation1–Citation4 Until recently, there has been no effective therapy to prevent or treat ISCH/REP-induced liver injury.Citation1
Several hypotheses have explained ISCH/REP-induced tissue damage including, microcirculatory injury, reactive oxygen species (ROS) and reactive nitrogen species generation, leukocyte adhesion, release of proinflammatory cytokines, and mast cell degranulation.Citation5–Citation8 Recently, there has been growing evidence that mast cells play an important role in ISCH/REP-induced damage in many organs, including the intestines, the heart, the lung, and the brain.Citation9,Citation10 However, the exact role of mast cells in ISCH/REP-induced liver damage has not been well clarified. A recent in vitro study concluded that mast cells are not involved in ISCH/REP-induced liver damage.Citation11 On the other hand, Yang et al recently provided evidence that mast cell degranulation was involved in ISCH/REP-induced hepatic injury in rats.Citation12
Ischemia-induced ROS generation causes mast cell degranulation and, hence, the release of histamine and many inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). The release of ROS as well as many inflammatory mediators has been found to contribute to and aggravate REP-induced tissue injury.Citation10,Citation12 This gives rise to the hypothesis that mast cells might play an important role in ISCH/REP-induced liver injury and, consequently, mast cell stabilizing agents could be an effective therapy that might protect against ISCH/REP-induced hepatic damage.
This study aimed first to reassess the role of mast cells in warm ISCH/REP-induced liver injury in rats. Second, it investigated and compared the supposed protective effect of two mast cell stabilizers, sodium cromoglycate (CROM) and ketotifen (KET), against ISCH/REP-induced liver injury. Finally, it studied the precise mechanisms of the probable hepatoprotective role of CROM and/or KET, focusing on ROS, nitric oxide (NO), and inflammatory cytokine production.
Materials and methods
Chemicals
Opticrom (4% CROM) (Sanofi-Aventis, Cairo, Egypt) and Zaditen (1 mg KET) (Novartis, Cairo, Egypt) were used in this study. All other chemicals are of analytical grades.
Animals
Twenty-four male albino rats (180–200 g) were used in this study. The rats were maintained under controlled conditions and had free access to food and water. The animals were fasted 12 hours before ISCH/REP induction. The animals were cared for according to institutional guidelines for the care and use of laboratory animals at King Fahd Medical Research Center. All experiments were performed according to the rules and regulations of the Saudi National Committee of Ethics with regard to animal research.
Experimental protocol
Four groups of rats (n=6) were assigned as follows: Group I: SHAM (normal saline, iv); Group II: ISCH/REP; Group III: CROM + ISCH/REP (CROM 50 mg/kg, iv), and Group IV: KET + ISCH/REP (KET 1 mg/kg, iv).Citation9,Citation13
Induction of liver, acute warm ISCH/REP
Total hepatic ISCH was induced by clamping the common hepatic artery and portal vein for 45 minutes, followed by 2 hours REP.Citation7,Citation12 The rats were treated with normal saline, CROM, or KET 0 minute before ISCH induction and immediately after the REP. An additional CROM dose was injected 30 minutes after the REP because of its short half-life.Citation8,Citation9
Sample collection
At the end of the perfusion period, liver samples were collected and kept either frozen (−80°C) or in 10% buffered formalin solution. In addition, blood samples were withdrawn and plasma was separated.
Sample preparation for the biochemical analysis
Frozen livers (−80°C) were used for the analysis of lipid peroxidation (malondialdehyde [MDA]) and to determine the levels of glutathione (GSH) and NO. Homogenization of the frozen liver samples was made in 50 mM potassium phosphate (pH 7.5).Citation14
Assessment of plasma alanine aminotransferase, aspartate aminotransferase, and lactic dehydrogenase enzyme activity
Concentrations of plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were expressed in U/L according to the method recommended by Reitman and Frankel.Citation15 The U/L values were determined using kits from Biodiagnostic (Cairo, Egypt). The absorbance of the samples was read at 546 nm against a reagent blank.
Total plasma lactic dehydrogenase (LDH) activity was measured using kits from Biodiagnostic. Total LDH activity (U/L) was assessed according to the method recommended by Henry.Citation16 The increase in absorbance due to the formation of NADH was measured spectrophotometrically at 340 nm at 1-minute intervals for 3 minutes.
