Abstract
Background
The aim of this study was to evaluate the effects of a phosphodiesterase inhibitor pentoxifylline (PTX), electromagnetic fields (EMFs), and a mixture of both materials on bone fracture healing in a rat model.
Materials and methods
Eighty male Wistar rats were randomly divided into four groups: Group A, femur fracture model with no treatment; Group B, femur fracture model treated with PTX 50 mg/kg/day intraperitoneal injection; Group C, femur fracture model treated with EMF 1.5±0.2 Mt/50 Hz/6 hours/day; and Group D, femur fracture model treated with PTX 50 mg/kg/day intraperitoneal injection and EMF 1.5±0.2 Mt/50 Hz/6 hours/day.
Results
Bone fracture healing was significantly better in Group B and Group C compared to Group A (P<0.05), but Group D did not show better bone fracture healing than Group A (P>0.05).
Conclusion
It can be concluded that both a specific EMF and PTX had a positive effect on bone fracture healing but when used in combination, may not be beneficial.
Introduction
Successful treatment of maxillofacial fractures depends on reduction and fixation to restore the normal occlusion, function, and proper alignment of bones,Citation1 and the success of a wide variety of surgical procedures such as repairing jaw fractures, orthognathic surgery, and repairing bone defects also depends on the physiological process of bone fracture healing. Despite progressions in maxillofacial surgery, bone fractures do not always heal successfully.Citation2 Therefore, studies in recent years have researched different factors such as growth factors, the applied force values, drug injections, or electrical stimulation, which could be considered to be of benefit in more successful rapid bone remodeling.Citation3
Pentoxifylline (PTX) is a competitive non-selective phosphodiesterase inhibitor (PDEI),Citation4 which reduces inflammation, and enhances microcirculation, blood flow, and tissue oxygenation.Citation5 In addition, PTX improves red blood cell deformability and decreases the potential for platelet aggregation and thrombus formation.Citation6 In a previous study, daily injections of PTX have been shown to stimulate bone formation and to increase systemic bone mass in mice.Citation7 In another study, Labib and Farid suggested that PTX could be regarded as a valid approach in the management of osseointegration.Citation8 In addition, many studies have demonstrated the positive effects of PTX in healing of mandibular.Citation9,Citation10 The efficacy of PTX in treatment is essentially due to its potential to increase blood flow and tissue oxygenation with its hemorheologic effects.Citation11 Since a vascular response is a prerequisite for successful bone healing physiology,Citation12,Citation13 it was hypothesized that in the current research that PTX might also be beneficial for bone fracture healing.
A variety of non-invasive bone fracture treatment techniques have been used for biophysical stimulation to accelerate and finalize bone healing.Citation14 One of these methods is electromagnetic field (EMF) stimulation, a method which has been used in cases of bone fracture healing for over three decades.Citation15–Citation18 Although the US Food and Drug Administration have approved EMF therapy as a safe and effective method for treating osteoporosis and as therapy for bone non-union,Citation19,Citation20 there are still some controversial results.Citation21,Citation22 Despite a long history of clinical use of EMFs and research, there is still poor understanding of the underlying action mechanisms of EMFs and the optimal parameters for usage. However, all researchers have emphasized that in the treatment of various diseases, it is necessary to determine the appropriate frequency and level of EMFs to be able to create gold-standard treatment protocols.Citation23,Citation24
To obtain normal functioning of the bone fracture and its surrounding structures, the swift recovery to normal anatomy after reduction is crucial, and an increasing number of studies are researching this subject.Citation25 The purpose of the current study was to undertake histological evaluations of the effects of PTX as a peripheral vasodilator, EMF therapy as a biophysical stimulator, and a combination of both materials on the repair process of a femur fracture in a rat model.
