Abstract
This report describes a case of reversible topiramate-induced maculopathy in a 32-year-old female patient with IgG4-related disease. The patient presented with decreased vision associated with anterior uveitis and cystoid macula edema, which was unresponsive to oral and topical steroids. Following topiramate cessation, both cystoid macula edema and vision improved. The ocular side effects of topiramate and putative pharmacological mechanisms for topiramate-induced maculopathy in the context of IgG4-related disease are discussed. This report highlights that neurologists and ophthalmologists should be aware that patients presenting with topiramate-associated maculopathy should be advised to discontinue topiramate promptly to prevent irreversible loss of vision.
Keywords:
Introduction
Topiramate, a sulfamate substituted monosaccharide, is widely used in the treatment of epileptic syndromes and migraine.Citation1 It has also been used in the management of migraine, depression, and neuropathic pain, and to aid weight loss. However, topiramate therapy has also been associated with several adverse effects. Since 2001, a range of adverse ocular effects associated with topiramate therapy have been described related to acute glaucoma and acute myopia.Citation2 More recently, the effects of topiramate on macula function have been reported. Topiramate-induced anterior uveitis has also been described in association with hypopyon formation.Citation2
The objective of this report was to describe and discuss a case of reversible topiramate-induced maculopathy in a patient with IgG4-related disease.
Case report
A 32-year-old female of Egyptian origin presented with a 2-week history of decreased vision in the left eye. There was no past history of eye problems, injury, or previous eye surgery. She was under the care of a neurologist with a 2-year history of IgG4 systemic fibrosclerosis with mediastinal involvement. A chest X-ray taken after a nonresolving chest infection revealed a pulmonary infiltrate and the patient underwent thoracotomy and biopsy of the lesion. The diagnosis of IgG4-related disease was confirmed histologically. IgG4 serum level was elevated at 410 mg/dL. Inflammatory markers demonstrated a raised erythrocyte sedimentation rate. She had also completed empirical tuberculosis treatment. Her past medical history included epilepsy, chronic migrainous headache, low mood, lumbosacral back pain secondary to intervertebral disc protrusion at the level of L5/S1, and disturbed sleep with episodic sleep paralysis with possible restless leg syndrome. She had been consuming 75 mg of Topamax® (topiramate) for 12 months on the advice of her neurologist and had previously been prescribed azathioprine and rituximab. Current treatment included pregabalin, duloxetine, and oral prednisolone with alendronic acid weekly, and calcichew and lansoprazole daily. The presenting best corrected vision was 6/6 in the right eye and vision of 6/9 in the left eye. Clinical examination showed bilateral anterior uveitis with bilateral cystoid macular edema. Intraocular pressures were within normal limits with deep anterior chambers. Pupils were equal and reactive with no relative afferent pupillary defect. Color vision was decreased in the left eye. Dilated fundal examination revealed cystoid macula edema, optic discs were normal, and retinal vasculature was normal. No vitritis was present. Magnetic resonance imaging showed normal intracranial and orbital appearances. Systemic workup demonstrated increased levels of inflammatory markers: erythrocyte sedimentation rate and C reactive protein. Antinuclear antibody, rheumatoid factor, anti-neutrophil cytoplasmic antibody, angiotensin converting enzyme, and syphilis testing were negative.
The patient was initially treated with dexamethasone 0.1% qds to both eyes. Vision subsequently deteriorated to 6/13 in the right eye and 6/38 in the left eye over the next few weeks. Spectral domain optical coherence tomography (OCT) (Cirrus, Carl Zeiss Meditec, Dublin, CA, USA) revealed bilateral intraretinal fluid cysts. Fundus fluoroscein and indocynanine green angiography showed no vasculitis or vascular leakage at the macula. The patient was known to be a long-standing myopic with a refraction of −8.00 D sphere bilaterally. Goldmann visual field testing showed bilateral central scotomas.
Electrodiagnostic testing showed normal bilateral pattern and flash visually evoked potentials. Pattern electroretinograms (ERGs) were moderately subnormal in both eyes. Multifocal ERGs showed reduced bilateral responses. The findings suggested bilateral central macular dysfunction, with no electrophysiological evidence of generalized retinal dysfunction or optic nerve dysfunction. The findings were consistent with bilateral macular edema ().
