Abstract
Osteoimmunology represents a large area of research resulting from the cross talk between bone and immune systems. Many cytokines and signaling cascades are involved in the field of osteoimmunology, originating from various cell types. The RANK/receptor activator of nuclear factor Kappa-B ligand (RANKL)/osteoprotegerin (OPG) signaling has a pivotal role in osteoimmunology, in addition to proinflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1, IL-6, and IL-17. Clinically, osteoimmunological disorders, such as rheumatoid arthritis, osteoporosis, and periodontitis, should be classified according to their pattern of osteoimmunological serum biomarkers. Paget’s disease of bone is a common metabolic bone disorder, resulting from an excessively increased bone resorption coupled with aberrant bone formation. With the exception of the cellular responses to measles virus nucleocapsid protein and the interferon-gamma signature, the exact role of the immune system in Paget’s disease of bone is not well understood. The cytokine profiles, such as the increased levels of IL-6 and the interferon-gamma signature observed in this disease, are also very similar to those observed in other osteoimmunological disorders. As a potential osteoimmunological disorder, the treatment of Paget’s disease of bone may also benefit from progress made in targeted therapies, in particular for receptor activator of nuclear factor Kappa-B ligand and IL-6 signaling inhibition.
Introduction
Osteoimmunology at a glance
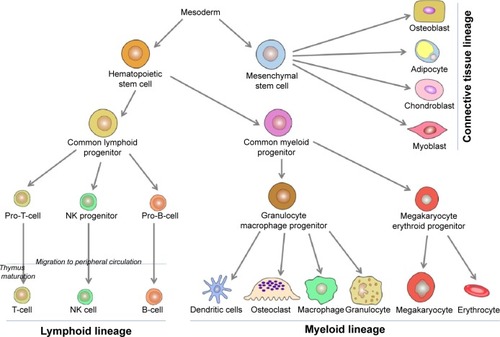
This narrative review of the literature presents first, data on osteoimmunology and osteoimmunological disorders, and second, discusses why Paget’s disease of bone should be considered as a potential osteoimmunological disease. Osteoimmunology is an emerging research area that is the result of the cross talk regarding the relationship between bones and the immune systems. Various cell types are involved in osteoimmunology processes, but most of them originate from the hematopoietic tissue. At the third week of human embryonic development, stem cell lineages are formed in the yolk sac.Citation1,Citation2 These lineages multiply asymmetrically, maintaining their original population and differentiating to other types of more specialized blood cells.Citation3 The immunological basic steps begin with the primary formation of hematopoietic stem cells lineage, which first appears in the yolk sac, followed by mesoderms of aorta, gonads, and nephrons (mesonephrons), and from there will migrate later to liver, spleen, and lymph nodes. Around the fourth month of fetal life, these hematopoietic stem cells migrate to bone marrow, and at the time of birth, bone marrow is responsible for all hematopoietic function.Citation4,Citation5 Likewise, bone marrow pluripotent stem cells – which are stimulated by growth factors – are divided into two types of multipotent progenitor stem cells: common lymphoid cells and common myeloid cells ().
Lymphoid cells
Common lymphoid cells are further divided into three cell types: committed pro-natural killer stem cells, pro-T stem cells, and pro-B stem cells. Pro-natural killer stem cells migrate to peripheral circulation, where they become natural killer cells. Natural killer cells differ from B-cells and T-cells by not having clusters of differentiation 3 (CD3) like T-cells, nor CD19 and CD20 like B-cells. Pro-T stem cells (naïve T-cells) migrate from bone marrow to peripheral circulation, mature in thymus gland, and then some return to peripheral circulation where they become mature T-cells. Some of these mature cells will become T helper (TH) naïve (containing markers CD3, CD4) lymphocytes or T cytotoxic (containing markers CD3, CD8) lymphocytes. TH naïve cells differentiate into TH1 cells and TH2 cells. TH1 cells when activated by interleukin (IL)-4 and IL-5 contribute to convert B-cells to plasma cells called active B-cells producing immunological antibodies. TH2, when activated by interferon-gamma (IFN-γ) and tumor necrosis factor-α (TNF-α), contribute to activate monocytes to be highly active macrophages, epithelioid cells (modified monocytes), and giant cells. IFN-γ receptor knockout mice showed exaggerated bone destruction in inflammatory arthritis in comparison with normal mice. IFN-γ induces TNF receptor-associated factor 6 (TRAF6) ubiquitination and degrades proteolytic TRAF6, ultimately leading to the inhibition of receptor activator of nuclear factor Kappa-B ligand (RANKL)-mediated osteoclastogenesis.Citation6,Citation7 Activities of TH2 cells constitute the humoral immunity, while activities of TH1 cells and T cytotoxic cells create the cellular immunity. Pro-B stem cells differentiate in turn to be mature B-cells in peripheral blood circulation.
