Abstract
Objective:
To increase awareness of the limitations of high-risk human papillomavirus (hrHPV) laboratory-developed testing (LDT) widely used in US cervical cancer screening.
Methods and results:
A young woman in her 30s was diagnosed and treated for stage 1B1 cervical squamous cell carcinoma in which HPV 16 DNA was detected using polymerase chain reaction testing. Both 1 month before and 42 months before cervical cancer diagnosis, the patient had highly abnormal cytology findings; however, residual SurePath™ (Becton, Dickson and Company, Franklin Lakes, NJ) vial fluid yielded negative Hybrid Capture 2 (HC2; Qiagen NV, Hilden, Germany) hrHPV LDT results from each of the two specimens. This prompted questions to be asked concerning the performance characteristics of hrHPV LDT. A review of the available data indicates that (1) purification of DNA from SurePath specimens requires complex sample preparation due to formaldehyde crosslinking of proteins and nucleic acids, (2) HC2–SurePath hrHPV testing had not been Food and Drug Administration-approved after multiple premarket approval submissions, (3) detectible hrHPV DNA in the SurePath vial decreases over time, and (4) US laboratories performing HC2–SurePath hrHPV LDT testing are not using a standardized manufacturer-endorsed procedure.
Conclusion:
Recently updated cervical screening guidelines in the US recommend against the use of hrHPV LDT in cervical screening, including widely used HC2 testing from the SurePath vial. The manufacturer recently issued a technical bulletin specifically warning that use of SurePath samples with the HC2 hrHPV test may provide false negative results and potentially compromise patient safety. Co-collection using a Food and Drug Administration-approved hrHPV test medium is recommended for HPV testing of patients undergoing cervical screening using SurePath samples.
Introduction
Since 2001, adjunctive high-risk human papillomavirus (hrHPV) testing has become increasingly integrated along with cytologic testing as a part of routine US cervical cancer screening, initially as a “preferred” reflex test after atypical cells of undetermined significance liquid-based cytology results and on a more widespread basis after 2003 Food and Drug Administration (FDA) approval for routine cytology and HPV cotesting of women 30 years and older.Citation1,Citation2 Recently updated cervical screening guidelines from the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology have proposed significantly lengthened screening intervals, particularly for patients with negative hrHPV test results and either negative or equivocally abnormal (atypical cells of undetermined significance) cytology findings.Citation3 For women 30 years and older with either hrHPV-negative atypical cells of undetermined significance or “double negative” results, a screening interval of 5 years has for the first time been recommended.Citation3 The guidelines, however, emphasize that the new extended screening intervals following negative hrHPV test results are based on HPV tests with performance characteristics similar to HPV tests used in the supporting evidence.Citation3 Since at least one-third of all US hrHPV tests use laboratory-developed test (LDT) methodology, largely exempt from regulatory oversight by the FDA and unlikely to have undergone rigorous evaluation using grade 3+ or grade 2+ cervical intraepithelial neoplasia clinical endpoints in properly designed trials,Citation3,Citation4 the guidelines publications specifically recommend against the use of HPV LDTs for cervical cancer screening.Citation3
The most common form of hrHPV LDT to date has been Hybrid Capture 2 (HC2; Qiagen NV, Hilden, Germany) performed on residual SurePath™ vial fluid (Becton, Dickinson and Company, Franklin Lakes, NJ).Citation5–Citation7 Although HC2 hrHPV testing is FDA-approved from both the Digene® (Qiagen) specimen transport medium tube (Qiagen) and the methanol-based PreservCyt® vial (Hologic, Inc, Bedford, MA), HC2 hrHPV testing from the SurePath vial to date has not been able to obtain FDA approval, despite multiple premarket approval submissions, beginning in 2002.Citation8 In a 2002 press release, the manufacturer stated: “We remain hopeful that resolution of the FDA’s issues will not significantly alter our prior expectations for introduction in 2003.”Citation8 Qiagen investigators have acknowledged that purification of DNA from SurePath specimens requires complex sample preparation due to the formaldehyde crosslinking of proteins and nucleic acids.Citation9 Three additional hrHPV tests have now also gained FDA approval from either the PreservCyt vial or also from proprietary manufacturer’s collection media, but none of these newer FDA-approved hrHPV tests have been approved using the SurePath vial.Citation10–Citation12
Recently, the authors encountered a patient diagnosed with invasive cervical cancer with two prior significantly abnormal Pap tests and two negative hrHPV LDT cotest results. Since virtually all cervical cancers are now thought to be due to persistent carcinogenic hrHPV infections,Citation13,Citation14 this case was investigated to better understand the possible causes of negative hrHPV LDT in screened patients developing cervical cancer.