Materials and methods
Study design
The study included 80 male Wistar albino rats (mean age, 12 weeks; weight 300±20 g) and was conducted at the Health Institution Research Centre, Dicle University, Diyarbakır, Turkey. All animals were acclimatized to laboratory conditions for 2 weeks before beginning the experiments. The animals were individually housed in flexiglass cages in a controlled environment (22°C±2°C; 12-hour light–dark cycle) with free access to drinking water and a diet of standard laboratory rat food pellets. The experimental protocol of the study was approved by the Animal Experiment Ethics Committee of Dicle University. The animals were maintained and used in accordance with the animal welfare act and guidance for the care and use of laboratory animals.Citation26 The rats were randomly divided into four groups:
Group A (control group), femur fracture model with no treatment (n=20);
Group B (PTX group), femur fracture model treated with PTX (50 mg/kg/day) intraperitoneal injection (n=20);
Group C (EMF group), femur fracture model treated with EMF (1.5±0.2 Mt/50 Hz/6 hours/day) (n=20); and
Group D (PTX + EMF group), femur fracture model treated with PTX (50 mg/kg/day) intraperitoneal injection and EMF (1.5±0.2 Mt/50 Hz/6 hours/day) (n=20).
Group A and Group C received a daily intraperitoneal injection in order to simulate any possible influence of psychical trauma or physical stress on bone healing caused by injection.
Chemicals
PTX (Trental®; 100 mg ampoules) was obtained from Sanofi-Aventis, Bridgewater, NJ, USA; cefazolin sodium (1 g vials) was purchased from Astellas Pharma Inc, Tokyo, Japan; ketamine chlorhydrate (10 mL) was acquired from Pfizer Inc, New York, NY, USA; and xylazine chlorhydrate (25 mL) was procured from Bayer AG, Leverkusen, Germany.
Rat femur fracture model and surgical procedure
Surgical procedures were performed under general anesthesia consisting of a combination of ketamine chlorhydrate (0.08 mL/100 g body weight) and xylazine chlorhydrate 2% (0.04 mL/100 g body weight). In the present study, the open surgery model of experimental fracture healing models was used. In this technique, the fracture is created with a hammer osteotomy, Gigli saw, or electric saw. This technique provides a transverse fracture line and in this respect, is more controlled than closed models.Citation27 However, in a previous study of different gap sizes, the callus geometry, tissue differentiation patterns, and fracture stiffness predicted by the model were similar to experimental observations for every analyzed situation.Citation28
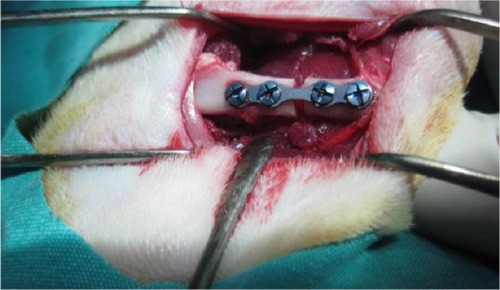
After positioning the right and left legs in flexion, a longitudinal cutaneous, subcutaneous, and periosteal incision was made 20–25 mm in length to reach the medial surface of the left femur. Exposing the medial surface of the femur with blunt dissection, the soft tissue was then retracted. Before making the bone incision, a four-holed microplate was adapted to the bone. After fixing the microplate, a bicortical bone incision was made with a 1 mm diameter fissure and a size 12 stainless steel dental burr (Meisinger, Nuess, Germany) on a low-speed handpiece, under constant sterile saline irrigation starting from the buccal cortical bone. A gap was left between the bone segments up to the thickness of the burr (1 mm) (). Then, the rigid stabilization of the fracture segments with the microplate and screws was checked again. Subcutaneous tissue layers were sutured with resorbable sutures, and the skin was sutured with silk. Upon completion of the surgical procedure, each animal received a single dose of 100 mg/kg cefazolin sodium antibiotic by intramuscular injection. Following surgery, ten rats per study group were killed at Day 21 and at Day 30 using high-dose ketamine.
EMF and PTX administration
Published data on the affirmative efficacy of EMF and PTX in rats are in agreement with the daily doses of 50 HzCitation29 and 50 mg/kg,Citation30 respectively, that animals received in the present study. To avoid any outside interference to the magnetic field (MF) during the application of the EMF, the experimental mechanism was placed inside an installed Faraday cage. For the EMF to reach the groups without hindrance, flexiglass cages were used. The EMF was produced by a pair of Helmholtz coils with a wrap-around number of 125, with internal diameter of 47 cm, and at distance intervals of 70 cm, placed vertically in a Faraday cage. Two identical Helmholtz coils inside the Faraday cage were attached to two identical power sources producing the same voltage and current (48 V, 40 A). Throughout the application of the EMF to the rats, measurements were regularly taken with a digital teslameter (FW Bell Corporation, Orlando, FL, USA) at 15 different points within the flexiglass cage. As a result of the measurements, the mean peak force was determined as 1.5±0.2 mT. After the pulse generator was switched off, the EMF measured in the cage environment, ie, the background, was found to be 1.5±0.2 mT, 50 Hz frequency and peak duration 1.3 ms.