Myeloid cells
Common myeloid progenitor cells differentiate into granulocyte/macrophage progenitor and megakaryocyte erythroid progenitor (MKEP) cells (). Granulocyte/macrophage progenitor cells are divided into monocytes and granulocytes. Later, monocytes migrate to some tissues, reside there, and change their name depending on the tissue, such as monocytes that migrate to inflammation sites are called macrophages, monocytes that migrate to skin are called Langerhans cells, and monocytes that migrate and reside in bone tissue, which will be differentiated into bone resorbing cells are called osteoclasts.Citation8,Citation9 Osteoclasts work mainly at the bone resorption activity controlling the bone turnover cycle.Citation10 Osteoclasts are large multinucleated cells of hematopoietic origin. They have the capability of removing organic and mineral components of bone. The macrophage lineages and the myeloid dendritic cell originate hematopoietically, and they are affected by the cytokines and produce many of them. On the other hand, osteoblasts originate from mesenchymal stems cells and play important roles in bone formation, osteoclasts differentiation, and hematopoietic cell growth and differentiation.Citation11,Citation12 The interaction or cross talk between osteoblast and osteoclasts play a central role in the osteoimmunological processes. Osteoblasts can control the osteoclastogenesis by two important cytokines; first the RANKL, which is a TNF member superfamily of proteins (). RANKL is a protein produced by Tumor Necrosis Factor ligand Super-Family member 11 (TNFSF11) gene. It has also been called TNF-related activation-induced cytokine or osteoprotegerin (OPG) ligand because it can be a ligand for osteoprotegerin decoy receptor. RANKL binds to RANK receptors, which normally are present at the pre-osteoclasts’ cell membrane. Its crucial role as a transmembrane protein synthesized by osteoblasts is to perform maturation, differentiation, and activation of osteoclasts.Citation13 Second, OPG is also a member of the TNF superfamily and plays a role of a decoy receptor of RANKL leading to inhibition of osteoclasts maturation, differentiation, and activation and then leading to osteoclast apoptosis (). So, the balance between RANKL and OPG can modulate the level of bone resorption.Citation14–Citation16 OPG works like a brake against the excessive bone resorption activity. A new inhibitory mechanism against OPG via autoantibodies has been revealed by studies of Riches et al. Indeed, they discovered autoantibodies against OPG in a man with celiac disease, severe osteoporosis, and high bone turnover.Citation17
Table 1 Main cytokines involved in osteoimmunology processes, cells of production, and main roles in osteoimmunology
Osteoimmunological cytokines
IL-1 is a very essential cytokine in osteoimmunological processes. The analysis of supernatants from phytohemagglutinin-stimulated peripheral blood monocytes in healthy humans suggested that IL-1 acts as the main stimulus of osteoclast-activating factor, which has a central role in osteoclastogenic activity. Subsequently, the same bone resorbing stimulating activity was found in TNF-α and IL-6. Indeed, IL-1, IL-6, and TNF-α increase the osteoclasts response to RANKL and consequently osteolysis (). Estrogen withdrawal after menopause has the same stimulating effect, increasing osteoclastic activity through IL-1, IL-6, and TNF-α effects.Citation18 Proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-17 () are also elevated in patients with rheumatoid arthritis, contributing to increased RANKL expression and subsequent osteolysis.Citation19 Schett et al have reviewed the important relation between autoimmunity and joint erosion in rheumatoid arthritis, revealing the presence of anti-citrullinated protein antibodies and anti-carbamylated protein antibodies in serum of the patients with rheumatoid arthritis. Molecular interaction between anti-citrullinated protein antibodies and the surface of osteoclast precursor cells via citrullinated vimentin induces differentiation and production of bone-resorbing osteoclasts, resulting in excessive bone resorption.Citation20 Vitamin D3, prostaglandin E2, parathyroid hormone, in addition to IL-1, IL-6, IL-11, and TNF-α, can also induce RANKL expression, leading to excessive osteoclastogenesis ().Citation21 Activated T-cells were also reported to regulate bone loss and activation of osteoclastogenesis in vitro through RANKL.Citation22 Contrariwise, TNF-stimulated gene 6 protein is an inflammation-induced protein that can inhibit osteoblastogenesis and osteoclast activation.Citation23 In addition, immunoreceptor tyrosine-based activation motif (ITAM) pathway may contribute to the relationship between immune system and bone as a co-stimulatory pathway in osteoclasts. ITAM-dependent receptors regulate myeloid-derived cells functions. Furthermore, ITAM-containing adapter proteins such as DNAX activation protein-12 and the Fc epsilon receptor I gamma chain (FCER1G) play an essential role in osteoclast differentiation. Suppression of calcineurin–nuclear factor of activated T-cells signaling can reduce the activity of ITAM pathway in the late stage of osteoclast differentiation, leading to the reduction of osteoclast differentiation and activity.Citation24,Citation25 Calcium signaling induces the calmodulin-dependent kinase pathway role in osteoclast formation and plays a crucial role in the autoamplification of the transcription factor nuclear factor of activated T-cells cytoplasmic-1. Further, activation of TRAF6 and c-Fos pathways by RANKL leads to autoamplification of nuclear factor of activated T-cells cytoplasmic-1 and enhances osteoclastogenesis.Citation21
Most frequent rheumatic osteoimmunological disorders and their related serum biomarkers
The most frequent rheumatic osteoimmunological disorders regroup bone metabolic diseases, such as osteoporosis and Paget’s disease of bone, systemic autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus and systemic sclerosis, and other rheumatic diseases including osteoarthritis and spondyloarthropathies, whereas periodontitis is frequently associated with systemic rheumatic conditions (). In almost all these disorders, serum levels of osteoimmunological biomarkers have been characterized in the literature (), and they can be combined to define a specific osteoimmunological pattern associated with a given rheumatic disease. For example, bone formation markers are usually increased in all these diseases, except in osteoporosis and rheumatoid arthritis (decreased level) and in systemic lupus erythematosus and systemic sclerosis (normal level). Bone resorption markers are also usually increased except for osteoarthritis and systemic lupus erythematosus (normal level). In addition, some proinflammatory cytokines may be of paramount importance at differentiating different rheumatic disorders. IL-17 and IL-6 levels are usually simultaneously increased in the same disorders, except for Paget’s disease of bone and systemic sclerosis; in both diseases, IL-6 is elevated but not IL-17. Finally, the pattern of IFN-γ serum levels in rheumatic diseases is very interesting: it is increased in almost all diseases, with the exception of osteoporosis and psoriatic arthritis (decreased level), and rheumatoid arthritis and ankylosing spondylitis (normal level). Overall, a combination of one serum biomarker of bone formation, one bone resorption biomarker, two proinflammatory cytokines such as IL-17 and IL-6 in addition to IFN-γ serum levels would be able to classify with a good sensitivity the most frequent rheumatic osteoimmunological disorders. Adding other already available biomarkers in clinical practice, such as autoantibodies, would increase the specificity of such a combination, and its clinical utility (ie, combination of markers for outcome/prognosis prediction and/or a pharmacogenomic test to guide the choice of any targeted biotherapy) may further be validated in prospective cohorts.
