Abstract
Background
Vancomycin resistance has raised concerns about its effectiveness prospect in the treatment of patients with Gram-positive infections. The Healthcare Infection Control Practices Advisory Committee (HICPAC) has recently established guidelines to delineate improper use of vancomycin. In this light, we sought out to determine the appropriateness of vancomycin prescription using the HICPAC guidelines.
Setting
The study was carried out in two university-affiliated hospitals, Valiasr and Imam Reza, with 297 and 234 beds, respectively, from May 2012 to May 2013.
Methods
This retrospective study evaluated the vancomycin prescription and usage in the hospitals. Total vancomycin use was determined and expressed as vancomycin courses per 298 admitted patients. The patient information was collected on a data collection sheet as follows: demographic variables, etiology and localization of infection, microbiological data, duration of vancomycin treatment, reasons for vancomycin prescription, prescribed antibiotic dosing, and patient regimen.
Results
The average age of the patients and vancomycin treatment duration were 55.965 years and 10.5 days, respectively. Septicemia (15.7%) was the most common cause of vancomycin administration. Vancomycin use was documented to be appropriate and inappropriate in 236 (89.4%) and 28 (10.6%) patients, respectively. No statistically significant differences were found among the wards and hospitals (P values =0.66 and 0.54, respectively) in terms of appropriateness of vancomycin use based on the HICPAC criteria. In addition, 29.21% and 62% of all patients exhibited complete and partial recovery, respectively. We found that 90% of the cases showed compliance with the HICPAC recommendations.
Conclusion
Comprehensive programs are required to improve the vancomycin use in the hospitals. Vancomycin use should be monitored due to its large-scale empiric use. The rate of improper use of vancomycin in the infection and intensive care unit services may be high, and pharmacists must take appropriate action to optimize the use of the drug.
Introduction
Bacterial resistance to antibiotics has recently been considered as a “fundamental threat” to global health according to United Nations.Citation1,Citation2 Over the past ten years, it has been evident that several highly resistant bacterial pathogens have acquired effective mechanisms against different therapeutic agents.Citation3,Citation4 Staphylococcus aureus and Staphylococcus epidermidis are bacterial pathogens known as considerable points to healthcare professionals around the world. In the past eight decades, S. aureus and S. epidermidis have been shown to be the most prevalent multi drug resistant pathogens worldwide,Citation5 showing resistance to several classes of antibiotics, including macrolides,Citation6 fluoroquinolones,Citation7 β-lactam antibiotics,Citation8 oxazolidinones,Citation9 and glycopeptides.Citation10
Vancomycin is a glycopeptide antibiotic used as early stage of therapy for the methicillin-resistant S epidermidis, methicillin-resistant S. aureus (MRSA), and penicillin-resistant enterococci.Citation11 Vancomycin prevents biosynthesis of the bacterial cell wall by binding to D-alanyl-D-alanine precursors.Citation12 The antibiotic may be promising in the prevention of healthcare-associated infections (HAIs); However, widespread and irrational use of the antibiotic has led to a few serious drawbacks.Citation13
The improper use of vancomycin augments various infections caused by multidrug-resistant organisms, which are correlated with adverse therapy outcomes, longer hospitalization, and higher treatment costs.Citation14 Reduced sensitivity to vancomycin observed in an outbreak has crucial negative effect on the staff and patients,Citation16–Citation18 leading to considerable costs for healthcare.Citation18 Therefore, there is an urgent need for surveillance of vancomycin use for targeted interventions to deal with such troubles.
Several research studies have been published on vancomycin application both in critically ill adult and pediatric patients from developed countries.Citation19–Citation22 The studies identified inappropriate use of antibiotics in most of the patients and suggested strategies to reduce inappropriate prescription of antibiotics. This kind of surveillance results in antibiotic stewardship programs and appropriate use of the antibiotic. On the contrary, such data are not available in hospitals of developing countries, including Iran.