Case report
The patient was a young woman in her 30s, a gravida 3, para 3 cigarette smoker with a long history of abnormal Pap test results and inconsistent follow-up due to medical appointment cancellations which the patient attributed to intermittent lack of insurance coverage. Forty-two months before her diagnosis of cervical cancer, the patient had a SurePath Pap test interpreted as “atypical squamous cells, cannot rule out a high grade squamous intraepithelial lesion” (). Because the patient’s gynecologist had ordered routine cytology and HPV cotesting in a woman 30 years and older, the residual SurePath vial fluid was sent to a regional laboratory facility of a large national commercial laboratory. The hrHPV test result was reported as “not detected.” In that report, an additional comment stated that “patients without hrHPV rarely have cervical cancer.” There was no comment concerning the performance characteristics of this LTD. A cervical biopsy obtained 4 months later reported koilocytosis and an endocervical curetting as benign. A second SurePath Pap test obtained 19 months later was reported as “high-grade squamous intraepithelial lesion.” The patient failed to return for scheduled colposcopic evaluation. Eighteen months later, the patient presented with irregular painful periods that were getting worse. A third SurePath Pap test was obtained and reported as “high-grade squamous intraepithelial lesion” (). The patient’s gynecologist had again ordered routine cytology and hrHPV cotesting in a woman 30 years or older, and therefore the residual SurePath vial fluid was again sent to the regional laboratory that had previously performed hrHPV testing. The hrHPV test, which utilized hybrid capture with signal amplification, was – as before – reported as “not detected.” In this report, however, an additional comment stated that “the analytical performance characteristics of this assay, when used to test SurePath or vaginal specimens, have been determined by (the laboratory).” The patient’s SurePath Pap test slides were reviewed and photographed for documentation.
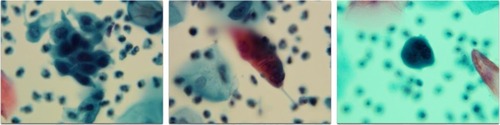
Figure 1 Abnormal SurePath Pap test cells interpreted as “atypical squamous cells, cannot rule out a high-grade squamous intraepithelial lesion” (original magnification 400×); residual SurePath vial fluid tested negative using the Hybrid Capture 2 test for high-risk human papillomavirus.
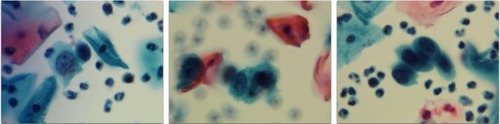
Figure 2 Abnormal SurePath Pap test cells interpreted as “high-grade squamous intraepithelial lesion” (original magnification 400×); residual SurePath vial fluid tested negative using the Hybrid Capture 2 test for high risk human papillomavirus.
A cold knife conization was performed 1 month later and the presence of a poorly differentiated squamous cell carcinoma (SCC) measuring 1 × 1 × 0.5 cm and extending to multiple biopsy margins was documented. One month later at an outside cancer referral center, the patient underwent a radical hysterectomy, bilateral pelvic lymph node dissection, bilateral salpingectomy, and left oophorectomy. Final pathologic diagnosis was of a cervical SCC (1.5 cm maximum tumor dimension) invading the upper third of the cervix with no lymphovascular invasion identified, and negative lymph nodes – stage 1B1 (). No further therapy was recommended. Twenty-six months later, at last follow-up, the patient was reported as alive and well with no evidence of disease.