Determination of MDA
A Biodiagnostic kit was used to measure the lipid peroxidation product, MDA (nmol/g tissue), according to the method recommended by Uchiyama and Mihara.Citation17 Briefly, thiobarbituric acid was added to the tissue homogenate and boiled in a water bath, then the color that was formed was extracted with n-butanol and measured at two distinct wavelengths: 535 nm and 525 nm.
Determination of reduced GSH
The level of GSH (nmol/g tissue) was determined according to the method described earlier by EllmanCitation18 using a Biodiagnostic kit. This assay is based on the reduction of bis-(3-carboxy-4-nitrophenyl) disulfide reagent by the thiol group to form 2-nitro-5-mercaptobenzoic acid. The absorbance of the formed yellow color was measured spectrophotometrically at 412 nm.Citation19
Determination of NO
A Biodiagnostic kit was used to measure NO (µmol/g tissue). NO was assayed spectrophotometrically (550 nm) by measuring total nitrate plus nitrite (NO3− + NO2−) using Griess reagent.Citation20
Determination of IL-6 and TNF-α
Liver tissues were homogenized in ice-cold phosphate-buffered saline containing protease inhibitor cocktail and 0.05% Tween-20. The samples were centrifuged at 12,074× g for 10 minutes. The resulting supernatant was used to analyze the IL-6 and TNF-α levels in an ELISA as a part of the Assaymax IL-6 and TNF-α kits (Gentaur, Dublin, Ireland) using monoclonal antibodies specific for IL-6 and TNF-α, respectively. Cytokine concentrations were calculated using standard purified recombinant cytokines.
Histopathologic examination of the liver sections
The liver tissues were fixed in buffered formalin and embedded in paraffin wax. Then, 3–5 µm sections were stained with hematoxylin and eosin (H&E). Assessment of the histopathologic changes was done microscopically. Liver mast cell degranulation analysis (toluidine blue staining) The liver sections were stained with 0.5% toluidine blue in 0.5 M of hydrochloric acid. Degranulated mast cells were defined as the cells with reduced granule density.
Immunohistochemical determination of inducible nitric oxide synthase expression
Inducible nitric oxide synthase (iNOS) expression was detected by the immunostaining of the tissue sections prepared from formalin-fixed, paraffin-embedded liver using a kit obtained from Lab Vision (Fremont, CA, USA). An immunoperoxidase (PAP, peroxidase/anti-peroxidase) technique was adopted. In this way, the cytoplasm of each iNOS (+) cell was stained brown. The brown staining was graded as follows: no brown color (−) (negative staining), mild brown staining (+) (mild positive), moderate brown staining (++) (moderate positive), and severe brown staining (+++) (severe positive).
Semiquantitative analysis of antibody immunoreactivity was assessed using Image-Pro Plus from Media Cybernetics-USA software version 6.0. The software used in the analysis is labeling intensity (mean intensity) and extension of the reaction (area percentage) of iNOS in 30 field ×40 objective lens and ×10 ocular lens.
Statistical analysis
One-way analysis of variance test was used for comparison between different groups followed by Dunnett’s t-test (two- sided) multiple comparisons to detect significant differences among individual mean values of all groups. Results are expressed as the mean ± standard deviation. The level of significance was set at P≤0.05. Statistical analysis was generated using SPSS software for windows, version 14.0 (SPSS Inc., Chicago, IL, USA).
Results
Effect of CROM and KET on ISCH/REP-induced injury changes in the microscopic appearance of liver sections after H&E staining
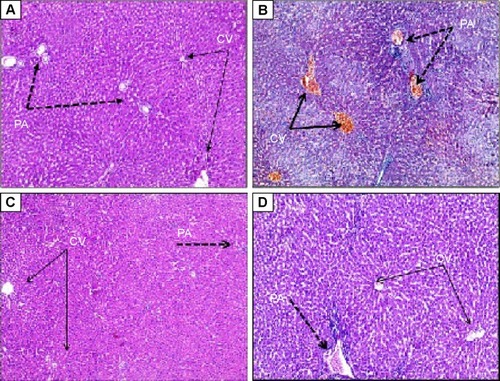
As shown in , induction of ISCH/REP resulted in severe congestion and dilation of the portal tract vessels and central veins, with a mild inflammatory reaction in the portal tract (). Pretreatment of the ISCH/REP rats with CROM protected the liver against ISCH/REP-induced pathological changes (). On the other hand, the liver sections of the KET-pretreated ISCH/REP rats showed a mild inflammatory reaction of the portal tract together with a mild dilation and congestion of the portal tract and central veins ().