Table 2 Pattern of serum biomarkers in most frequent rheumatic osteoimmunological disorders
Paget’s disease of bone as a potential osteoimmunological disorder
Paget’s disease of bone
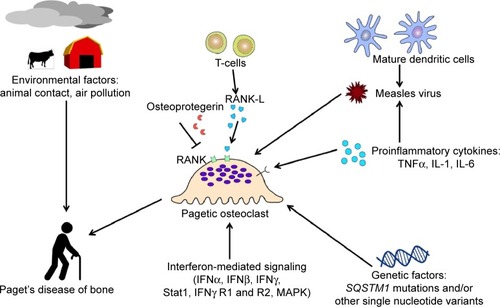
Paget’s disease of bone is the second most frequent metabolic bone disorder after osteoporosis,Citation26 where more than 3% of Caucasians older than 55 years are affected. This disorder is characterized by an excessive increased bone resorption by osteoclasts accompanied by aberrant osteoblastic bone formation. This aberrant bone remodeling causes fragile and weaker bones. To date, about 30 mutations in SQSTM1/p62 gene have been reported in familial forms and unrelated patients with Paget’s disease of bone. Furthermore, several common single nucleotide polymorphisms have been associated with Paget’s disease of bone, in genome-wide association study, in particular in CSF1, OPTN, TNFRSF11A, PML, RIN3, and NUP205 genes.Citation26–Citation28 The consequences of these polymorphisms on osteoclast phenotype and activity are yet unknown.
SQSTM1/p62 role and importance of osteoclastogenesis in Paget’s disease
The SQSTM1/p62 protein anatomical structure has some important domains that regulate their essential functions such as Phox and Bem1p (PB1), ZZ, TRAF6 binding domain, LIR, KIR, and ubiquitin-associated (UBA) domains.Citation29 PB1 plays a role in adipogenesis by inhibiting ERK1, and it also activates NF-κB pathway through interaction with PKCζ. ZZ domain activates NF-κB through the interaction with receptor interacting protein. Interaction of TRAF6 with the TRAF6 binding domain can activate NF-κB pathway. SQSTM1/p62 can activate autophagy by interaction of LC3 with LIR domain, and autophagy can be inhibited by mTOR. The UBA domain of SQSTM1/p62 has a very important role for this protein function; it interacts non-covalently with ubiquitin protein to perform post-transcriptional modifications and degradation by 26S multisubunit protease or by autophagy. The UBA domain also has an important role in induction and activation of some transcription factors such as NF-kB. In osteoclasts, NF-kB–RANK signaling pathway is very important for osteoclastogenesis. With impairment of UBA functions, ubiquitin protein cannot interact with its domain in SQSTM1/p62 disrupting the autophagy and NF-kB signaling pathways, and consequently, osteoclastogenesis.Citation26,Citation30,Citation31 The KIR domain of SQSTM1/p62 plays a role in oxidative stress with Keap1 (cysteine-rich protein) that has antioxidant elements such as antioxidant response elements/electrophile response element in Nrf2 pathway. Keap1-Nrf2 has a cytoprotective action against oxidative stress, and Keap1 can be downregulated by SQSTM1/p62.Citation32 Autophagy dysfunction may result as a consequence of SQSTM1 gene mutations and proteasomal pathway impairment. Ubiquitinated proteins are usually directed by SQSTM1/p62 protein to autophagosome. After binding to Atg8/LC3 at the surface of the autophagosome, they aggregate into polyubiquitinated non-functional proteins within the autophagosome in the autophagosome. Mutation in SQSTM1/p62 can induce abnormal autophagy process, leading to the accumulation of aggregated ubiquitinated proteins, which stimulate osteoclastogenesis by triggering of NF-kB pathway, and may contribute to Paget’s disease of bone.Citation33 Athanasiou’s group reported very interesting data on pathogenic excessive osteoclastogenesis in Paget’s disease of bone.Citation34 Neale et al found that macrophage-colony stimulating factor and IL-6 induce osteoclast formation and bone resorption in Paget’s disease. Furthermore, elevated macrophage-colony stimulating factor in the serum may correlate with disease activity in patients with Paget’s disease.Citation34 Kudo et al have also shown that IL-6 and IL-11 can enhance osteoclast formation and bone resorption through RANKL-independent mechanism. Then, the role of dexamethasone in osteoclastogenesis was suggested.Citation35 Indeed, dexamethasone can enhance proliferation and differentiation of human osteoclast precursors and suppress the bone resorption by mature osteoclasts.Citation36
Role of Optineurin in Paget’s disease of bone
Mutations of Optineurin (OPTN) gene have been reported in glaucoma and amyotrophic lateral sclerosis, as well as common genetic variants; in particular, the intronic variant rs1561570 was found to be associated with Paget’s disease of bone.Citation37 The OPTN gene encodes a 67 kDa cytosolic protein that consists of 577 amino acids. The importance of OPTN was first raised after discovering disease-causing mutations in primary open-angle glaucoma.Citation38 In addition, OPTN may be involved in neurofibrillary tangles and dystrophic neuritis that leads to Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, Creutzfeldt–Jakob disease, and glial cytoplasmic inclusions that lead to a rare neurological disorder called multiple system atropy.Citation39 Although the exact role of OPTN in Paget’s disease of bone pathogenesis is unknown yet, interaction between OPTN and TANK-binding kinase 1 may suggest a connection with the immune system. Indeed, TANK-binding kinase 1 is a TNF-α-activated protein kinase, which may be activated by viral double-stranded DNA or by lipopolysaccharide. On the other hand, induction of IFN-β due to RNA virus infection can be suppressed by OPTN.Citation39–Citation41
Environmental factors