An increased incidence of vancomycin-resistant entero-coccus (VRE) was highly correlated with large university-affiliated hospitals (>200 beds).Citation23 The Healthcare Infection Control Practices Advisory Committee (HICPAC) developed and published recommendations to prevent and control the spread of VRE as part of the continuing efforts to decline the inappropriate use of vancomycin and in response to the rapid emergence of VRE.Citation24 The committee introduced statements that describe protocols considered to prevent and control HAIs, with three principal goals: protecting the healthcare workers, visitors, and others in the healthcare environment; protecting the patients; and accomplishing this in a cost-effective manner whenever possible.Citation25 They are informed through a systematic review of evidence, and an assessment of the benefits and harms of alternative care options.Citation26 In fact, the HICPAC of the Centers for Disease Control and Prevention has issued guidelines and criteria to prevent VRE spread, including measurements to ensure the careful use of vancomycin and outlining specific situations in which vancomycin therapy is appropriate or inappropriate.Citation24 HICPAC criteria offer better treatment results, decrease risks, and allow cost-effective clinical care.
Aim of the study
The goal of this study was to assess the usage of vancomycin according to HICPAC criteria in university-affiliated hospitals in Southern Khorasan Province (East Iran). This evaluation would help us develop appropriate strategies for the prevention of the improper use of vancomycin, resulting in reduced cost and improved outcome of vancomycin therapy.
Ethics approval
The procedures used in this study were approved by the Ethics Committee of Birjand University of Medical Sciences, Birjand, Iran (ir.bums.REC.1394.420). In addition, written informed consent was obtained from all participants included in the study. The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the European Commission.
Patients and methods
Hospital setting
The present study was carried out in two university-affiliated hospitals, Valiasr and Imam Reza, with 297 and 234 beds, respectively. In this study, we studied 298 patients treated with vancomycin in Valiasr Hospital (infectious diseases, internal medicine, pediatrics, and general intensive care unit [ICU] departments) and Imam Reza Hospital (surgical, orthopedic, and surgical ICU departments). Hospital physicians have to write all antibiotic orders for standard pharmacy protocols, but there are no restrictions for antibiotic use. The pharmacy maintains a permanent computerized record of all antimicrobial agents dispensed.
Design
We conducted a retrospective study to evaluate the vancomycin prescription and usage in the hospitals.
Vancomycin use
The patient information was collected on a structured data collection sheet as follows: demographic variables (age and gender), clinical (etiology and localization of infection), laboratory, microbiological data, duration of vancomycin treatment, reasons for vancomycin prescription, prescribing physician specialty, prescribed antibiotic dosing, patient regimen, serum creatinine, comorbidity, and prescription of other drugs according to the HICPAC criteria. A clinical pharmacist reviewed the medical records for indication of vancomycin use, medical services prescribing vancomycin, as well as culture and susceptibility data. The number of prescribed vancomycin was recorded for each patient during hospital length of stay. The indication for vancomycin use was classified by a clinical pharmacist as appropriate or inappropriate according to the HICPAC guidelines. These criteria were analyzed for each patient to assess the appropriateness of the vancomycin treatment. Inappropriate vancomycin use (based on HICPAC) was described in the cases, such as wrong dosage, duration, and indication. All concomitant medications were recorded.
Statistical analysis
Descriptive statistics were performed and data were analyzed using chi-squared test or Fisher’s exact test. IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, USA) was used for the analysis of significant differences. Data are presented as the mean and SD. A P-value of ≤0.05 considered to be statistically significant.
Results
Study groups included 201 (67.4%) and 97 (32.6%) patients recruited from the Valiasr and Imam Reza hospitals, respectively. The study was conducted from May 2012 to May 2013. The average age of the patients was 55.96±12.6 years. The average weight of the patients was 58.79±27.5 kg and the average hospital length of stay was 11.4±10.3 days. The average dosing of vancomycin was 875±125 mg and the mean length of vancomycin treatment was 10.54±7.88 days (a range of 1–21 days). The average of creatinine clearance in patients before and after vancomycin use was 1.06±0.88 and 80.73±42.65 mL/min, respectively (). Vancomycin use was documented as appropriate and inappropriate in 236 (89.4%) and 28 (10.6%) of the patients, respectively. No sufficient information was available for 35 cases (). No statistically significant difference was observed among studied wards and hospitals (P value =0.66 and 0.54, respectively) regarding appropriateness of vancomycin use based on the HICPAC criteria. It is also worth noting that 29. 21% and 62% of all patients were completely and partially recovered, respectively. Other medications used together with vancomycin are listed in . The most common wards where vancomycin was prescribed were infection disease, ICU, and internal medicine ().