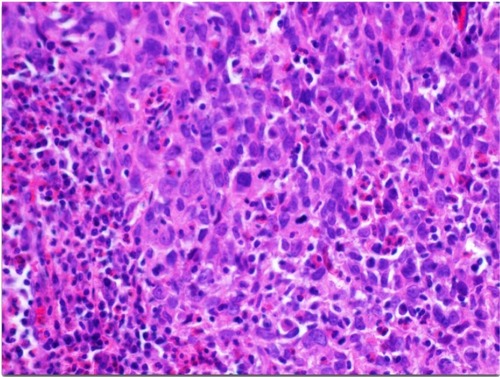
Figure 3 Invasive poorly differentiated cervical squamous carcinoma (original magnification 200×); diagnosed after two negative Hybrid Capture 2 human papillomavirus tests from SurePath vial fluid.
Paraffin sections of SCC samples from the patient’s cold knife conization specimen were used for hrHPV testing by polymerase chain reaction methods.Citation15 HPV in tumor sections was initially tested for using M09/M11 PCR primers, which amplify an approximately 450 base pair conserved region of the L1 gene of HPV. HPV type-specific PCR was also performed by PCR amplification of portions of E6 and E7 followed by automated DNA sequencing of the amplified products. Using these methods, presence of HPV 16 type-specific E6 was documented along with L1 deletion. Thus, the hrHPV tests from the SurePath vials were true false negative results. Fortunately, in the case of this patient, the false negative results did not affect her course or management.
Discussion
Confirmation of the presence of HPV 16 DNA by PCR in this patient’s invasive cervical SCC is consistent with the current understanding that persistent infections with a group of approximately a dozen carcinogenic HPV genotypes cause virtually all cases of cervical cancer worldwide. However, negative HC2 hrHPV test results from residual SurePath vial fluid 42 months and 1 month before tumor diagnosis were unexpected. Since both Pap tests contained highly abnormal cells (atypical squamous cells, cannot rule out a high-grade squamous intraepithelial lesion and high-grade squamous intraepithelial lesion), the discrepancy cannot be attributed to a failure to sample lesional cells. In fact, it has been argued that one advantage of HC2 cotesting is that HC2 may detect hrHPV DNA in patients with occult invasive cervical cancer, even when lesional cells are not sampled.Citation16
In the largest study of HC2 tests collected in FDA-approved specimen transport medium, positive hrHPV HC2 results were reported in 185 of 198 (93.4%) samples collected from patients with simultaneous histopathologic diagnoses of invasive cervical cancer.Citation17 In the same study, similar positive hrHPV HC2 test results were reported in 246 of 264 (93.2%) specimen transport medium tubes collected from patients with simultaneous histopathologic diagnoses of grade 3 cervical intraepithelial neoplasia.Citation17 In contrast, the very limited data from the most widely cited US laboratory self-validation study of HPV testing from the SurePath vial showed positive hrHPV HC2 results for patients with cancer in only 33% (one of three tests) or 50% (one of two patients). Also in contrast to the above cited data, the authors puzzlingly asserted that false negative hrHPV screening test results in patients with invasive cervical cancer are “not surprising.”Citation7
HC2 uses a positive cut point of 1.0 relative light units per positive control, a cut point which corresponds to greater than or equal to 5000 HPV DNA copies per test well, based on a receiver operating characteristic curve analysis versus cervical intraepithelial neoplasia grade 2+, to minimize the detection of lower viral load HPV infections that are mostly benign.Citation18–Citation20 Nevertheless, a subset of invasive cervical cancers associated with low viral load have been described,Citation21 and low viral loads in patients with developing invasive cervical cancer may fall below the detection cut point of FDA-approved hrHPV tests such as HC2.Citation17,Citation22 In one of the authors’ own laboratories (RMA), three of 31 (10%) patients diagnosed with invasive cervical SCC and tested within the prior 12 months for hrHPV by HC2 from FDA-approved PreservCyt vial fluid had negative HC2 results.Citation23 All three patients had HPV 18 detected by PCR in SCC sampled in paraffin sections, and two also had detectible HPV 16.Citation23