Figure 1 Effect of sodium cromoglycate (CROM) and ketotifen (KET) on ISCH/REP injury-induced changes in microscopic appearance of liver sections after hematoxylin and eosin staining (H&E ×100).
Abbreviations: ISCH/REP, ischemia/reperfusion; CV, central vein; PA, portal artery.
Effect of CROM and KET on plasma ALT, AST, and LDH levels in ISCH/REP-induced liver injury
As shown in , induction of ISCH/REP significantly increased the plasma ALT, AST, and LDH levels (approximately 16-, six-, and twofold, respectively) as compared with the rats in the SHAM group (P=0.000, 0.000, and 0.030, respectively). Pretreatment of the ISCH/REP rats with CROM significantly decreased the plasma ALT, AST, and LDH levels (62%, 25%, and 39%, respectively) compared with the ISCH/REP rats (P=0.001, 0.006, and 0.046, respectively). In contrast, pretreatment of the ISCH/REP rats with KET did not change the plasma ALT, AST, and LDH levels compared with the ISCH/REP rats (P=0.789, 0.855, and 0.54, respectively).
Table 1 Effect of sodium cromoglycate (CROM) and ketotifen (KET) on plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactic dehydrogenase (LDH) levels in rats subjected to ischemia/reperfusion (ISCH/REP)-induced injury
Effect of CROM and KET on liver MDA, reduced GSH, and NO concentrations in ISCH/REP-induced liver injury
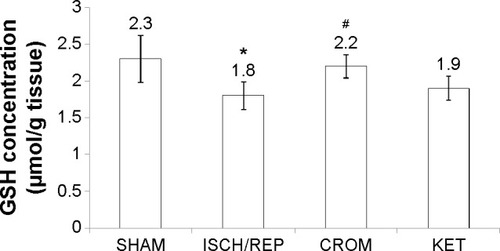
Induction of ISCH/REP significantly increased both liver MDA and NO (81% and 18%, respectively) as compared with the SHAM rats (P=0.003 and P=0.012, respectively) ( and ). On the other hand, ISCH/REP significantly decreased the level of GSH (21%) as compared with the SHAM rats (P=0.01) (). Pretreatment of the ISCH/REP rats with CROM significantly decreased liver MDA (29%) and increased liver GSH (22%) as compared with the ISCH/REP rats (P=0.025 and 0.009, respectively) ( and ). On the other hand, CROM did not affect NO formation in comparison with the ISCH/REP rats (P=0.119) (). Pretreatment of the ISCH/REP rats with KET did not change either the hepatic MDA or GSH content in comparison with the ISCH/REP rats (P=0.48 and 0.341, respectively) ( and ). On the other hand, pretreatment of the ISCH/REP rats with KET significantly increased the liver NO (27%) content as compared with the ISCH/REP rats (P=0.003) ().
Figure 2 Effect of sodium cromoglycate (CROM) and ketotifen (KET) on liver lipid peroxide (MDA) concentration in rats subjected to ischemia/reperfusion (ISCH/REP)-induced injury. Each point represents the mean ± SD of six rats.
Abbreviation: MDA, malondialdehyde.
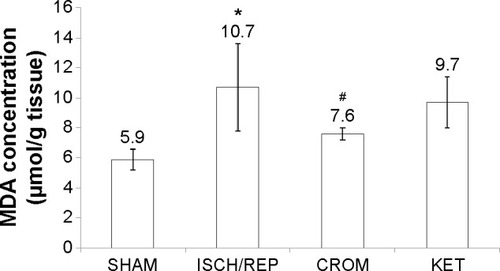
Figure 3 Effect of sodium cromoglycate (CROM) and ketotifen (KET) on liver nitric oxide (NO) concentrations in rats subjected to ischemia/reperfusion (ISCH/REP)-induced injury. Each point represents the mean ± SD of six rats.