The application of the EMF was started on postoperative Day 1, and was continued once a day every day at 1.5±0.2 mT intensity and at a frequency of 50 Hz for 6 hours. Throughout all the applications, an observer visited the study room regularly each hour, and the procedures were continued without interruption. The application of PTX was started on postoperative Day 1, was applied intraperitoneally at 50 mg/kg/day as a single dose of PTX (Trental® ampoule [100 mg]) once a day every day.
Histopathological evaluations
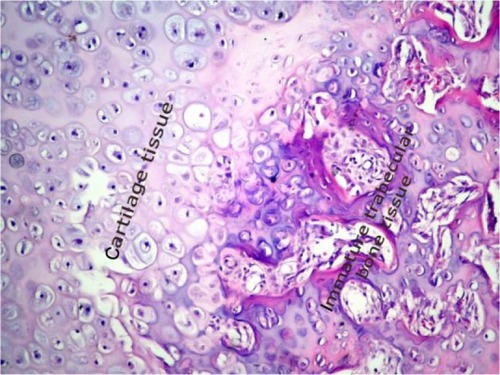
Bone regeneration and fibrotic healing were evaluated histopathologically. The rat femurs were fixed in 10% formaldehyde solution for 48 hours and were decalcified with 5% nitric acid. The specimens were washed with distilled water, dehydrated with increasing increments of concentrations of ethyl alcohol, cleaned in xylene, and were then embedded in paraffin. Beginning from the center of each specimen, sequential parts were cut parallel to the mid-sagittal defect using a microtome. Transverse sections of 4–5 μm thickness were prepared for each tibia defect and were stained with hematoxylin and eosin. The tissue samples were assessed by a pathologist using a light microscope. The pathologist was blinded to the treatment group that each specimen came from. A 10-point scaleCitation31 was used for evaluation of bone healing, where tissue was scored according to the histopathological findings in the healing zone, as follows:
1 point, only fibrous tissue;
2 points, predominantly fibrous tissue;
3 points, equal amounts of fibrous and cartilage tissue;
4 points, predominantly cartilage tissue with little fibrous tissue;
5 points, only cartilage tissue;
6 points, predominantly cartilage tissue with little immature bone;
7 points, equal amounts of cartilage and immature bone tissue;
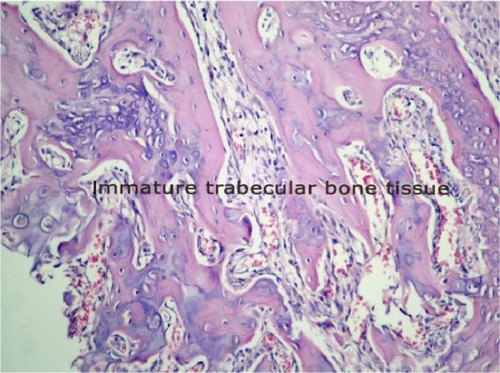
8 points, predominantly immature bone with little cartilage tissue;
9 points, healing with immature bone; and
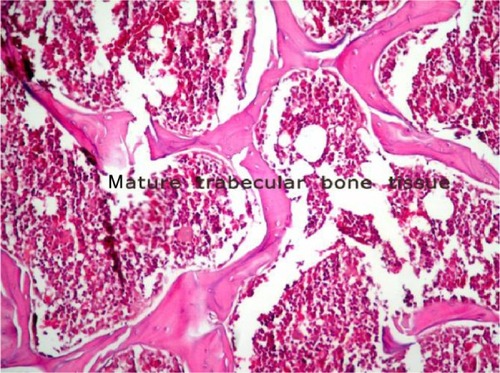
10 points, healing with mature bone.
Four slides were examined from each fracture. The mean histological score was calculated for each treatment group.
Statistical analysis
The data obtained for all the parameters to be evaluated were transferred to SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL, USA) program for statistical analysis. For the comparison of paired parameters for independent variables, the Mann–Whitney U-test was used as a two-way nonparametric test. In all tests, a value of P<0.05 was accepted as statistically significant.