Figure 1 Appropriateness of vancomycin utilization by hospitals (based on Healthcare Infection Control Practices Advisory Committee criteria).
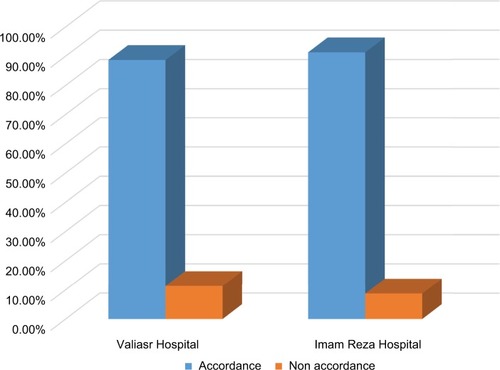
Table 1 Variables and values of the study
Table 2 Concomitant use of other antibiotics and drugs with vancomycin
Table 3 Appropriateness of vancomycin utilization by different wards (based on HICPAC criteria)
Discussion
Results from our study showed that vancomycin use was documented to be appropriate and inappropriate in 236 (89.4%) and 28 (10.6%) patients, respectively. Sufficient data were unavailable for 35 cases. No statistically significant differences were found among the wards and hospitals (P values =0.66 and 0.54, respectively) in terms of appropriateness of vancomycin use based on the HICPAC criteria. It is also worth noting that 29.21% and 62% of all patients exhibited complete and partial recovery, respectively. It was found that 90% of the cases (73) showed compliance with the HICPAC recommendations.
Vancomycin is currently one of a few effective drugs available for some life-threatening infections caused by an expanding list of resistant Gram-positive pathogens.Citation27 Of note, vancomycin, daptomycin, teicoplanin (antibiotics of choice), ceftaroline, telavancin, and linezolid (alternative agents) are the most frequently used drugs for life-threatening infections. The reason why vancomycin was chosen for this study was that vancomycin is an old antibiotic used for staphylococcal infections; in addition, the antibiotic is cheaper and more accessible than other antibiotics, which is used extensively, especially in developing countries.Citation28,Citation29 Studies conducted at large universities and teaching hospitals have reported a significant increase in the frequent use of vancomycin. Some institutions reported that more resources were invested on vancomycin when compared with other drugs on formulary.Citation30,Citation31 Otherwise, combinations of vancomycin with other antibiotics lead to increased toxicity in the study population, as depicted in . Vancomycin is frequently prescribed in an inappropriate manner, especially as empirical treatment. However, poor outcomes were documented for patients with MRSA bacteremia for whom appropriate therapy was delayed. In the guidelines of the Centers for Disease Control and Prevention (CDC) and the Infectious Diseases Society of America, it is strongly recommended that empirical vancomycin treatment be stopped when available culture results fail to reveal β-lactam-resistant Gram-positive bacterial infections; however, it is well known that empirical vancomycin use is inappropriately continued for a proportion of patients.Citation32 Several studies evaluated the pattern of use of vancomycin, most of which were conducted in developed countries, and data on the utilization of vancomycin in developing regions remain to be elusive.Citation33,Citation34 Possible explanation for this practice is that physicians may have decided to continue empirical use of vancomycin because MRSA might have not been undetected in cultures. Another reason for continuing vancomycin is convenience of use, as its spectrum covers many Gram-positive bacteria, especially in developing countries.Citation35
Table 4 Drug–drug interaction with vancomycin that may lead to increased nephrotoxicity in the study population (n=298)
Recent investigations have described that enhanced antibiotic resistance has been associated with increased virulence of several Gram-positive pathogens. This is, in part, associated with the fact that some of these pathogens have acquired resistance to vancomycin.Citation31 In addition, various studies demonstrated that vancomycin use is a key determinant in the development of vancomycin-resistant pathogens.Citation36 Because treatment options are finite and death due to such resistant bacteria may be significantly higher in patients,Citation37 studies on preventive strategies of VRE appear to ameliorate the survival of the patients.