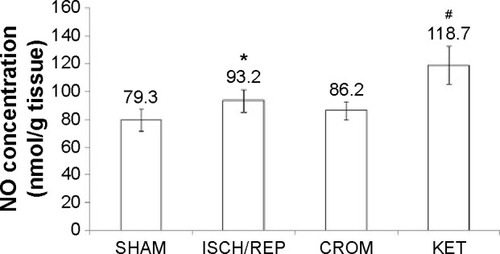
Figure 4 Effect of sodium cromoglycate (CROM) and ketotifen (KET) on reduced glutathione (GSH) content in the liver of rats subjected to ischemia/reperfusion (ISCH/REP)-induced injury. Each point represents the mean ± SD of six rats.
Effect of CROM and KET on liver TNF-α and IL-6 concentrations in ISCH/REP-induced liver injury
As shown in , induction of ISCH/REP significantly increased liver TNF-α and IL-6 (61% and 44%, respectively) (P=0.044 and 0.008, respectively) as compared with the SHAM rats. Pretreatment of the ISCH/REP rats with both CROM and KET significantly decreased liver TNF-α (38% and 44%, respectively) (P=0.050 and 0.026, respectively) and IL-6 (87% and 44%, respectively) (P=0.019 and 0.038, respectively) as compared with the ISCH/REP rats.
Table 2 Effect of sodium cromoglycate (CROM) and ketotifen (KET) on liver tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) concentrations in rats subjected to ischemia/reperfusion (ISCH/REP)-induced injury
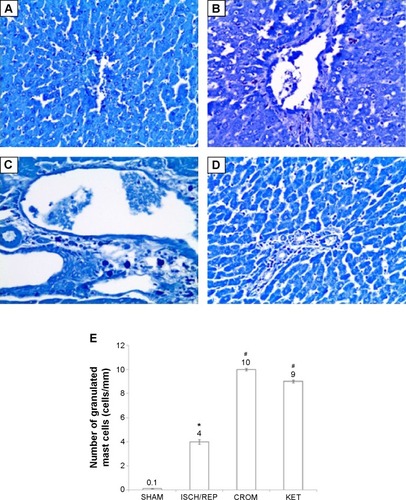
Effect of CROM and KET on ISCH/REP-induced injury changes in mast cell recruitment and granulation in liver sections after toluidine blue staining
showed normal mast cells around the portal tract. Induction of ISCH/REP resulted in increased drainage and degranulation of the mast cells around the portal tract ( and E). Pretreatment of the ISCH/REP rats with both CROM and KET stabilized the mast cell membranes and protected them from degranulation ().
Figure 5 Effect of sodium cromoglycate (CROM) and ketotifen (KET) on ISCH/REP-induced injury changes in mast cell recruitment and granulation in liver sections after toluidine blue staining: (A) SHAM; (B) 45 minutes following ISCH and 60 minutes following REP; (C) CROM + ISCH/REP; and (D) KET + ISCH/REP.
Abbreviation: ISCH/REP, ischemia/reperfusion.
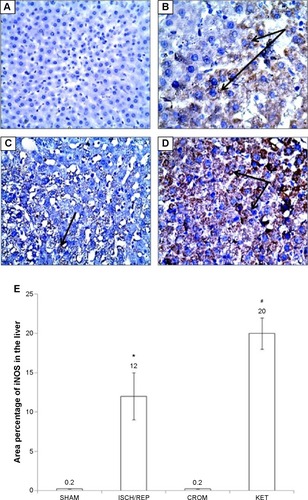
Effect of CROM and KET on ISCH/REP-induced injury changes in liver iNOS immunoreactivity
showed normal liver with no iNOS immunoreactivity. The liver sections of the ISCH/REP rats showed moderately positive immunoreactivity toward iNOS (P=0.0001) (). Pretreatment of the ISCH/REP rats with CROM resulted in negative iNOS immunoreactivity (). However, pretreatment of the ISCH/REP rats with KET resulted in a severe increase in iNOS immunoreactivity (P=0.0003) ().