Figure 1 OCT macular images of right (A) and left (B) eyes in a patient treated with topiramate.
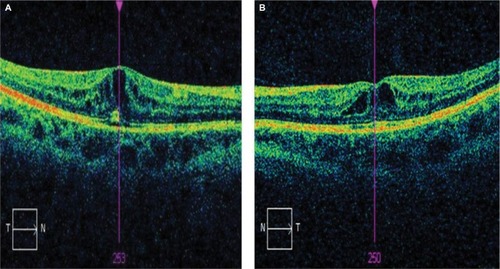
After neurology consultation, the patient was advised to stop taking topiramate and subsequently OCT macular changes were completely resolved ().
Figure 2 OCT macular images of right (A) and left (B) eyes after cessation of topiramate treatment.
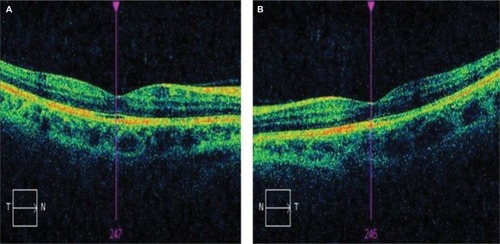
Due to weight gain after cessation of therapy, the patient recommenced topiramate treatment with recurrence of maculopathy. Vision decreased to 6/24 on the right and 6/19 on the left over a 6-month period. She was advised again to cease topiramate and subsequently macular changes resolved completely.
The resolution of the symptoms and macular signs in this patient after cessation of therapy strongly suggest that topiramate was the relevant etiological factor. This patient’s maculopathy was reversible on cessation of topiramate.
The patient provided written consent for publication. The patient was not enrolled in a clinical trial and did not receive any experimental treatment, hence ethical approval was not sought from the institutional review board.
Discussion
Topiramate is a monosaccharide related to fructose, containing a substituted sulfamate moiety, first synthesized in 1979.Citation3 The sulfamate moiety is considered an essential component for anticonvulsant activity of the drug. The efficacy of topiramate has been established as monotherapy or adjunctive therapy in the treatment of adult and pediatric patients with generalized tonic-clonic seizures, partial seizures with or without generalized seizures, and seizures associated with Lennox–Gastaut syndrome.Citation1 The safety of topiramate in epilepsy was assessed in a prospective add-on trial of topiramate therapy to standard therapy in patients with epilepsy.Citation4 A total of 106 patients were studied.Citation4 The drug was well tolerated in >65% of cases for side effects such as psychosis, giddiness, and anorexia. Mild side effects were seen in <35% of cases.Citation4
The most common reported ocular side effects of topiramate are the induction of acute myopia and secondary angle closure glaucoma,Citation5 which is reported to have occurred within the first month of commencing topiramate and is associated with increase in dosage from 25 to 50 mg/day.Citation5 The patient may report acute onset of blurred vision with a red painful eye. On examination, the intraocular pressure is found to be raised, along with increased myopia, choroidal effusion, and an anterior displacement of the iris-lens diaphragm associated with narrowing of the anterior chamber angle, which is the space between the iris and cornea. Carbonic anhydrase inhibition by topiramate is not considered to be the pharmacological mechanism for these effects.Citation5 Carbonic anhydrase inhibitors are well-established therapeutic agents to treat glaucoma. It is likely that the effect of topiramate on the function of aquaporins, water channels that regulate intraocular fluid formation and hence intraocular pressure, may be responsible for these adverse ocular effects.Citation5 The exact pathophysiological mechanisms are yet to be fully elucidated.
Asenio-Sanchez et alCitation6 reported a case of irreversible visual loss secondary to maculopathy related to topiramate use. Vision did not recover 1 year after the cessation of topiramate. Tsui et alCitation7 reported a case of electronegative ERG associated with topiramate toxicity and coexistent vitelliform maculopathy. The patient had ceased therapy with 400 mg topiramate, which she had been taking for 4 years and 9 months prior to the onset of decreased vision in both eyes.Citation7
The mechanism by which topiramate may cause maculopathy remains uncertain.