In the past two decades, several studies assessed the vancomycin use according to the indications. In those studies, investigators reported inappropriate use of vancomycin up to 65% when compared with specific institution criteria and/or CDC recommendations.Citation38,Citation39 In a recent study from Brazil, application of the same guidelines has exhibited that vancomycin use within the first 24 hours and after 72 hours was appropriate only in 34.3% and 33.0% of the patients, respectively.Citation40 A recent evaluation of prospective drug use in 50 patients at a private hospital in the Minneapolis–St. Paul area has reported that vancomycin use is inappropriate based on the CDC criteria in 69% of the patients. Inconsistent with aforementioned studies, 10.6% of the vancomycin orders evaluated in our hospitals were due to inappropriate indications, which is considerably lower than the rates reported from previous studies. There are several possible explanations for these different findings, including implementation of a restriction policy to prescribe in some services and educational programs on institutional prescribing patterns.
Results from studies, in Nemazee and Tabriz hospitals, showed that vancomycin was used inappropriately in 68.63% and 69.3% of the patients, respectively. In addition, some authors reported 65% of inappropriate empiric vancomycin prescription.Citation41 Other studies showed that 69.3% of patients received vancomycin inappropriately. Fahimi et al reported that 97.7% of their study population had inappropriate indication and dosing regimen of vancomycin and they concluded that vancomycin irrational use was high compared with other countries.Citation45 In a similar study, Misan et al reported that 97% of the patients receiving vancomycin for prophylaxis purposes were classified as inappropriate use.Citation42 Studies conducted in Western countries showed that inappropriateness in the use of vancomycin did not exceed 40% of guidelines recommendations even in the absence of restriction policies.Citation43
Studies showed that the interventions conducted by the clinical pharmacists and infectious diseases specialists lead to improvement of the antibiotic prescription to the hospitalized patients, subsequent promising outcomes, as well as decreased antimicrobial resistance and hospital-acquired infections. The most common interventions include implementation of guidelines and compulsory order forms, acceleration of laboratory tests, expert approval (clinical pharmacists and infectious disease specialists), restriction or removal of drugs, review and correction of prescription by clinical pharmacists, as well as monitoring of the therapeutic drug.Citation16,Citation17 In our study, surprisingly, most of the inappropriate prescribing cases were observed in the infection and ICU services, demonstrating that the greatest vancomycin consumers are in these units and excessive use of vancomycin was observed in many cases. This finding is probably due, in part, to the fact that the number of patients varies in different wards, ranging from 1 (neonatal intensive care unit) to 82 (infection). The authors suggested that the initiation of vancomycin therapy should be assessed in high-use services to reduce vancomycin use. Continued intensive education for hospital personnel and monitoring will result in further improvement. For example, the restriction of antibiotic use has been also found to be effective for reducing inappropriate vancomycin use in inpatient settings.Citation39 Although there are several approaches for antibiotic restriction, approval by the infectious disease specialists is the most common mechanism.Citation44
Limitations of the study
There were several limitations in the present study. First, the study was a single-center study accomplished in a large university hospital. Therefore, our results might not be representative to the centers that did not share similar characteristics. Second, it is possible that our methodology for evaluating the appropriateness of vancomycin use will lead to some misclassification based on the objective criteria. This is due to the fact that our categorization should be viewed as a retrospective proxy estimate of appropriate vancomycin use, in the absence of reproducible prospective way to measure appropriateness.