Figure 6 Effect of sodium cromoglycate (CROM) and ketotifen (KET) on liver inducible nitric oxide synthase (iNOS) immunoreactivity examined in rats subjected to ischemia/reperfusion (ISCH/REP) injury: (A) rat liver under control conditions (SHAM); (B) 45 minutes following ISCH and 60 minutes following REP; (C) CROM + ISCH/REP; and (D) KET + ISCH/REP.
Discussion
This study’s results revealed increased mast cell degranulation in the liver during the acute phase of warm ISCH/REP. Moreover, CROM protected the liver against ISCH/REP-induced acute injury, which was confirmed by the decrease in the activity of ALT, AST, and LDH and the normal liver tissue histology revealed by H&E staining. On the other hand, KET did not protect against acute phase ISCH/REP-induced liver injury. Both CROM and KET protected against mast cell degranulation in the liver that was induced during the acute phase of ISCH/REP. In addition, both CROM and KET decreased the levels of IL-6 and TNF-α that were induced in the liver during the acute phase of ISCH/REP. Taken together, CROM-induced inhibition of mast cell degranulation and proinflammatory cytokine release are not the key mechanisms accounting for its protective role against the acute phase of warm ISCH/REP in the liver. CROM, but not KET, decreased MDA formation and increased the GSH content in the liver during the acute phase of ISCH/REP, suggesting that CROM protected the liver against the toxic action of ISCH/REP through an antioxidant mechanism and that the same protective effect did not occur with KET. Furthermore, KET, but not CROM, increased both NO formation and iNOS expression in the liver during the acute phase of ISCH/REP. From our results, we could suggest that KET increased the reactive nitrogen species and NO through iNOS; hence, it promoted liver injury during the acute phase of ISCH/REP.
Our results appear to be consistent with Yang et alCitation12 who found increased mast cell degranulation in the liver and a protective effect of CROM against ISCH/REP-induced liver injury. In addition, Huang et alCitation21 recently reported on the protective role of CROM against liver injury triggered by small intestinal ISCH/REP. Conversely, Shibamoto et alCitation11 found ISCH/REP-induced injury in the isolated perfused liver of genetically mast cell deficient (Ws/Ws) rats. The Shibamoto group analyzed ISCH/REP-induced injury 1 hour after REP, whereas we analyzed ISCH/REP-induced injury 2 hours after REP.Citation11 Most of the research studies used the 2 hours or greater time frame to investigate the role of mast cells during ISCH/REP.Citation12,Citation22,Citation23 To the best of our knowledge, no study has reported on the effect that KET has on ISCH/REP-induced liver injury; nonetheless, our results appear to be contrary to the findings from a previous study conducted by Huang et alCitation21 who reported a protective role of KET against liver injury triggered by small intestinal ISCH/REP.
On the one hand, CROM produced a hepatoprotective effect while, on the other hand, KET did not have any effect on ISCH/REP-induced liver injury. Interestingly, this type of paradoxical effect could be explained, depending on the nature and mechanism of action of each agent. CROM is a mast cell membrane stabilizer, so it could inhibit mast cell degranulation and the release of histamine, TNF-α, and other inflammatory mediators.Citation24 In contrast, KET is a second-generation histamine H1-receptor antagonist.Citation25,Citation26 In various experimental and clinical conditions, KET has been noted to reduce mast cell degranulation and to decrease the release of histamine, mast cell proteases, myeloperoxidase, leukotrienes, platelet-activating factor, and various prostaglandins.Citation25,Citation27–Citation29 Depending on these facts and our results, we could conclude that both CROM and KET stabilized the mast cell membrane during ISCH/REP; hence, we expected that both would decrease histamine release. As KET, but not CROM, blocked the H1 receptor, our results suggested that the small amount of histamine released after mast cell stabilization played a protective role via the H1 receptor. A preliminary in vitro study showed that histamine, at slightly elevated concentrations, aggravated hypoxia/reoxygenation-induced rat liver BRL-3A cell injury.Citation4 Therefore, it was reasonable to speculate that ISCH/REP might mediate liver injury primarily via mast cell activation and the subsequent elevated release of histamine.Citation21
