Abstract
Autism spectrum disorder (ASD) is a behaviorally defined disorder which has increased in prevalence over the last two decades. Despite decades of research, no effective treatment is currently available. Animal models, as well as other lines of evidence, point to abnormalities in the balance of cortical excitation to inhibition in individuals with ASD, with this imbalance resulting in an overall increase in cortical excitation. To reduce cortical excitatory glutamate pathways, arbaclofen, a selective agonist of the gamma aminobutyric acid receptor type B, has been developed. This article reviews the evidence for this treatment for ASD using a systematic review methodology. Overall, a systematic search of the literature revealed 148 relevant references with the majority of these being review papers or news items that mentioned the potential promise of arbaclofen. Five original studies were identified, four of which used STX209, a form of arbaclofen developed by Seaside Therapeutics, Inc., and one which used R-baclofen. In an animal model, treatment of Fragile X, a genetic disease with ASD features, demonstrated a reversal of behavioral, neurological, and neuropathological features associated with the disease. One double-blind, placebo-controlled study treated children and adults with Fragile X. Results from this study were promising, with signs of improvement in social function, especially in the most severely socially impaired. Two studies, one open-label and one double-blind, placebo-controlled, were conducted in children, adolescents, and young adults with ASD. These studies suggested some improvements in socialization, although the effects were limited and may have been driven by individuals with ASD that were higher-functioning. These studies and others that have used arbaclofen for the treatment of gastroesophageal reflux suggest that arbaclofen is safe and well-tolerated. Clearly, further clinical studies are needed in order to refine the symptoms and characteristics of children with ASD that are best treated with arbaclofen.
Introduction
The autism spectrum disorders (ASD) are a group of behaviorally defined neurodevelopmental disorders that have potentially life-long consequences. They are defined by impairments in communication and social interaction, along with restrictive and repetitive behaviors.Citation1 The definition of ASD has recently undergone revision due to the difficulty with subtyping ASD. Previously, The Diagnostic and Statistical Manual of Mental Disorders (DSM) Version IV Text Revision divided ASD into several diagnoses with the most prevalent diagnoses being autistic disorder, Asperger syndrome, and pervasive developmental disorder-not otherwise specified. However, the current revision of the DSM, the DSM version 5, does not differentiate between these subtypes of ASD and considers communication and social impairments together, rather than separate symptom classes.Citation2 It is believed that this change may decrease the overall prevalence of ASD.Citation3 However, most research has used the former version of the DSM to define ASD, and currently, ASD has been estimated to affect one out of 68 individuals in the United States of America,Citation4 with four times more males than females being affected.Citation5 Over the past two decades, the incidence of the ASDs has grown dramatically, although the reasons for this increase are continually debated.
Despite decades of research, the etiology of ASD is unclear at this time. Several genetic syndromes, such as Fragile X syndrome (FXS) and Rett syndrome, have been associated with ASD, yet empirical studies have estimated that single gene and chromosomal defects only account for a minority of ASD cases.Citation6 Evidence has accumulated that implicates a role of environmental factors in ASD.Citation7 For example, one recent study of 192 twin pairs reported that environmental factors were estimated to account for 55% of the risk of developing autistic disorder compared with 37% for genetic factors; a similar risk pattern was also observed for developing the broader diagnosis of ASD.Citation8
Despite a better understanding of some of the genetic, environmental, and physiologic abnormalities associated with ASD, a better understanding of how these factors influence brain function and development is still lacking.Citation9 One of the more promising avenues of research has converged on the excitatory–inhibitory balance of cerebral cortical circuits. Indeed, ASD may be associated with cortical hyperexcitability, due to deficits in cortical inhibitory circuits or glutamate receptor pathway abnormalities.Citation10–Citation12
Some of the strongest evidence of excitatory-inhibitory imbalances in the brains of individuals with ASD is derived from the Fmr1-knockout mouse. This is an animal model of FXS, the most prevalent single gene disorder in the ASD population, which accounts for approximately 1%–5% of boys with ASD.Citation7 FXS includes many cognitive and behavioral deficits that are seen as comorbid conditions in ASD, including intellectual disability, epilepsy, anxiety, aggression, and attention deficit hyperactivity disorder,Citation13 and approximately 20% of boys with FXS meet the criteria for ASD.Citation14 FXS is a trinucleotide repeat disorder that results from a failure to transcribe the FMR1 gene, a gene that encodes the FXS mental retardation protein. This protein is essential for regulating protein synthesis, particularly at excitatory synapses, and is intimately involved in neural plasticity. The Fmr1-knockout mouse model shows similar neuropathology to that found in the human brain of FXS boys, including increased dendritic spine density and excessive protein synthesis.