Conclusion
In summary, the present study demonstrated that vancomycin was appropriately prescribed in most patients, compared with the other studies. Only 10.6% of the patients showed inappropriate vancomycin use according to the HICPAC criteria. Continued surveillance and larger patient samples, as well as multicenter studies, are recommended to overcome the limitations.
Acknowledgments
The authors would like to convey their appreciation to all patients who made this study possible. This work was supported by a research grant (623) from Birjand University of Medical Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
- HolpuchAUN Meeting Tackles the’ Fundamental Threat’ of Antibiotic-Resistant SuperbugsUKThe Guardian2016
- SalehifarENasehiMEslamiGSahraeiSAlizadeh NavaeiRDetermination of antibiotics consumption in Buali-Sina Pediatric Hospital, Sari 2010–2011Iran J Pharm Res201413399525276201
- LaxminarayanRDuseAWattalCAntibiotic resistance-the need for global solutionsLancet Infect Dis201313121057109824252483
- NaderiAKasra-KermanshahiRGharaviSImani FooladiAAAbdollahpourAMSaffarianPStudy of antagonistic effects of Lactobacillus strains as probiotics on multi drug resistant (MDR) bacteria isolated from urinary tract infections (UTIs)Iran J Basic Med Sci201417320120824847423
- DenoonDDrug-resistant staph may get nastier: experts call for increased efforts to halt MRSAWebMD Medical News2008
- MoranGJKrishnadasanAGorwitzRJMethicillin-resistant S. aureus infections among patients in the emergency departmentN Engl J Med2006355766667416914702
- FrazeeBWLynnJCharleboisEDLambertLLoweryDPerdreau-RemingtonFHigh prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infectionsAnn Emerg Med200545331132015726056
- ChambersHFCommunity-associated MRSA—resistance and virulence convergeMass Medical Soc20053521414851487
- WilsonPAndrewsJACharlesworthRLinezolid resistance in clinical isolates of Staphylococcus aureusJ Antimicrob Chemother200351118618812493812
- HagrasMMohammadHMandourMSInvestigating the antibacterial activity of biphenylthiazoles against methicillin- and vancomycin-resistant Staphylococcus aureus (MRSA and VRSA)J Med Chem20176094074408528436655
- YeZKLiCZhaiSDGuidelines for therapeutic drug monitoring of vancomycin: a systematic reviewPLoS One201496e9904424932495
- JavorskaLKrcmovaLKSolichovaDSolichPKaskaMModern methods for vancomycin determination in biological fluids by methods based on high-performance liquid chromatography–a reviewJ Sep Sci201639162026351070
- AbbasQUl HaqAKumarRAliSAHussainKShakoorSEvaluation of antibiotic use in pediatric intensive care unit of a developing countryIndian J Crit Care Med201620529127275078
- ErbayAÇolpanABodurHÇevikMASamoreMHErgönülOEvaluation of antibiotic use in a hospital with an antibiotic restriction policyInt J Antimicrob Agents200321430831212672575
- ArdaBSipahiORYamazhanTShort-term effect of antibiotic control policy on the usage patterns and cost of antimicrobials, mortality, nosocomial infection rates and antibacterial resistanceJ Infect2007551414817512598
- Garnacho-MonteroJGutiérrez-PizarrayaAEscoresca-OrtegaADe-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shockIntensive Care Med2014401324024026297
- DaveyPBrownECharaniEInterventions to improve antibiotic prescribing practices for hospital inpatientsCochrane Database Syst Rev201344
- SpauldingTJRaghuTSImpact of CPOE usage on medication management process costs and quality outcomesInquiry-J Health Car2013503229247
- DingHYangYChenYWangYFanSShenXAntimicrobial usage in paediatric intensive care units in ChinaActa Paediatr200897110010418076718
- BlinovaELauEBitnunAPoint prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unitPediatr Crit Care Med2013146e280e28823823209
- StockerMFerraoEBanyaWCheongJMacraeDFurckAAntibiotic surveillance on a paediatric intensive care unit: easy attainable strategy at low costs and resourcesBMC Pediatr201212119623259701