Toxic substances released after ISCH/REP, including MDA, ROS, neutrophils, pro-inflammatory cytokines, and adhesion molecules, contribute to liver injury through portal circulation and induced oxidative stress and inflammatory responses in the liver.Citation21 Excessive degranulation of mast cells elicited by a severe ischemic insult has been shown to cause the release of certain biochemical mediators to an extent that was cytotoxic; thus, it mediated ischemic cell death and the accompanying secondary inflammatory processes.Citation30 Mast cells were abundant in the microcirculatory beds in which the inflammatory effects of ISCH were demonstrated and they are known to degranulate and release various inflammatory mediators, including leukotriene B4 and platelet activating factor. Mast cells have been shown to play a role in leukocyte recruitment into tissue after ISCH/REP.Citation31,Citation32 The present study showed significantly increased levels of TNF-α and IL-6 in the liver of the ISCH/REP rats as compared with the SHAM rats. These results are consistent with the findings of many previous studies.Citation33–Citation35 Similar to our results, previous studies have found that treatment with either CROM or KET could significantly decrease the levels of TNF-α and IL-6 in the intestine in comparison with an intestinal ISCH/REP model.Citation10,Citation36
The small amount of NO, which was generated from endothelial NOS, was essential to maintain microvascular circulation. Consequently, it could protect the liver cells against early ISCH/REP-induced damage.Citation37 The large amount of NO, which was formed by iNOS, has been shown to have a significant role in numerous immune and inflammatory reactions.Citation38 However, the impact of iNOS in liver warm ISCH/REP injury is still an issue of debate.Citation39 Previously, it was reported that aminoguanidine, a selective inhibitor of iNOS, reduced liver warm ISCH/REP injury in rats.Citation40 Another study reported that a selective inhibitor of iNOS exacerbated liver damage during warm ISCH/REP.Citation41 As a product of iNOS, NO might act as an antioxidant and/or a prooxidant molecule.Citation42 NO reacted with a superoxide radical to form peroxynitrite anion, a potent nitrogen-free radical that damages cells.Citation43 However, as an antioxidant, NO reacted with the superoxide anion and removed it.Citation44,Citation45 The results of the present study showed that ISCH/REP induced an increase in iNOS immunoreactivity, which was further increased upon the administration of KET. CROM inhibited ISCH/REP-induced iNOS immunoreactivity. In addition, both ISCH/REP and KET increased the NO content in the liver. Similar to our results, Trocha et alCitation46 reported a significant increase in iNOS protein concentration caused by ISCH/REP in the liver of rats. In addition, Jiang et alCitation47 reported activated iNOS gene transcripts in warm ISCH/REP rats. It has also been previously reported that KET enhanced iNOS activity in the renal cortex of rats and in human colonic tissue homogenates.Citation48 Taken together, CROM might protect against warm ISCH/REP-induced liver through the inhibition of iNOS immune expression; moreover, KET might maintain warm ISCH/REP-induced liver injury by increasing the NO content in the liver and by increasing iNOS immune expression.
The present study results showed that CROM, but not KET, decreased MDA and increased GSH in the liver after ISCH/REP. The lipid peroxidation product, MDA, was used as a quantitative measure of liver oxidative stress. In the present study, ISCH/REP increased liver MDA, which was accompanied by increased NO and iNOS, indicating that iNOS plays a role in liver damage during warm ISCH/REP via ROS. Non-enzymatic (GSH) and enzymatic (superoxide dismutase and glutathione peroxidase) antioxidants have been found to be involved in minimizing the damaging effects of ROS on the liver.Citation49 Our results showed that CROM increased GSH in the liver after ISCH/REP. This finding might suggest that GSH plays a protective role in ISCH/REP-induced liver injury through improved antioxidant defense.
In conclusion, this study clearly demonstrated mast cell degranulation in warm ISCH/REP in the liver of rats. More importantly, CROM, but not KET, was found to ameliorate the injury effect of ISCH/REP in the liver of rats. CROM may protect the liver through mast cell stabilization, inhibition of TNF-α, IL-6, MDA, and iNOS, and increased GSH. Moreover, KET may maintain ISCH/REP-induced liver injury through the NO/iNOS pathway.
Recommendation
More studies are needed to resolve the actual role that the mast cell stabilizers, CROM and KET, play in warm ISCH/REP-induced liver injury.