The Fmr1-knockout mouse model has provided insights into the neuropathology of FXS and ASD, particularly the abnormalities in the excitatory-inhibitory balance associated with ASD. Specifically, studies have demonstrated that mGluR1 and mGluR5, two metabotropic excitatory glutamate receptors, when stimulated by glutamate, promote protein synthesis, thereby worsening the already ongoing excessive protein synthesis at excitatory synapses.Citation15 Studies have demonstrated that reducing mGluR5 stimulation results in a reduction in the abnormal behavior characteristic of the Fmr1-knockout mouse model.Citation16,Citation17 Two avenues have been developed to address this abnormality in the balance of excitatory-inhibitory neurotransmission. First is the development of glutamate antagonistsCitation16,Citation18 in order to reduce cortical excitation. The second is to increase neural inhibition. The major cortical inhibitory pathway relies on gamma-aminobutyric acid (GABA) signaling. GABAB agonists like STX209Citation19 are particularly interesting as they act both pre- and post-synaptically. Pre-synaptically, GABAB agonists can actually inhibit the release of glutamate into the synaptic cleft.
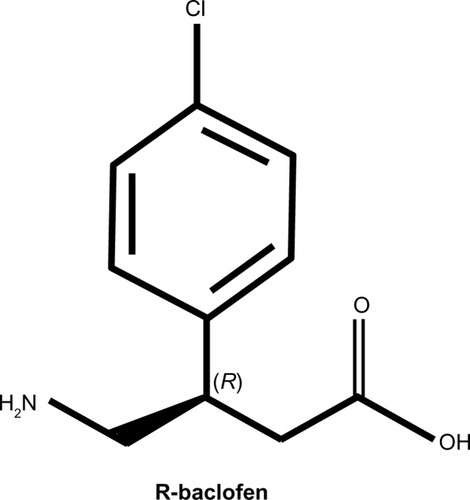
For several decades, baclofen, a GABA receptor type B agonist, has been utilized both orally and intrathecally to treat spasticity by increasing the GABAergic pathways that participate in the monosynaptic and polysynaptic spinal reflex loop. Oral baclofen, which is the racemic mixture of the R and S isomers, can have significant adverse effects. In particular, baclofen, especially at high doses, tends to cause somnolence.Citation20,Citation21 Several forms of baclofen have been recently developed to improve its tolerability. Arbaclofen is the active R-enantiomer of baclofen (). STX209, developed by Seaside Therapeutics, Inc. (Cambridge, MA, USA), has been used in clinical studies investigating treatments for FXS and ASD. The pharmacodynamics and metabolites of arbaclofen are slightly different than racemic baclofen. This is because only the S-enantiomer produces a metabolic byproduct.Citation20 Thus, the R-enantiomer does not have a metabolic byproduct, hastening its elimination and simplifying the pharmacodynamics of its systemic and central effects.