Electronegative ERGs caused by topiramate have been reported in rabbitsCitation8 but not in humans. Topiramate has been demonstrated to modify several receptor-gated and voltage-sensitive ion channels,Citation9 which may result in accumulation of gamma-aminobutyric acid in the inner segment layer.
Gualtieri and JanulaCitation10 reported a case of isolated topiramate-induced maculopathy. This was unassociated with well-documented adverse ocular effects such as acute glaucoma, myopia, and choroidal effusions described in relation to topiramate therapy. It was suggestedCitation10 that the macular striae and OCT changes could be interpreted as a result of an effusion syndrome confined to macular choriocapillaris, which had not yet progressed to a full ocular syndrome.
Severn et alCitation11 reported a case of topiramate maculopathy secondary to dose titration. The patient reportedlyCitation11 discontinued topiramate of his own volition after the onset of visual symptoms related to a prescribed increase in the dose from 75 to 100 mg. This was associated with a resolution of bilateral retinal macula striae and improved vision. Kozner et alCitation12 described the development of an acute myopia syndrome secondary to topiramate discontinuation, a dose decrease. Kozner et alCitation12 hypothesized that sudden changes in the plasma levels of topiramate may result in abnormal carbonic anhydrase activity and subsequent accumulation of fluid within the uveal tissue. This, in turn, alters permeability of the choriocapillaris, providing a pathophysiological mechanism for the aetiology of choroidal folds.Citation12
This report agrees with this suggestion that an isolated maculopathy associated with topiramate therapy may be the initial reversible sign of the full ocular syndrome which, when established, may be irreversible. An additional factor of the patient in our report is the presence of IgG4-related disease. IgG4-related disease is a disorder of unknown aetiology associated with lymphoplasmacytic tissue infiltration with a predominance of IgG4-positive plasma cells and T lymphocyctes, usually accompanied by fibrosis, obliterative phlebitis, and elevated serum levels of IgG4.Citation13
Ocular involvement in IgG4-related conditions has been described in relation to orbital disease;Citation13 however, no specific reports of isolated maculopathy in relation to this condition have been published to date. The pathogenesis of IgG4-related disease is poorly understood with findings of both an autoimmune disorder and an allergic disorder present.Citation13,Citation14 A specific autoantigenic target has not been identified yet and it is not clear whether the IgG4 autoantibodies are pathogenic.Citation15 IgG4 levels >135 mg/L are widely accepted for the diagnosis of IgG4-related disease.Citation16 However, high serum levels of IgG4 have been found in patients with other autoimmune diseases, carcinoma, and other conditions.Citation17 Carrunthers et alCitation17 studied the diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Their conclusionsCitation17 were that a substantial subset of patients with biopsy-proven IgG4-related disease did not have elevated serum IgG4. Auto-antibodies have been described against carbonic anhydrase in IgG4-related pancreatitis, a condition considered to be the prototype for IgG4-related disorder;Citation18 however, there is no definitive evidence to support the role of autoimmunity in this disorder. Intraocular inflammation is well recognized to be associated with cystoid macula edema, and the patient in this report was noted to have bilateral anterior uveitis. Bilateral anterior uveitis, in addition to features of acute angle closure glaucoma associated with topiramate use, has been described by Jabbarpoor Bonyadi et alCitation2 Topiramate was discontinued and 3 days after the intraocular pressure was controlled, the patient developed a severe anterior uveitis with hypopyon and posterior synechia formation between the iris and lens.Citation2
The patient in Jabbarpoor Bonyadi et al’s reportCitation2 was fully investigated for uveitis and no cause was found. Jabbarpoor Bonyadi et alCitation2 speculated that the high intraocular pressure may have inhibited aqueous flow and hence, hypopyon formation in the early stages of disease. The patient responded to a tapering course of oral steroids. Jabbarpoor Bonyadi et alCitation2 considered that the case reported was drug-induced anterior uveitis secondary to topiramate.
Osmotic retinal hemostasis is maintained by water-selective channels, the aquaporins, and osmotic stress has been shown to decrease aquaporin 4 production in culture.Citation19 Topiramate is suggested to inhibit the function or expression of aquaporins 1 and 4.Citation5 This provides a putative pathophysiological mechanism to explain how an autoimmune disease such as IgG4-related inflammatory disease might influence the pharmacokinetics of topiramate. In addition, it is postulated that an autoantibody reaction in IgG4-related disease may stimulate carbonic anhydrase, thereby disturbing the normal fluid dynamics of the choroid and retina. The exact mechanisms are yet to be fully elucidated.