Acknowledgments
This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No 166-529-D1435. The authors therefore, gratefully acknowledge the DSR technical and financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
- RenFDuanZChengQInhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanismHepatology201154268769621567437
- DaiYCuiJCunYShiATetrahydrobiopterin ameliorates hepatic ischemia-reperfusion Injury by coupling with eNOS in miceJ Surg Res20121762e65e7122475351
- FengMWangHWangQGuanWMatrix metalloprotease 9 promotes liver recovery from ischemia and reperfusion injuryJ Surg Res2013180115616123157925
- WuTGanXZhouSGeMZhangZHeiZHistamine at low concentrations aggravates rat liver BRL-3A cell injury induced by hypoxia/reoxygenation through histamine H2 receptor in vitroToxicol In Vitro201327137838622926047
- JessupJMBattlePWallerHReactive nitrogen and oxygen radicals formed during hepatic ischemia-reperfusion kill weakly metastatic colorectal cancer cellsCancer Res19995981825182910213485
- HanJYFanJYHorieYAmeliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusionPharmacol Ther2008117228029518048101
- OzkanEYardimciSDulunduEProtective potential of montelukast against hepatic ischemia/reperfusion injury in ratsJ Surg Res2010159158859419515388
- DattaGFullerBJDavidsonBRMolecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout modelsWorld J Gastroenterol201319111683169823555157
- CordeiroPGLeeJJMastorakosDHuQYPintoJTSantamariaEPrevention of ischemia-reperfusion injury in a rat skin flap model: the role of mast cells, cromolyn sodium, and histamine receptor blockadePlast Reconstr Surg200010565465910697173
- HeiZGanXLuoGLiSCaiJPretreatment of cromolyn sodium prior to reperfusion attenuates early reperfusion injury after the small intestine ischemia in ratsWorld J Gastroenterol200713385139514617876882
- ShibamotoTTsutsumiMKudaYOhmukaiCZhangWKurataYMast cells are not involved in the ischemiareperfusion injury in perfused rat liverJ Surg Res201217411421227466
- YangMQMaYYTaoSFMast cell degranulation promotes ischemia-reperfusion injury in rat liverJ Surg Res2014186117017824139633
- VuralKMLiaoHOzMCPinskyDJEffects of mast cell membrane stabilizing agents in a rat lung ischemia-reperfusion modelAnn Thorac Surg200069122823210654519
- UrazSTahanVAygunCRole of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicityDig Dis Sci20085341071107717934844
- ReitmanSFrankelSColorimetric method for aspartate and alanine amino transferasesAm J Clin Pathol195728566313458125
- HenryRJColorimetric determination of lactic dehydrogenaseHeneryRJClinical Chemistry: Principles and Techniques2nd edHagerstown, NJHarper and Row1974819831
- UchiyamaMMiharaMDetermination of malondialdehyde precursor in tissues by thiobarbituric acid testAnal Biochem197986271278655387
- EllmanGLTissue sulfhydryl groupsArch Biochem Biophys195974214226
- TheodorusPAkerboomMSiesHAssay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samplesMeth Enzymol1981773733837329314
- TarpeyMMWinkDAGrishamMBMethods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerationsAm J Physiol Regul Integr Comp Physiol2004286R431R44414761864
- HuangPGanXLiuJLiuDWangYHeiZEffects of anti-histamine treatment on liver injury triggered by small intestinal ischemia reperfusion in ratsChin J Physiol201457527127825241987
- HeiZQGanXLHuangPJWeiJShenNGaoWLInfluence of ketotifen, cromolyn sodium, and compound 48/80 on the survival rates after intestinal ischemia reperfusion injury in ratsBMC Gastroenterol200884218808687
- SantenSWangYMengerMDJeppssonBThorlaciusHMast-cell-dependent secretion of CXC chemokines regulates ischemia-reperfusion-induced leukocyte recruitment in the colonInt J Colorectal Dis20082352718193431
- HemmatiAANazariZMotlaghMEGoldastehSThe role of sodium cromolyn in treatment of paraquat-induced pulmonary fibrosis in ratPharmacol Res200246322923412220965