Results
None of the rats died in any of the groups during the course of the experiment. No unwanted condition developed in any rat, and none of the rats was excluded from the study. All of the animals tolerated the procedure well and demonstrated good homeostasis and rapid recovery from anesthesia.
Histopathological results
Comparison of histopathological bone fracture healing scores among all groups at Day 21 and Day 30 is shown in and . In all groups ten of the rats were sacrificed at day 21 and other ten rats were sacrificed at day 30. In the histological examination, a larger increase was observed in the cartilage and bone volumes in the EMF and PTX groups than in the control groups (–). Using pairwise comparisons, there was no statistically significant difference between Group B (PTX) at Day 21 and Day 30 and Group C (EMF) at Day 21 and Day 30 (P>0.05). The level of bone healing in Group B (PTX) and Group C (EMF) was significantly higher than that of Group A (control) at Day 21 (P<0.05). There was also significantly better healing in Group B (PTX) and Group C (EMF) than in Group A (control) at Day 30 (P<0.05).
Table 1 Statistical analysis of bone healing at Day 21 and Day 30
Figure 2 Comparison of histopathological bone-healing scores among groups according to the Huo scale.
Abbreviations: EMF, electromagnetic field; PTX, pentoxifylline; MIX, both EMF and PTX treatment.
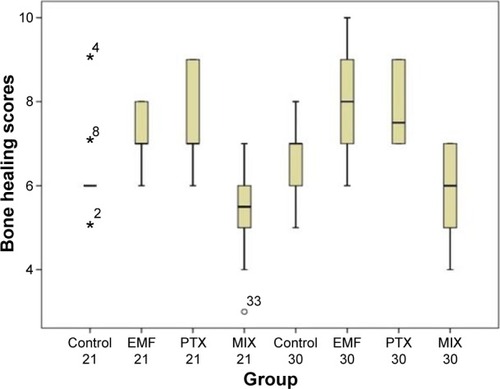
Figure 3 Histopathological sample of bone tissue. The sample shows mostly cartilage and a little newly formed, immature bone tissue.
Discussion
Swift bone fracture healing after traumas and surgical procedures is crucial in order to regain normal functioning of the bone and its surrounding structures.Citation32 There have been an increasing number of studies on this subject.Citation25,Citation32 Although the outcome of various clinical and surgical procedures in bone healing is affected by several factors, which may be patient-, defect-, or surgery-related variables,Citation32 blood flow and circulation play a significant role in the process of bone remodeling and the reparative regeneration of bone tissue.Citation33,Citation34 After the third day of the fracture in our current study, there was a significant rise in local blood flow, which is associated with vascular proliferation in the formation of fracture callus. Such changes in flow are likely to be secondary to locally controlled vasoreactivity of pre-existing vessels, as opposed to that of newly formed angiogenic vessels.Citation34
The present study aimed to evaluate the possible effects of PTX as a PDEI and EMFs on bone fracture healing. The efficacy of PTX and a specific EMF on the bone-healing stage of a femoral fracture model in rats was evaluated through histopathological analyses.
PDEIs provides increased blood flow via the nitric oxide (NO) pathway in the process of bone healing.Citation34 NO is a highly reactive free radical involved in inflammation, arthritis, and bone fracture healing. It is known that NO has a regulatory role in blood flow and perfusion pressure to isolate vascular beds in response to appropriate physiological stimuli.Citation35 Therefore, treatment modalities applied to improve the blood circulation may improve the outcomes of bone healing. Thus, the current study focused on the impact of PTX as a peripheral vasodilator on bone fracture healing. The results of this study have shown that PTX had a positive effect on bone fracture healing.
Studies that have examined the role of PDEIs involved in vascular response related to bone healing have reported results comparable to the findings of the present investigation. The administration of two PDEIs, tadalafil and vardenafil, have been shown to result in reduced bone mass, evidenced by in vitro cell culture and in vivo animal models,Citation36,Citation37 and the beneficial effects of the PDEI sildenafil on bone healing have been previously shown.Citation34,Citation37 In a study by Aydın et alCitation30 in which the same dose of PTX was applied as in the current study, it was suggested that the hematoma stage of fracture healing, which is the first phase, might be promoted by PTX. At Day 21, it was thought that the anti-inflammatory effects of PTX might have delayed fracture healing.Citation30 However, in the current study, in complete contrast, positive results of bone healing were obtained at Day 21. Despite the numerous studies on the subject, there is insufficient knowledge about the effects of PDEI use on bone healing metabolism.