Environmental factors may also contribute to Paget’s disease of bone.Citation26 Although controversial in the literature, several studies have found a relationship between viral infections and excessive enhanced osteoclasts activity.Citation42 Inclusion bodies contained in osteoclasts were reported to be similar to Paramyxoviral nucleocapsids. Measles virus, respiratory syncytial virus, and canine distemper virus may play a role in Paget’s disease of bone.Citation43 The expression of measles virus nucleocapsid protein (MVNP) in osteoclasts was reported to lead to the formation of pagetic-like osteoclasts. MVNP is known to increase the production of IL-6 that in turn leads to increase the production of TAFII-17 and increase the sensitivity of osteoclasts to 1,25-(OH)2D3. The pagetic phenotype of osteoclast is characterized by hypermultinucleation and hypersensitivity to 1,25-(OH)2D3. NF-kB signaling can be increased in cells by increasing the production of IL-6 and IL-1.Citation44
Paget’s disease as a potential osteoimmunological disorder
Paget’s disease of bone should be considered as a potential osteoimmunological disorder for several reasons. First, the RANKL-NF-κB signaling has a major role in pagetic osteoclast differentiation and activation, and the cytokine profile observed in this disease is very similar to those observed in other osteoimmunological diseases (). However, the exact role of the immune system in Paget’s disease of bone is not very well understood, except for cellular responses to MNVP. Second, dendritic cells may also play a role in the pathogenesis of Paget’s disease of bone.Citation45 Immature myeloid dendritic cells express CDw150, a signaling lymphocyte activation molecule acting as a receptor for measles virus. Dendritic cells matured by stimulation of Toll-like receptors 2 and 4 will overexpress CDw150 up to fivefold. Then, human dendritic cells may increase the expression of measles virus, the latter contributing to Paget’s disease of bone ().Citation46 In addition, Nagy et al were the first authors to show in the literature a remarkable increase in IFN-mediated signaling in Paget’s disease of bone. Increasing expression of IFN-α, IFN-β, and IFN-γ messenger (m)RNA, STAT-1, IFN-γ receptors 1 and 2, and mitogen-activated protein kinase were found in monocytes and lymphocytes from patients with Paget’s disease in comparison with healthy controls, suggesting a possible post-viral reaction in Paget’s disease of bone.Citation47
Main osteoimmunological cytokines as therapeutic targets
A large majority of the already known osteoimmunological cytokines or their receptors have been targeted by monoclonal antibodies, humanized or not, and they are now indicated in several rheumatic disorders, mostly in rheumatoid arthritis (). The treatment of Paget’s disease of bone mostly relies on bisphosphonates to control disease activity, and no monoclonal antibodies have been investigated in this indication yet. But as a potential osteoimmunological disorder, the treatment of Paget’s disease of bone may also benefit from progresses in targeted therapies. For instance, denosumab, a monoclonal antibody inhibiting RANKL, could be relevant to treat Paget’s disease of bone as it prevents the osteoclastogenesis and promotes the apoptosis of mature osteoclasts. A case report has now been published on using denosumab in a patient with Paget’s disease of bone and impaired renal function, which contraindicated the use of bisphosphonates. In this case, the authors reported that denosumab 60 mg subcutaneously administrated at 0 month, 6 months, 9 months, 12 months, and 15 months has rapidly decreased the activity of Paget’s disease of bone as measured by biochemical markers and bone scan.Citation48 It is worth mentioning that IL-6 was found to be elevated in pagetic osteoclasts in bone marrow and in peripheral blood of patients with Paget’s disease of bone.Citation32 However, some other studies by Neale et al found low serum levels of IL-1 beta, IL-6, and TNF-α in 13 patients with Paget’s disease of bone in comparison with eight healthy controls.Citation34 As the IL-6 signaling is induced by MVNP, inhibition of IL-6 signaling should inhibit the development of pageitc osteoclasts.Citation49 Then, considering the high levels of IL-6 that usually characterize Paget’s disease of the bone, inhibiting the IL-6 signaling by drugs should also be considered as a future therapeutic avenue that is as yet unexplored.Citation50
Table 3 Overview of the main clinical trials or case reports in which a cytokine or its receptor, involved in osteoimmunological process related to a rheumatic or musculoskeletal disorder, was targeted
Conclusion
In conclusion, Paget’s disease of bone should be considered as a new addition to the large family of osteoimmunological disorders. The cytokine profiles observed in this disease are also very similar to those observed in other osteoimmunological disorders that should probably be classified accordingly. The treatment of Paget’s disease of bone may also benefit from progresses in osteoimmunology-targeted therapies, in particular, RANKL and IL-6 signaling inhibition.
Acknowledgments
MSN was supported in part by an MSc student award from the Department of Medicine (Université Laval). NA is supported by a postdoctoral award from The Arthritis Society. LM is supported by a career award from the FRQ-S. The authors thank Mr Thomas Pornin for technical help with and . We acknowledge fundings from the Canadian Institute of Health Research (Catalyst grant: environments, genes and chronic diseases), the Fondation du CHU de Québec, the Canadian Foundation for Innovation, the FRQ-S, the Laval University, and the CHU de Québec Research Centre.
LM declares one grant per year for a congress attendance from Amgen Canada and in kind contribution for bone biochemical markers’ detection kits by Roche Diagnostics Canada. JPB declares research grants and/or consulting or speaking fees from Amgen Inc., Eli Lilly, Novartis, Radius.
Disclosure
The authors report no conflicts of interest in this work.