HPV testing from the non-FDA-approved SurePath vial is thought to be more challenging, primarily due to the formaldehyde crosslinking of proteins and nucleic acids.Citation9,Citation24 Although recovery of DNA and ribonucleic acid is largely unaffected by long-term storage in PreservCyt,Citation25,Citation26 storage in SurePath preservative fluid (Becton Dickinson) has been shown to affect the recovery of both DNA and ribonucleic acid.Citation27,Citation28 Upon exposure to SurePath media, recovery of both DNA and ribonucleic acid rapidly diminished. This reduction was most apparent in the 0–150 hours range (ie, up to around 6 days).Citation27 The websites of four large national laboratories that offer HC2 testing of referred SurePath samples all indicated that SurePath samples are stable at room temperature for HC2 testing for ≤1 month compared to 90 days/3 months for the FDA-approved ThinPrep® vial (Hologic) (). Interestingly, none of the laboratories, when queried by telephone, could produce independent SurePath–HC2 stability data; instead, laboratories referred to the FDA-approved Becton Dickinson package insert which states that SurePath preservative fluid preserves cells (for cytologic testing) for up to 4 weeks at room temperature (15°C–30°C).Citation29 Even in the most widely cited “validation” study mentioned previously, the authors referred to the digene package insert for specimen stability parameters.Citation7 ACCURUN® 372 series 400; (SeraCare Life Sciences, Inc, Milford, MA),Citation31 the HPV proficiency testing vendor for the College of American Pathologists has reported that HPV 16-positive control samples shipped in SurePath fluid degraded so rapidly that detectible HPV DNA was lost after 1 day.Citation30 The vendor concluded that only use of a two tube methodology, separating the HPV 16 sample from the SurePath sample until the time of testing, could be used for laboratory HPV proficiency testing.Citation31,Citation32
Table 1 Testing times allowed for of samples collected for Hybrid Capture 2 human papillomavirus testing as indicated on national laboratory websites
There is at present no standardized SurePath HPV protocol that all laboratories use and no literature with sufficient detail to represent an agreed upon standard.Citation6,Citation7,Citation33–Citation36 Furthermore, the manufacturer cannot under current regulations recommend standardized procedures for non-FDA-approved hrHPV testing. As a result of continued ongoing widespread off-label HPV LDT use and related patient safety concerns, on June 8, 2012 the manufacturer of SurePath released a technical bulletin which stated:
“The Becton Dickinson SurePath sample medium has not been approved by the FDA for use with the HC2 test and use [...] may under certain conditions provide false negative results. False negative results could lead to inappropriate patient management and potentially compromise patient safety.”
Conclusion
The authors echo the cautions of the new US screening guidelines that emphasize that extended screening intervals following negative hrHPV test results be based on HPV tests with performance characteristics similar to HPV tests used in the supporting evidence. Such supporting evidence is so far lacking for the hrHPV LDTs described here. Given those new cervical screening guidelines and manufacturer communications that caution against non-FDA-approved LDT hrHPV testing from the SurePath vial, it is reasonable to view continued widespread use of this nonstandardized off-label testing as a patient safety issue. With new, extended 5-year screening intervals proposed for many women, an increasing number of women will be screened every 5 years. An avoidable increase in false negative hrHPV results in women with both precancer and early invasive cervical cancer will place patients at unnecessary risk. The use of screening methods that have not been validated should be strongly discouraged.Citation37,Citation38 With four FDA-approved alternatives, it is difficult to justify the use of anything but rigorously clinically validated specimens. The College of American Pathologists should discontinue offering its current form of laboratory proficiency testing for HPV testing out of the SurePath vial, as it could mislead participants to believe that their methodology is currently safe and acceptable. For laboratories that use SurePath for cytology, co-collection of a second sample for hrHPV testing in an FDA-approved collection medium provides a safe and effective alternative.