- CrapsLPNeyUMKetotifen: current views on its mechanism of action and their therapeutic implicationsRespiration19844544114216206536
- GrantSMGoaKLFittonASorkinEMKetotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disordersDrugs1990403412448 Review2226222 ErratumDrugs1991412192
- KarmeliFEliakimROkonERachmilewitzDGastric mucosal damage by ethanol is mediated by substance P and prevented by ketotifen, a mast cell stabilizerGastroenterology19911005 Pt 1120612161707383
- PothoulakisC1KarmeliFKellyCPKetotifen inhibits Clostridium difficile toxin A-induced enteritis in rat ileumGastroenterology199310537017078395445
- GrönbechJELacyERSubstance P attenuates gastric mucosal hyperemia after stimulation of sensory neurons in the rat stomachGastroenterology199410624404497507874
- StrbianDKarjalainen-LindsbergMLTatlisumakTLindsbergPJCerebral mast cells regulate early ischemic brain swelling and neutrophil accumulationJ Cereb Blood Flow Metab20062660561216163296
- KubesPGrangerDNLeukocyte-endothelial cell interactions evoked by mast cellsCardiovasc Res1996326997088915188
- KuroseIArgenbrightLWWolfRLianxiLGrangerDNIschemia/reperfusion-induced microvascular dysfunction: role of oxidants and lipid mediatorsAm J Physiol Heart Circ Physiol1997272H2976H2982
- CollettiLMRemickDGBurtchGDKunkelSLStrieterRMCampbellDAJrRole of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the ratJ Clin Invest1990856193619432161433
- ScalesWECampbellDAJrGreenMERemickDGHepatic ischemia/reperfusion injury: importance of oxidant/tumor necrosis factor interactionsAm J Physiol19942676 Pt 1G1122G11277810659
- El-MahdyNAEl-SisiAEDewidarBIEl-DesoukyKIHistamine protects against the acute phase of experimentally-induced hepatic ischemia/re-perfusionJ Immunotoxicol201310191622793375
- Zi-qingHXiao-liangGPin-jieHJingWNingSWan-lingGInfluence of Ketotifen, Cromolyn Sodium, and Compound 48/80 on the survival rates after intestinal ischemia reperfusion injury in ratsBMC Gastroenterol200884218808687
- CauwelsANitric oxide in shockKidney Int20077255756517538569
- KotiRSTsuiJLobosEYangWSeifalianAMDavidsonBRNitric oxide synthase distribution and expression with ischemic preconditioning of the rat liverFASEB J2005191155115715870170
- ShahVKamathPSNitric oxide in liver transplantation: pathobiology and clinical implicationsLiver Transpl2003911112514766
- YaylakFCanbazHCaglikulekciMLiver tissue iNOS expression and lipid peroxidation in experimental hepatic ischemia reperfusion injury stimulated with lipopolysaccharide: the role of aminoguanidineJ Surg Res200814821422318222473
- HsuCMWangJSLiuCHChenLWKupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanismShock20021728028511954827
- CasiniAGalliGSalzanoRRotellaCMSurrentiCAcetaldehyde-protein adducts, but not lactate and pyruvate, stimulate gene transcription of collagen and fibronectin in hepatic fat-storing cellsJ Hepatol1993193853928151099
- CrowJPSpruellCChenJOn the pH-dependent yield of hydroxyl radical products from peroxynitriteFree Radic Biol Med1994163313388063196
- KoppenolWHMorenoJJPryorWAIschiropoulosHBeckmanJSPeroxynitrite, a cloaked oxidant formed by nitric oxide and superoxideChem Res Toxicol199258348421336991
- RubboHRadiRTrujilloMNitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivativesJ Biol Chem199426926066260757929318
- TrochaMMerwid-LadASzubaAEffect of simvastatin on nitric oxide synthases (eNOS, iNOS) and arginine and its derivatives (ADMA, SDMA) in ischemia/reperfusion injury in rat liverPharmacol Rep201062234335120508290
- JiangWKongLLiGWangXExpression of iNOS in early injury in a rat model of small-for-size liver transplantationHepatobiliary Pancreat Dis Int20098214615119357027
- HeymanSNKarmeliFBrezisMRachmilewitzDThe effect of ketotifen on nitric oxide synthase activityBr J Pharmacol1997120154515519113377
- FanCZwackaRMEngelhardtJFTherapeutic approaches for ischemia/reperfusion injury in the liverJ Mol Med19997757759610543390