References
- TavianMPeaultBEmbryonic development of the human hematopoietic systemInt J Dev Biol2005492–324325015906238
- Regenerative MedicineIn Stem Cell Information [World Wide Web Site] Chapter 5Bethesda, MDNational Institutes of Health, U.S. Department of Health and Human Services2011 [cited Monday, May 18, 2015]. Available from: http://stemcells.nih.gov/info/scireport/Pages/2006report.aspxAccessed February 11, 2015
- JonesDLWagersAJNo place like home: anatomy and function of the stem cell nicheNat Rev Mol Cell Biol200891112118097443
- OrkinSHDevelopment of the hematopoietic systemCurr Opin Genet Dev1996655976028939717
- OrkinSHZonLIHematopoiesis and stem cells: plasticity versus developmental heterogeneityNat Immunol20023432332811919568
- JonesDHKongYYPenningerJMRole of RANKL and RANK in bone loss and arthritisAnn Rheum Dis200261suppl 2ii32ii3912379618
- ZhaoBIvashkivLBNegative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressorsArthritis Res Ther201113423421861861
- YinTLiLThe stem cell niches in boneJ Clin Invest200611651195120116670760
- ImaiYYounMYInoueKTakadaIKouzmenkoAKatoSNuclear receptors in bone physiology and diseasesPhysiol Rev201393248152323589826
- HadjidakisDJAndroulakisIIBone remodelingAnn N Y Acad Sci2006109238539617308163
- LorenzoJChoiYOsteoimmunologyImmunol Rev20052085616313336
- Bar-ShavitZThe osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cellJ Cell Biochem200710251130113917955494
- WongBRRhoJArronJTRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cellsJ Biol Chem19972724025190251949312132
- WuytsWVan WesenbeeckLMorales-PigaAEvaluation of the role of RANK and OPG genes in Paget’s disease of boneBone200128110410711165949
- RoodmanGDGalsonDLThe Origins of Bone Cells: Osteoclasts Chapter 2, OsteoimmunologyVol Osteoimmunology1st ed2011
- LorenzoJHorowitzMChoiYSchettGTakayanagiHOsteoimmunology: Interactions of the Immune and Skeletal Systems Chapter 2Academic Press ElsevierLondon2010
- RichesPLMcRorieEFraserWDDetermannCvan’t HofRRalstonSHOsteoporosis associated with neutralizing autoantibodies against osteoprotegerinN Engl J Med2009361151459146519812402
- AndersonDMMaraskovskyEBillingsleyWLA homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell functionNature199739066561751799367155
- JungSMKimKWYangCWParkSHJuJHCytokine-mediated bone destruction in rheumatoid arthritisJ Immunol Res2014201426362525295284
- SchettGGravalleseEBone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatmentNat Rev Rheumatol201281165666423007741
- DanksLTakayanagiHImmunology and boneJ Biochem20131541293923750028
- KongYYFeigeUSarosiIActivated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligandNature1999402675930430910580503
- MahoneyDJMikeczKAliTTSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activationJ Biol Chem200828338259522596218586671
- ZawawiMSDharmapatniAACantleyMDMcHughKPHaynesDRCrottiTNRegulation of ITAM adaptor molecules and their receptors by inhibition of calcineurin-NFAT signalling during late stage osteoclast differentiationBiochem Biophys Res Commun2012427240440923000414
- HumphreyMBLanierLLNakamuraMCRole of ITAM-containing adapter proteins and their receptors in the immune system and boneImmunol Rev2005208506516313340
- NumanMSBrownJPMichouLImpact of air pollutants on oxidative stress in common autophagy-mediated aging diseasesInt J Environ Res Public Health20151222289230525690002
- MichouLColletCLaplancheJLOrcelPCornelisFGenetics of Paget’s disease of boneJoint Bone Spine200673324324816574459
- AlbaghaOMWaniSEViscontiMRGenetic Determinants of Paget’s Disease (GDPD) ConsortiumGenome-wide association identifies three new susceptibility loci for Paget’s disease of boneNat Genet201143768568921623375
- ReaSLWalshJPLayfieldRRatajczakTXuJNew insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget’s disease of boneEndocr Rev201334450152423612225
- LayfieldRCianiBRalstonSHStructural and functional studies of mutations affecting the UBA domain of SQSTM1 (p62) which cause Paget’s disease of boneBiochem Soc Trans200432pt 572873015493999
- GoodeALayfieldRRecent advances in understanding the molecular basis of Paget disease of boneJ Clin Pathol201063319920319858527
- CoppleIMListerAObengADPhysical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathwayJ Biol Chem201028522167821678820378532
- MathewRKarpCMBeaudoinBAutophagy suppresses tumorigenesis through elimination of p62Cell200913761062107519524509
- NealeSDSchulzeESmithRAthanasouNAThe influence of serum cytokines and growth factors on osteoclast formation in Paget’s diseaseQJM200295423324011937650
- KudoOSabokbarAPocockAItonagaIFujikawaYAthanasouNAInterleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanismBone20033211712584029
- HirayamaTSabokbarAAthanasouNAEffect of corticosteroids on human osteoclast formation and activityJ Endocrinol2002175115516312379499
- AlbaghaOMViscontiMRAlonsoNGenome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of boneNat Genet201042652052420436471
- RezaieTChildAHitchingsRAdult-onset primary open-angle glaucoma caused by mutations in optineurinScience200229555571077107911834836
- YingHYueBYCellular and molecular biology of optineurinInt Rev Cell Mol Biol201229422322364875
- MankouriJFragkoudisRRichardsKHOptineurin negatively regulates the induction of IFNβ in response to RNA virus infectionPLoS Pathog201062e100077820174559
- MortonSHessonLPeggieMCohenPEnhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucomaFEBS Lett20085826997100218307994
- SingerFRThe etiology of Paget’s disease of bone: viral and genetic interactionsCell Metab20111315621195342
- RalstonSHLayfieldRPathogenesis of Paget disease of boneCalcif Tissue Int20129129711322543925
- EhrlichLARoodmanGDThe role of immune cells and inflammatory cytokines in Paget’s disease and multiple myelomaImmunol Rev200520825226616313353