For many years, EMFs have been researched as a stimulation method to enhance bone regeneration in maxillofacial surgery, including dental implants, distraction osteogenesis, and bone grafts.Citation21,Citation38,Citation39 The knowledge from recent studiesCitation40,Citation41 has conclusively demonstrated that the contribution of EMFs to the bone healing process is based on stimulating the expression of growth factors, such as vascular endothelial growth factor and bone morphogenetic protein. EMFs also induce angiogenesis, migration, proliferation, and differentiation of stem cells from the surrounding mesenchymal tissues into cartilage and bone-forming cells in an area of injury.Citation40,Citation41 The beneficial effects of the methods of electromagnetic stimulation have been widely documented. Selecting the parameters (intensity, frequency, etc) likely to have maximum benefits in EMF therapy has been especially complicated,Citation42 because EMFs may influence bone healing through a variety of pathways. In addition, EMF therapy has been reported to have benefits in fracture healing,Citation22 and healing of osteotomies,Citation43 pseudoarthrosis,Citation43 osteoporosis, and the deleterious effects of poor bone mineral density.Citation44
Most published studies suggest that EMFs have a positive effect on bone healing,Citation22,Citation43,Citation45–Citation48 while some studies have reported different results.Citation21,Citation49–Citation51 The scientific evidence regarding the efficacy and efficiency of EMFs is still controversial. However, in a study by Fredericks et alCitation38 it was claimed that low-frequency and low-intensity EMFs are powerful in the acceleration and finalization of bone fracture healing. Similarly, in the present study, it was observed that low-frequency and low-intensity EMF (1.5±0.2 mT, 50 Hz) significantly improved fracture healing in rats treated with EMF in the remodeling phase following treatment, compared with the control group. Controversial results regarding the effectiveness of EMFs on bone and other tissues may partly be due to the wide variations in type, frequency, duration, magnetic intensity, stimulation period length, and dose.Citation52 Differences in the results of the studies might also be related to the study design.
There are limited data about experimental studies that use the PTX and EMF combination for the treatment of bone fracture healing. In the current study, in all the groups to which combined therapy was applied, negative results were evident in bone fracture healing. These results may have arisen due to the unfavorable effects of MFs on NO metabolites. A study by Okano et alCitation53 demonstrated that MF decreased plasma levels of NO metabolites. From the results of this study, it could be considered that the regulatory role of NO on the blood flow was suppressed by the EMF, and therefore the bone fracture healing was not successful.
There are some limitations to this study. A single dose of PTX (50 mg/kg/day) and EMF (1.5±0.2 Mt/50 Hz/6 hours/day) was applied to the rats, and therefore there can be speculation as the effects on bone fracture healing of different dosages. However, due to ethical restrictions, a larger number of animals could not be used. Other limitations were that histopathological results could not be supported by biochemical and radiological findings; the monitoring period was relatively short; and there was no hemodynamic monitoring of the animals during the experiment. There is a need for further studies to clarify the mechanism of the effect of EMFs and PTX on bone remodeling.
Conclusion
It can be concluded that separate application of both PTX as a peripheral vasodilator and a specific EMF as a biophysical stimulator had a positive effect on bone fracture healing, but when used in combination, there may be no benefits. However, the efficacy of both treatments was modest, and the overall bone healing process could not be completely explained.