Disclosure
The authors report no conflicts of interest in this work.
References
- WrightTCJrCoxJTMassadLS2001 Consensus Guidelines for the management of women with cervical cytological abnormalitiesJAMA2002287162120212911966387
- US Food and Drug AdministrationFDA news: FDA approves expanded use of HPV test. FDA News3312003 Available from: http://www.fda.gov/ohrms/dockets/dockets/07p0210/07p-0210-ccp0001-01-FDA-News-vol3.pdf. Accessed August 9, 2012.
- SaslowDSolomonDLawsonHWAmerican Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancerAm J Clin Pathol2012137451654222431528
- VanceGHCollege of American Pathologists proposal for the oversight of laboratory-developed testsArch Pathol Lab Med2011135111432143522032569
- ClavelCMasureMPutaudIHybrid Capture II, a new sensitive test for human papillomavirus detection. Comparison with Hybrid Capture I and PCR results in cervical lesionsJ Clin Pathol1998511073774010023335
- SiddiquiMTCohenCNassarADetecting high-grade cervical disease on ASC-H cytology: role of BD ProEx C and digene Hybrid Capture II HPV DNA testingAm J Clin Pathol2008130576577018854269
- KoVTambouretRHKueblerDLBlack-SchafferWSWilburDCHuman papillomavirus testing using Hybrid Capture II with SurePath collection: initial evaluation and longitudinal data provide clinical validation for this methodCancer2006108646847417091508
- TriPath ImagingComments on the FDA review of Digene’s PMA application [press release]Burlington, NCTriPath Imaging7102002
- KupferCLindebaumKGilesJChendvankarRLeeYSprenger-HausselsMQIAsymphony AXpH sample preparation for automated conversion of liquid-based cytology specimens for use in the digene HC2 high-risk HPV DNA testPaper presented at: EUROGIN 2011 CongressMay 8–11, 2011Lisbon, Portugal
- BelinsonJLWuRBelinsonSEA population-based clinical trial comparing endocervical high-risk HPV testing using Hybrid Capture 2 and Cervista from the SHENCAST II studyAm J Clin Pathol2011135579079521502436
- StolerMHWrightTCJrSharmaAThe interplay of age stratification and HPV testing on the predictive value of ASC-US cytologyAm J Clin Pathol2012137229530322261457
- RatnamSCoutleeFFontaineDAptima HPV E6/E7 mRNA test is as sensitive as Hybrid Capture 2 assay but more specific at detecting cervical precancer and cancerJ Clin Microbiol201149255756421147950
- BoschFXLorinczAMunozNMeijerCJShahKVThe causal relation between human papillomavirus and cervical cancerJ Clin Pathol200255424426511919208
- BouvardVBaanRStraifKA review of human carcinogens – part B: biological agentsLancet Oncol200910432132219350698
- FerrisRLMartinezISirianniNHuman papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesisEur J Cancer200541580781515763658
- KinneyWFettermanBCoxJTLoreyTFlanaganTCastlePECharacteristics of 44 cervical cancers diagnosed following Pap-negative, high risk HPV-positive screening in routine clinical practiceGynecol Oncol2011121230931321276605
- KangWDKimCHCoMKComparison of Hybrid Capture II assay with the human papillomavirus DNA chip test for the detection of high-grade cervical lesionsInt J Gynecol Cancer200919592492819574786
- ShermanMESchiffmanMCoxJTEffects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS)J Natl Cancer Inst200294210210711792748
- KinneyWStolerMHCastlePESpecial commentary: patient safety and the next generation of HPV testsAm J Clin Pathol2010135219319920660320
- RoncoGGiorgi-RossiPCarozziFResults at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening testJ Natl Cancer Inst2008100749250118364502
- BouletGABenoyIHDepuydtCEHuman papillomavirus 16 load and E2/E6 ratio in HPV16-positive women: biomarkers for cervical intraepithelial neoplasia ≥2 in a liquid-based cytology settingCancer Epidemiol Biomarkers Prev200918112992299919861526
- KatkiHAKinneyWKFettermanBCervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practiceLancet Oncol201112766367221684207
- LiZAustinRMGuoMZhaoCScreening test results associated with cancer diagnoses in 287 women with cervical squamous cell carcinomaArch Pathol Lab Med8202012 [Epub ahead of print.]