- TakayanagiHOsteoimmunology: shared mechanisms and crosstalk between the immune and bone systemsNat Rev Immunol20077429230417380158
- MurabayashiNKurita-TaniguchiMAyataMMatsumotoMOguraHSeyaTSusceptibility of human dendritic cells (DCs) to measles virus (MV) depends on their activation stages in conjunction with the level of CDw150: role of toll stimulators in DC maturation and MV amplificationMicrobes Infect20024878579412270725
- NagyZBGergelyPDonathJBorgulyaGCsanadMPoorGGene expression profiling in Paget’s disease of bone: upregulation of interferon signaling pathways in pagetic monocytes and lymphocytesJ Bone Miner Res200823225325918197754
- SchwarzPRasmussenAQKvistTMAndersenUBJorgensenNRPaget’s disease of the bone after treatment with Denosumab: a case reportBone20125051023102522586699
- GalsonDLRoodmanGDPathobiology of Paget’s disease of boneJ Bone Metab2014212859825025000
- MichouLBrownJPEmerging strategies and therapies for treatment of Paget’s disease of boneDrug Des Devel Ther20115225239
- MillsBGFraustoACytokines expressed in multinucleated cells: Paget’s disease and giant cell tumors versus normal boneCalcif Tissue Int199761116219192505
- Francisco-CruzAAguilar-SantelisesMRamos-EspinosaOGranulocyte-macrophage colony-stimulating factor: not just another haematopoietic growth factorMed Oncol201431177424264600
- WangYNishidaSElaliehHZLongRKHalloranBPBikleDDRole of IGF-I signaling in regulating osteoclastogenesisJ Bone Miner Res20062191350135816939393
- SchoenbornJRWilsonCBRegulation of interferon-gamma during innate and adaptive immune responsesAdv Immunol2007964110117981204
- SokolCLBartonGMFarrAGMedzhitovRA mechanism for the initiation of allergen-induced T helper type 2 responsesNat Immunol20089331031818300366
- GarlisiCGFalconeABillahMMEganRWUmlandSPT cells are the predominant source of interleukin-5 but not interleukin-4 mRNA expression in the lungs of antigen-challenged allergic miceAm J Respir Cell Mol Biol19961534204288810648
- GarciaGTailleCLavenezianaPBourdinAChanezPHumbertMAnti-interleukin-5 therapy in severe asthmaEur Respir Rev20132212925125723997052
- ZhangQChenBYanFInterleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseasesBiomed Res Int2014201428483624696846
- HymowitzSGFilvaroffEHYinJPIL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor bindingEMBO J200120195332534111574464
- StanleyERBergKLEinsteinDBBiology and action of colony – stimulating factor-1Mol Reprod Dev19974614108981357
- UdagawaNTakahashiNJimiEOsteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligandBone199925551752310574571
- ChamouxEHoudeNL’ErigerKRouxSOsteoprotegerin decreases human osteoclast apoptosis by inhibiting the TRAIL pathwayJ Cell Physiol2008216253654218338379
- ItonagaISabokbarASunSGTransforming growth factor-beta induces osteoclast formation in the absence of RANKLBone2004341576414751563
- Online Mendelian Inheritance in Man® (OMIM®)Tumor Necrosis Factor (TNF), OMIM 1911602015 Available from: http://www.omim.org/entry/191160Accessed March 3, 2015
- ZhangJFuQRenZChanges of serum cytokines-related Th1/Th2/Th17 concentration in patients with postmenopausal osteoporosisGynecol Endocrinol201531318390 Epub 2014 Nov 1125384921
- Al-ZahraniMKElnasiehAMAleneziFMA 3-month oral vitamin D supplementation marginally improves diastolic blood pressure in Saudi patients with type 2 diabetes mellitusInt J Clin Exp Med20147125421542825664051
- AlvarezLPerisPGuañabensNLong-term biochemical response after bisphosphonate therapy in Paget’s disease of bone. Proposed intervals for monitoring treatmentRheumatology200443786987415054158
- MarshallMJEvansSFSharpCAPowellDEMcCarthyHSDavieMWIncreased circulating dickkopf-1 in Paget’s disease of boneClin Biochem20094210–1196596919389391
- PolyzosSAAnastasilakisADEfstathiadouZThe effect of zoledronic acid on serum dickkopf-1, osteoprotegerin, and RANKL in patients with Paget’s disease of boneHorm Metab Res2009411184685019670154
- Werner de CastroGRBussZDa RosaJSFrodeTSInflammatory cytokines in Paget’s disease of boneInt Immunopharmacol201418227728124355795
- YavropoulouMPvan LieropAHHamdyNARizzoliRPapapoulosSESerum sclerostin levels in Paget’s disease and prostate cancer with bone metastases with a wide range of bone turnoverBone201251115315722579776
- RossiniMViapianaOAdamiSIn patients with rheumatoid arthritis, dickkopf-1 serum levels are correlated with parathyroid hormone, bone erosions and bone mineral densityClin Exp Rheumatol2015331778325438096
- HongQXuJXuSLianLZhangMDingCAssociations between serum 25-hydroxyvitamin D and disease activity, inflammatory cytokines and bone loss in patients with rheumatoid arthritisRheumatology201453111994200124907153
- CortetBCottenABoutryNPercutaneous vertebroplasty in patients with osteolytic metastases or multiple myelomaRev Rhum1997643177183
- Al-AwadhiAOlusiSAl-ZaidNPrabhaKSerum concentrations of interleukin 6, osteocalcin, intact parathyroid hormone, and markers of bone resorption in patients with rheumatoid arthritisJ Rheumatol19992661250125610381038
- SerioloBFerrettiVSulliACarattoEFascioloDCutoloMSerum osteocalcin levels in premenopausal rheumatoid arthritis patientsAnn N Y Acad Sci200296650250712114311
- WongPKYoungLVaileJHTelopeptides as markers of bone turnover in rheumatoid arthritis and osteoarthritisIntern Med J2004349–1053954415482266
- LongLLiuYWangSDickkopf-1 as potential biomarker to evaluate bone erosion in systemic lupus erythematosusJ Clin Immunol201030566967520589421
- HeardBJRosvoldJMFritzlerMJEl-GabalawyHWileyJPKrawetzRJA computational method to differentiate normal individuals, osteoarthritis and rheumatoid arthritis patients using serum biomarkersJ R Soc Interface201411972014042824920114
- JiHILeeSHSongRSerum level of osteopontin as an inflammatory marker does not indicate disease activity or responsiveness to therapeutic treatments in patients with rheumatoid arthritisClin Rheumatol201433339740223995733