Acknowledgments
This study was supported by the Scientific Research Projects of Dicle University.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShokierHKhalifaGFawzyASallamMMExperimental use of autogenous bone grafts as an alternative method for bone plates in treatment of mandibular fractureAust J Basic Appl Sci2010414661472
- UçanMKoparalMAğaçayakSInfluence of caffeic acid phenethyl ester on bone healing in a rat modelJ Int Med Res2013411648165424065455
- CookJJSummersNJCookEAHealing in the new millennium: bone stimulators: an overview of where we’ve been and where we may be headingClin Podiatr Med Surg201532455925440417
- EssayanDMCyclic nucleotide phosphodiesterasesJ Allergy Clin Immunol200110867168011692087
- DelanianSPorcherRRudantJLefaixJLKinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosisJ Clin Oncol2005238570857916260695
- WardAClissoldSPPentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacyDrugs19873450973308412
- KinoshitaTKobayashiSEbaraSPhosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal miceBone20002781181711113392
- LabibGSFaridRMOsteogenic effect of locally applied Pentoxyfilline gel: in vitro and in vivo evaluationsDrug Deliv Epub2014220
- KahenasaNSungECNabiliVKellyJGarrettNNishimuraIResolution of pain and complete healing of mandibular osteoradionecrosis using pentoxifylline and tocopherol: a case reportOral Surg Oral Med Oral Pathol Oral Radiol2012113e18e2322668439
- DelanianSDepondtJLefaixJLMajor healing of refractory mandible osteoradionecrosis after treatment combining pentoxifylline and tocopherol: a phase II trialHead Neck20052711412315641107
- WardAClissoldSPPentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacyDrugs19873450973308412
- ColnotCLuCHuDHelmsJADistinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal developmentDev Biol2004269556915081357
- HausmanMSchafflerMMajeskaRPrevention of fracture healing in rats by an inhibitor of angiogenesisBone20012956056411728927
- ChaoEInoueNBiophysical stimulation of bone fracture repair, regeneration and remodellingEur Cell Mater20036728414722904
- NoltePAvan der KransAPatkaPJanssenIMRyabyJPAlbersGRLow-intensity pulsed ultrasound in the treatment of nonunionsJ Trauma20015169370311586161
- BassettCAMitchellSNGastonSRPulsing electromagnetic field treatment in ununited fractures and failed arthrodesesJAMA19822476236287054564
- MassariLCarusoGSollazzoVSettiSPulsed electromagnetic fields and low intensity pulsed ultrasound in bone tissueClin Cases Miner Bone Metab2009614915422461165
- WangYQinQHA theoretical study of bone remodelling under PEMF at cellular levelComput Methods Biomech Biomed Engin20121588589721604221
- ChaoEYInoueNKooTKKimYBiomechanical considerations of fracture treatment and bone quality maintenance in elderly patients and patients with osteoporosisClin Orthop Relat Res2004425122515292783
- FunkRHMonseesTOzkucurNElectromagnetic effects–From cell biology to medicineProg Histochem Cytochem20094317726419167986
- do NascimentoCIssaJPMelloASde Albuquerque JuniorRFEffect of electromagnetic field on bone regeneration around dental implants after immediate placement in the dog mandible: a pilot studyGerodontology201229e1249e125122612842
- ShiHFXiongJChenYXEarly application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: a prospective randomized controlled studyBMC Musculoskelet Disord2013143523331333
- RyabyJTClinical effects of electromagnetic and electric fields on fracture healingClin Orthop Relat Res1998355S205S2159917640
- TrockDHElectromagnetic fields and magnets: investigational treatment for musculoskeletal disordersRheum Dis Clin North Am200026516210680193
- AnituaESánchezMOriveGAndíaIThe potential impact of the preparation rich in growth factors (PRGF) in different medical fieldsBiomaterials2007284551456017659771
- ClarkJDGebhartGFGonderJCKeelingMEKohnDFThe 1996 guide for the care and use of laboratory animalsILAR journal1997381414811528046
- ÖztunaVOrtopedi ve Travmatolojide Kullanılan Deneysel Hayvan Modelleri (Temel ilkeler, Etik unsurlar ve Modeller) [Experimental Animal Models Used in Orthopedics and Traumatology (basic principles, ethical elements and Models)]2007 Cilt: 6 Sayi12Turkish Association of Orthopaedics and Traumatology Available from: http://www.totbid.org.tr/files/ONLIB/6_1-2/5.pdf. Turkish