- MoelansCBOostenrijkDMoonsMJvan DiestPJFormaldehyde substitute fixatives: effects on nucleic acid preservationJ Clin Pathol2011641196096721715573
- CuschieriKSBeattieGHassanSRobertsonKCubieHAssessment of human papillomavirus mRNA detection over time in cervical specimens collected in liquid based cytology mediumJ Virol Methods20051241–221121515664071
- LinWMAshfaqRMichalopoulosEAMaitraAGazdarAFMullerCYMolecular Papanicolaou tests in the twenty-first century: molecular analyses with fluid-based Papanicolaou technologyAm J Obstet Gynecol20001831394510920306
- PowellNSmithKFianderARecovery of human papillomavirus nucleic acids from liquid-based cytology mediaJ Virol Methods20061371586216828171
- ArbynMAnderssonKBergeronCBogersJPvon Knebel-DoebertitzMDillnerJCervical cytology biobanks as a resource for molecular epidemiologyMethods in Mol Biol201167527929820949396
- SurePath® collection [package insert]Franklin Lakes, NJBecton, Dickinson and Company2011
- AnekellaBDrygaSMoskowitzKStability of integrated HPV16 DNA in various sample transport mediaJ Mol Diagn20079679680
- SeraCare Life Sciences, Inc. ACCURUN® 372 series 400 HPV DNA positive control. Available from: http://www.seracarecatalog.com/Default.aspx?tabid=219&txtSearch=A3&List=1&SortField=ProductName%2CProductName&ProductID=121#LiveContent[ACC-P]. Accessed July 16, 2012.
- MoriartyATBentzJSWinklerBProficiency testing of high risk human papillomavirus DNA tests: the first 3 years of experience of the College of Pathologists CHPV surveysArch Pathol Lab Med Forthcoming 2012.
- Davis-DevineSDaySJFreundGGTest performance comparison of inform HPV and Hybrid Capture 2 high-risk DNA test using the SurePath liquid-based Pap test as the collection methodAm J Clin Pathol20051241243015923167
- SiddiqiASpataroMMcIntireHHybrid capture 2 human papillomavirus DNA testing for women with atypical squamous cells of undetermined significance Papanicolaou results in SurePath and ThinPrep specimensCancer2009117531832519693966
- ZhaoFHHuSYBianJJComparison of ThinPrep and SurePath liquid-based cytology and subsequent human papillomavirus DNA testing in ChinaCancer Cytopathol2011119638739421774094
- KellyRSPatnickJKitchenerHCMossSMHPV testing as a triage for borderline or mild dyskaryosis on cervical cytology: results from the Sentinel Sites studyBr J Cancer2011105798398821897395
- StolerMHCastlePESolomonDSchiffmanMThe expanded use of HPV testing in gynecologic practice per ASCCP-guided management requires the use of well-validated assaysAm J Clin Pathol2007127333533717276947
- NaryshkinSAustinRMLimitations of widely used high risk HPV DNA testing in patients with invasive cervical cancerPaper presented at: EUROGIN 2011 CongressMay 8–11, 2011Lisbon, Portugal