- FloretNThouverezMThalamyBEstavoyerJMTalonDEvaluation of vancomycin use in a large university-affiliated hospital in eastern France in 1999Pharm World Sci2001233939711468882
- Centers for Disease Control and Prevention (CDC)Nosocomial enterococci resistant to vancomycin–United States, 1989–1993MMWR Morb Mortal Wkly Rep199342305978336690
- SolomkinJSMazuskiJBlanchardJCIntroduction to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee guideline for the prevention of surgical site infectionsSurg Infect2017184385393
- WaleMKibseyPYoungLDobbynBArcherJNew approaches to infection prevention and control: implementing a risk-based model regionallyInt J Qual Health Care201628340541127194074
- PaiMPNeelyMRodvoldKALodiseTPInnovative approaches to optimizing the delivery of vancomycin in individual patientsAdv Drug Deliv Rev201477505724910345
- SpitzerEDTortoraGTVancomycin-resistant gram-positive cocciLab Med1990217411413
- BenvenutoMBenzigerDPYankelevSViglianiGPharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteersAntimicrob Agents Chemother200650103245324917005801
- FigueroaDAManginiEAmodio-GrotonMSafety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical programClin Infect Dis200949217718019500039
- FletcherCGieseRRodmanJPharmacist interventions to improve prescribing of vancomycin and tobramycinAm J Health Syst Pharm198643921982201
- AnagnostouTGoundanPHoynackEEvaluation of Vancomycin usage after Implementation of an Antibiotic Stewardship Program: Experience from a Community-Based Teaching HospitalOpen Forum Infectious DiseasesOxford University Press2015
- KimNHKooHLChoePGInappropriate continued empirical vancomycin use in a hospital with a high prevalence of methicillin-resistant Staphylococcus aureusAntimicrob Agents Chemother201559281181725403664
- AlfandariSLeventTDescampsDEvaluation of glycopeptide use in nine French hospitalsMed Mal Infect201040423223719959309
- RybakMLomaestroBRotschaferJCTherapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases PharmacistsAm J Health Syst Pharm2009661829819106348
- IstúrizRECarbonCAntibiotic use in developing countriesInfect Control Hosp Epidemiol200021639439710879571
- PowellSLLiebeltEAppropriate use of vancomycin in a pediatric emergency department through the use of a standardized electronic guidelineJ Pediatr Nurs201530349449725618611
- CetinkayaYFalkPMayhallCGVancomycin-resistant enterococciClin Microbiol Rev200013468670711023964
- AskarianMAssadianOSafaeeGGolkarANamaziSMovahedMRVancomycin use in a large teaching hospital in Shiraz, Islamic Republic of Iran, 2003East Mediterr Health J20071351195120118290414
- DibJGAl-TawfiqJAAl AbdulmohsinSMohammedKJendenPDImprovement in vancomycin utilization in adults in a Saudi Arabian medical center using the hospital infection control practices advisory committee guidelines and simple educational activityJ Infect Public Health20092314114620701874
- JuniorMSCorreaLMarraARCamargoLFPereiraCAAnalysis of vancomycin use and associated risk factors in a university teaching hospital: a prospective cohort studyBMC Infect Dis2007718817678541
- VazinAMahi BirjandMDarakeMEvaluation of vancomycin therapy in the adult ICUs of a teaching hospital in southern IranDrug Healthc Patient Saf201810212629670404
- MisanGMMartinEDSmithERSomogyiAABartholomeuszRCBochnerFDrug utilization review in a teaching hospital: experience with vancomycinEur J Clin Pharmacol19903954574612076737
- KernWVde WithKGonnermannCUpdate on glycopeptide use in German university hospitalsInfection200432315716215188076
- FridkinSKVancomycin-intermediate and -resistant Staphylococcus aureus: what the infectious disease specialist needs to knowClin Infect Dis200132110811511118389
- FahimiFSoleymaniFTavakoli-ArdakaniMVancomycin Utilization Evaluation in a teaching hospital: A case-series study in IranJ Pharm Care2013514