This case report is the first to describe topiramate-induced maculopathy in IgG4-related disease and highlights the mechanisms by which the molecular pharmacodynamics of topiramate can be modified by an autoimmune condition such as IgG4-related disease.
The limitations of this report is that it is difficult to draw inferences from a single case and IgG4-related disease may not be a single entity, rather a collection of conditions with variable presentations and levels of autoantibodies. Further research will determine the molecular pharmacological targets of topiramate and the interaction with specific autoantibodies.
In conclusion, ophthalmologists and neurologists should be aware that patients presenting with topiramate-associated maculopathy should be advised to discontinue topiramate promptly to prevent irreversible loss of vision.
Disclosure
The authors report no conflicts of interest in this work.
Reference
- Lyseng-WilliamsonKAYangLPTopiramate: a review of its use in the treatment of epilepsyDrugs200767152231225617927286
- Jabbarpoor BonyadiMHSoheilianRSoheilianMTopiramate induced bilateral anterior uveitis associated with hypopyon formationOcul Immunol Inflamm2011191868821034312
- MaryanoffBENorteySOGardockiJFShankRPDodgsonSPAnti-convulsant O-alkyl sulfamates. 2,3:4,5-Bis-O-(1-methyleethylidene)-beta-D-fructopyranose sulfamate and related compoundsJ Med Chem19873058808873572976
- GuptaPPThackerAKHaiderJDhawanSPandeyNPandeyAKAssessment of topiramate’s efficacy and safety in epilepsyJ Neurosci Rural Pract20145214414824966552
- ShankRPMaryanoffBEMolecular pharmacodynamics, clinical therapeutics and pharmacokinetics of topiramateCNS Neurosci Ther200814212014218482025
- Asensio-SánchezVMTorreblanca-AgüeraBMartínez-CalvoSCalvoMJRodríguezRToxicidad oftalmológica severa por Topamax. [Severe ocular side effects with Topamax]Arch Soc Esp Oftalmol2006816345348 Spanish16804780
- TsuiICasperDChouCLTsangSHElectronegative electroretinogram associated with topiramate toxicity and vitelliform maculopathyDoc Ophthalmol20081161576017912565
- KjellströmSBruunAIsakssonBErikssonTAndréassonSPonjavicVRetinal function and histopathology in rabbits treated with TopiramateDoc Ophthalmol2006113317918617111186
- WhiteHSMolecular pharmacology of topiramate: managing seizures and preventing migraineHeadache200545Suppl 1S48S5615833090
- GualtieriWJanulaJTopiramate maculopathyInt Ophthalmol201333110310623015022
- SevernPSSymesRRajendramRPalBTopiramate maculopathy secondary to dose titration: first reported caseEye201529798298425931169
- KoznerPSimonovaKBrozekBSinghKLate acute myopia syndrome induced by a combination of sulphonamide drugsJ Glaucoma2014232e119e12123661047
- CheukWChanJKIgG4-related sclerosing disease: a critical appraisal of an evolving clinicopathologic entityAdv Anat Pathol201017530333220733352
- ZenYFujiiTHaradaKTh2 and regulatory immune reactions are increased in immunoglobulin G4-related sclerosing pancreatitis and cholangitisHepatology20074561538154617518371
- FragoulisGEMoutsopoulosHMIgG4 syndrome: old disease, new perspectiveJ Rheumatol20103771369137020595288
- HamanoHKawaSHorinchAHigh serum IgG4 concentrations in patients with sclerosing pancreatitisN Engl J Med20013441073273811236777
- CarruthersMNKhosroshahiAAugustinTDeshpandeVStoneJHThe diagnostic utility of serum IgG4 concentrations in IgG4-related diseaseAnn Rheum Dis2015741141824651618
- AparisiLFarreAGomez-CambroneroLAntibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitisGut200554570370915831920
- WillermainFJanssensSArsenijevicTOsmotic stress decreases aquaporin-4 expression in human epithelial cell line, ARPE-19Int J Mol Med201434253353824888368