- KorczowskaIOlewicz-GawlikAHrycajPLackiJThe effect of long-term glucocorticoids on bone metabolism in systemic lupus erythematosus patients: the prevalence of its anti-inflammatory action upon bone resorptionYale J Biol Med2003762455415369631
- RulloOJWooJMParsaMFPlasma levels of osteopontin identify patients at risk for organ damage in systemic lupus erythematosusArthritis Res Ther2013151R1823343383
- Lyn-CookBDXieCOatesJIncreased expression of toll-like receptors (TLRs) 7 and 9 and other cytokines in systemic lupus erythematosus (SLE) patients: ethnic differences and potential new targets for therapeutic drugsMol Immunol2014611384324865418
- VincentFBNorthcottMHoiAMackayFMorandEFClinical associations of serum interleukin-17 in systemic lupus erythematosusArthritis Res Ther201315R9723968496
- La MontagnaGBaruffoAAbbadessaSMajaLTirriREvidence for bone resorption in systemic sclerosisJ Rheumatol19952247977997791196
- IštokRCzirjákLLukáčJStančíkováMRovenskýJIncreased urinary pyridinoline cross-link compounds of collagen in patients with systemic sclerosis and Raynaud’s phenomenonRheumatology200140214014611257149
- AtteritanoMSorbaraSBagnatoGBone mineral density, bone turnover markers and fractures in patients with systemic sclerosis: a case control studyPLoS One201386e6699123818972
- AllanoreYBorderieDLemaréchalHCherruauBEkindjianOGKahanACorrelation of serum collagen I carboxyterminal telopeptide concentrations with cutaneous and pulmonary involvement in systemic sclerosisJ Rheumatol2003301687312508392
- MackoRFGelberACYoungBAIncreased circulating concentrations of the counteradhesive proteins SPARC and thrombospondin-1 in systemic sclerosis (scleroderma). Relationship to platelet and endothelial cell activationJ Rheumatol200229122565257012465153
- CastellinoGCoralliniFBortoluzziAThe tumour necrosis factor-related apoptosis-inducing ligand-osteoprotegerin system in limited systemic sclerosis: a new disease marker?Rheumatology20104961173117620299382
- DovioADataVCarignolaRCirculating osteoprotegerin and soluble RANK ligand in systemic sclerosisJ Rheumatol200835112206221318843778
- MaekawaTKomineMMurataSOhtsukiMPeritoneal loose body: a case report and comparison with encapsulated fat necrosisJ Dermatol201340121058105924330174
- AndersenGNNilssonKNagaevaORantapaa-DahlqvistSSandstromTMincheva-NilssonLCytokine mRNA profile of alveolar T lymphocytes and macrophages in patients with systemic sclerosis suggests a local Tr1 responseScand J Immunol201174327228121535076
- WuMSchneiderDJMayesMDOsteopontin in systemic sclerosis and its role in dermal fibrosisJ Invest Dermatol201213261605161422402440
- Olewicz-GawlikADanczak-PazdrowskaAKuznar-KaminskaBInterleukin-17 and interleukin-23: importance in the pathogenesis of lung impairment in patients with systemic sclerosisInt J Rheum Dis201417666467024467649
- PehlivanYOnatAMCeylanNSerum leptin, resistin and TNF-alpha levels in patients with systemic sclerosis: the role of adipokines in sclerodermaInt J Rheum Dis201215437437922898217
- HasegawaMSatoSFujimotoMIhnHKikuchiKTakeharaKSerum levels of interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosisJ Rheumatol19982523083139489824
- KimWUMinSYChoMLElevated matrix metalloproteinase-9 in patients with systemic sclerosisArthritis Res Ther200571R71R7915642145
- PantsulaiaIKalichmanLKobylianskyEAssociation between radiographic hand osteoarthritis and RANKL, OPG and inflammatory markersOsteoarthr Cartil201018111448145320633673
- KummJTammALintropMDiagnostic and prognostic value of bone biomarkers in progressive knee osteoarthritis: a 6-year follow-up study in middle-aged subjectsOsteoarthr Cartil201321681582223523608
- DalbethNPoolBSmithTCirculating mediators of bone remodeling in psoriatic arthritis: implications for disordered osteoclastogenesis and bone erosionArthritis Res Ther2010124R16420796300
- GrisarJBerneckerPMAringerMAnkylosing spondylitis, psoriatic arthritis, and reactive arthritis show increased bone resorption, but differ with regard to bone formationJ Rheumatol20022971430143612136902
- AmitalHBarakVWinklerRERubinowAImpact of treatment with infliximab on serum cytokine profile of patients with rheumatoid and psoriatic arthritisAnn N Y Acad Sci20071110164966017911480
- TaylanASariIAkinciBBiomarkers and cytokines of bone turnover: extensive evaluation in a cohort of patients with ankylosing spondylitisBMC Musculoskelet Disord20121319123025387
- MarhofferWStrackeHMasoudIEvidence of impaired cartilage/bone turnover in patients with active ankylosing spondylitisAnn Rheum Dis19955475565597668898
- ToussirotERicard-BlumSDumoulinGCedozJWendlingDRelationship between urinary pyridinium cross-links, disease activity and disease subsets of ankylosing spondylitisRheumatology1999381212710334678
- MitraDElvinsDCollinsABiochemical markers of bone metabolism in mild ankylosing spondylitis and their relationship with bone mineral density and vertebral fracturesJ Rheumatol199926102201220410529140
- YilmazNOzaslanJBiochemical bone turnover markers in patients with ankylosing spondylitisClin Rheumatol2000192929810791618
- ParkMCChungSJParkYBLeeSKBone and cartilage turnover markers, bone mineral density, and radiographic damage in men with ankylosing spondylitisYonsei Med J200849228829418452267
- VosseDLandeweRGarneroPvan der HeijdeDvan der LindenSGeusensPAssociation of markers of bone-and cartilage-degradation with radiological changes at baseline and after 2 years follow-up in patients with ankylosing spondylitisRheumatology20084781219122218539620
- MeiYPanFGaoJIncreased serum IL-17 and IL-23 in the patient with ankylosing spondylitisClin Rheumatol201130226927321161669
- BalAUnluEBaharGAydogEEksiogluEYorganciogluRComparison of serum IL-1 beta, sIL-2R, IL-6, and TNF-alpha levels with disease activity parameters in ankylosing spondylitisClin Rheumatol200726221121516583185