- Gómez-BenitoMGarcía-AznarJKuiperJDoblaréMInfluence of fracture gap size on the pattern of long bone healing: a computational studyJ Theor Biol200523510511915833317
- SertCMustafaDDüzMZAkşenFKayaAThe preventive effect on bone loss of 50-Hz, 1-mT electromagnetic field in ovariectomized ratsJ Bone Miner Metab20022034534912434162
- AydınKŞahinVGürsuSMercanAŞDemirBYildirimTEffect of pentoxifylline on fracture healing: an experimental studyEklem Hastalik Cerrahisi20112216016522085352
- HuoMHTroianoNWPelkerRRGundbergCMFriedlaenderGEThe influence of ibuprofen on fracture repair: biomechanical, biochemical, histologic, and histomorphometric parameters in ratsJ Orthop Res199193833902010842
- AtalayYBozkurtMFGonulYThe effects of amlodipine and platelet rich plasma on bone healing in ratsDrug Des Devel Ther2015919731981
- RajkumarDSFaitelsonAVGudyrevOSDubrovinGMPokrovskiMVIvanovAVComparative evaluation of enalapril and losartan in pharmacological correction of experimental osteoporosis and fractures of its backgroundJ Osteoporos2013201332569323401845
- YamanFAtilganSGünesNPhosphodiesterase-5 inhibitors may facilitate bone defect recoveryEur Rev Med Pharmacol Sci2011151301130522195363
- CorbettSAHukkanenMBattenJMcCarthyIDPolakJMHughesSNitric oxide in fracture repair. Differential localisation, expression and activity of nitric oxide synthasesJ Bone Joint Surg Br19998153153710872379
- GongYXuCWangJRInhibition of phosphodiesterase 5 reduces bone mass by suppression of canonical Wnt signalingCell Death Dis20145e154425429621
- HistingTMarciniakKScheuerCSildenafil accelerates fracture healing in miceJ Orthop Res20112986787321246617
- FredericksDCPiehlDJBakerJTAbbottJNepolaJVEffects of pulsed electromagnetic field stimulation on distraction osteogenesis in the rabbit tibial leg lengthening modelJ Pediatr Orthop20032347848312826946
- PuricelliEDutraNBPonzoniDHistological evaluation of the influence of magnetic field application in autogenous bone grafts in ratsHead Face Med20095119134221
- HuangYHPolimeniGQahashMWikesjöUMBone morphogenetic proteins and osseointegration: current knowledge – future possibilitiesPeriodontol 200020084720622318412583
- FassinaLSainoEVisaiLElectromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto titanium fiber-mesh scaffoldsJ Biomed Mater Res A20088775075918200542
- ZhongCZhaoTXuZHeREffects of electromagnetic fields on bone regeneration in experimental and clinical studies: a review of the literatureChin Med J (Engl)201212536737222340573
- BoyetteMYHerrera-SotoJATreatment of delayed and nonunited fractures and osteotomies with pulsed electromagnetic field in children and adolescentsOrthopedics201235e1051e105522784899
- ElsisiHFMousaGSELdesokyMTElectromagnetic field versus circuit weight training on bone mineral density in elderly womenClin Interv Aging20151053954725834412
- JingDCaiJShenGThe preventive effects of pulsed electromagnetic fields on diabetic bone loss in streptozotocin-treated ratsOsteoporos Int2011221885189520976595
- HongJMKangKSYiHGKimSYChoDWElectromagnetically controllable osteoclast activityBone2014629910724556539
- CeccarelliGBloiseNMantelliMA comparative analysis of the in vitro effects of pulsed electromagnetic field treatment on osteogenic differentiation of two different mesenchymal cell lineagesBiores Open Access2013228329423914335
- EsmailMYSunLYuLXuHShiLZhangJEffects of PEMF and glucocorticoids on proliferation and differentiation of osteoblastsElectromagn Biol Med20123137538122676065
- BuzzáEPShibliJABarbeiroRHBarbosaJREffects of electromagnetic field on bone healing around commercially pure titanium surface: histologic and mechanical study in rabbitsImplant Dent20031218218712861888
- van der JagtOPvan der LindenJCWaarsingJHVerhaarJAWeinansHElectromagnetic fields do not affect bone micro-architecture in osteoporotic ratsBone Joint Res2014323023525015993
- JingDCaiJWuYModerate-intensity rotating magnetic fields do not affect bone quality and bone remodeling in hindlimb suspended ratsPLoS One20149e10295625047554
- AslanAKirdemirVKocakAInfluence of 1800 MHz GSM-like electromagnetic radiation exposure on fracture healingArch Med Res20144512513124508290
- OkanoHMasudaHOhkuboCDecreased plasma levels of nitric oxide metabolites, angiotensin II, and aldosterone in spontaneously hypertensive rats exposed to 5 mT static magnetic fieldBioelectromagnetics20052616117215768432