- FranckHMeurerTHofbauerLCEvaluation of bone mineral density, hormones, biochemical markers of bone metabolism, and osteoprotegerin serum levels in patients with ankylosing spondylitisJ Rheumatol200431112236224115517638
- HouLTLiuCMLiuBYLinSJLiaoCSRossomandoEFInterleukin-1beta, clinical parameters and matched cellular-histopathologic changes of biopsied gingival tissue from periodontitis patientsJ Periodontal Res200338324725412753361
- TakeichiOHaberJKawaiTSmithDJMoroITaubmanMACytokine profiles of T-lymphocytes from gingival tissues with pathological pocketingJ Dent Res20007981548155511023273
- KawaiTMatsuyamaTHosokawaYB and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal diseaseAm J Pathol2006169398799816936272
- OhyamaHKato-KogoeNKuharaAThe involvement of IL-23 and the Th17 pathway in periodontitisJ Dent Res200988763363819605880
- MalhotraRGroverVKapoorAKapurRAlkaline phosphatase as a periodontal disease markerIndian J Dent Res201021453121187620
- MarcacciniAMNovaesABJrMeschiariCACirculating matrix metalloproteinase-8 (MMP-8) and MMP-9 are increased in chronic periodontal disease and decrease after non-surgical periodontal therapyClin Chim Acta20094091–211712219751716
- NakajimaTHondaTDomonHPeriodontitis-associated upregulation of systemic inflammatory mediator level may increase the risk of coronary heart diseaseJ Periodontal Res201045111612219602107
- DuartePMda RochaMSampaioESerum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot studyJ Periodontol20108171056106320192617
- LiuKMengHTangXElevated plasma calcifediol is associated with aggressive periodontitisJ Periodontol20098071114112019563291
- ZhangXMengHSunXElevation of vitamin D-binding protein levels in the plasma of patients with generalized aggressive periodontitisJ Periodontal Res2013481747922803589
- MiricescuDTotanACalenicBSalivary biomarkers: relationship between oxidative stress and alveolar bone loss in chronic periodontitisActa Odontol Scand2014721424723869629
- OzcakaONalbantsoyABicakciNKoseTBuduneliNPlasma levels of C-telopeptide pyridinoline cross-links of type I collagen and osteocalcin in chronic periodontitisInflammation201134320320820577791
- GursoyUKKönönenEPradhan-PalikhePSalivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitisJ Clin Periodontol201037648749320507371
- HansSMaliAMEstimation and comparison of osteopontin levels in plasma in subjects with healthy periodontium and generalized chronic periodontitis and its assessment after scaling and root planingJ Indian Soc Periodontol201216335435723162328
- Kone-PautIGaleottiCAnakinra for cryopyrin-associated periodic syndromeExpert Rev Clin Immunol201410171824308832
- CavalliGFranchiniSAielloPEfficacy and safety of biological agents in adult-onset Still’s diseaseScand J Rheumatol201516
- SayginCUzunaslanDHatemiGCurrently used biologic agents in the management of Behcet’s syndromeCurr Med Chem201522161976198525666786
- Highlights of prescribing information, Arcalyst [rilonacept]Regeneron Pharmaceuticals, Inc Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125249lbl.pdfAccessed October 20, 2014
- European Medicines Agency Docs2015 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001109/WC500031680.pdfAccessed February 28, 2015
- CantariniLLopalcoGCasoFEffectiveness and tuberculosis-related safety profile of interleukin-1 blocking agents in the management of Behcet’s diseaseAutoimmun Rev20151411925151975
- PRODUCT MONOGRAPH, ACTEMRA® [tocilizumab]2015 Available from: http://www.rochecanada.com/fmfiles/re7234008/Research/ClinicalTrialsForms/Products/ConsumerInformation/Monographsand-PublicAdvisories/Actemra/Actemra_PM_E.pdfAccessed February 28, 2015
- MPRIxekizumab vs Etanercept for Plaque Psoriasis: Phase 3 Results Announced2015 Available from: http://www.empr.com/ixekizumab-vs-etanercept-for-plaque-psoriasis-phase-3-results-announced/article/367354/Accessed February 28, 2015
- Novartis Pharmaceuticals Canada IncSecukinumab [AIN457]2015 Available from: http://www.novartis.ca/cs/www.novartis.ca-v2/downloads/en/News/Novartis_PR_Nov172014_EN.pdfAccessed February 28, 2015
- MPRBrodalumab Demonstrates Efficacy in Plaque Psoriasis Study2015 Available from: http://www.empr.com/ixekizumab-vs-etanercept-for-plaque-psoriasis-phase-3-results-announced/article/367354/Accessed February 28, 2015
- JANSSEN, STELARA®2015 Available from: http://www.janssen.ca/product/190Accessed February 28, 2015
- CundyTDavidsonJRutlandMDStewartCDePaoliAMRecombinant osteoprotegerin for juvenile Paget’s diseaseN Engl J Med2005353991892316135836
- PRODUCT MONOGRAPH PROLIA [denosumab]2015 Available from: https://www.amgen.ca/Prolia_PM.pdfAccessed February 28, 2015
- PRODUCT MONOGRAPH XGEVA [denosumab]2015 Available from: https://www.amgen.ca/Xgeva_PM.pdfAccessed February 28, 2015
- GrasemannCSchündelnMMHövelMEffects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget’s diseaseJ Clin Endocrinol Metab20139883121312623788687
- PolyzosSASinghellakisPNNaotDDenosumab treatment for juvenile Paget’s disease: results from two adult patients with osteoprotegerin deficiency (“Balkan” mutation in the TNFRSF11B gene)J Clin Endocrinol Metab201499370370724433001
- PRODUCT MONOGRAPH ENBREL [etanercept]2015 Available from: https://www.amgen.ca/Enbrel_PM.pdfAccessed February 28, 2015
- PRODUCT MONOGRAPH REMICADE [infliximab]2015 Available from: http://www.mun.ca/pharmacy/aboutpharmacy/REMICADEPME.pdfAccessed February 28, 2015
- PRODUCT MONOGRAPH, HUMIRA® [adalimumab]2015 Available from: http://www.abbvie.ca/content/dam/abbviecorp/ca/english/docs/HUMIRA_PM_EN.pdfAccessed February 28, 2015
- PRODUCT MONOGRAPH SIMPONI [golimumab]2015 Available from: http://www.mun.ca/pharmacy/aboutpharmacy/SIM11182011-CPM.SNDS.pdfAccessed February 28, 2015
- PRODUCT MONOGRAPH, CIMZIA® [certolizumab pegol];2015 Available from: http://www.ucb-canada.ca/_up/ucbpharma_ca_en/documents/cimzia_pm_en_15jan2014.pdfAccessed February 28, 2015