Arbaclofen has been developed for several other conditions besides ASD and FXS. For example, arbaclofen is being used in ongoing clinical trials to treat spasticity in multiple sclerosis. Arbaclofen placarbil has been developed for gastroesophageal reflux. It is a prodrug with significantly superior absorption as compared to baclofen, thus improving the stability of blood levels.Citation21 Several double-blind, placebo-controlled (DBPC) clinical trials have demonstrated some efficacy of arbaclofen placarbil for gastroesophageal reflux in specific subgroups of patients.Citation22–Citation24 Arbaclofen placarbil has also been shown to demonstrate efficacy for spasticity in patients with spinal cord injuries in a DBPC crossover study.Citation25
Since this is a promising treatment for these neurodevelopmental disorders that have no alternative US Food and Drug Administration (FDA)-approved medical treatment for the symptoms that define the disorders, it is important to understand the evidence for this novel treatment. Thus, this review systematically examines the literature on the use of arbaclofen for treating ASD and related disorders, reviewing both the animal and human studies that provide support for its use as a novel treatment.
Methodology
The PICO (patient problem or population [P], intervention [I], comparison [C], and outcome(s) [O]) framework was used to conduct this review. The goal was to find research studies that address the use of arbaclofen in patients or models of ASD. Arbaclofen was not compared to any other treatment and all study designs were considered. The primary goal was to consider improvement in any symptom or pathological marker associated with ASD as a result of arbaclofen treatment.
A prospective protocol for this systematic review was developed a priori, and the search terms and selection criteria were chosen in an attempt to capture all pertinent publications. A computer-aided search of PubMed, Google Scholar, CINAHL, Embase, Scopus, and ERIC databases from inception through to February 2014 was conducted to identify pertinent publications using the search terms “autism”, “autistic”, “Asperger”, “ASD”, “pervasive”, and “PDD” in combination with the term “arbaclofen” or “R-baclofen”. The references cited in identified publications were also searched to locate additional studies. Studies were screened by reviewing abstracts of all potentially relevant publications. Studies were included if they: 1) involved individuals or animal models of ASD and 2) reported arbaclofen as an intervention. Articles were excluded if: 1) they were conference proceedings without a published abstract, 2) did not present new or unique data, or 3) presented duplicate data.
An analysis of the level of evidence for the treatment of arbaclofen for ASD was also provided in order to quantitatively analyze the strength of evidence for this treatment. The strengths and weaknesses of the included studies were ranked using a well-established level of evidence scale.Citation26 Using this scale, each study was individually assessed to determine the corresponding level of evidence, ranging from level 1 to 5 (). After assessing all identified studies for each treatment, a grade of recommendation was derived for arbaclofen () ranging from A (solid evidence) to D (troublingly, inconsistent, or inconclusive evidence). We summarized and synthesized the information about arbaclofen regarding its efficacy but also then reviewed the evidence for its tolerability and safety. Finally, we provided recommendations for further studies.
Table 1 Levels of evidence
Table 2 Grade of recommendation
Results and discussion
Literature related to arbaclofen
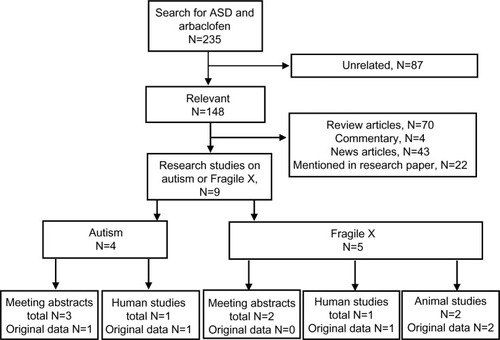
provides the results of the systematic review, which revealed 148 unique, relevant articles concerning arbaclofen and ASD, with the majority of these articles being review articles or news articles. Only nine articles were original research reports, with four of these reports specifically concerning ASD, with the other five reports considering FXS, which is regarded as a model of ASD. For the reports on FXS, one published paper concerned human participants, while two published papers concerned animal models. The other two studies were meeting abstracts that reported the same data as the published papers. For the reports on ASD, there was only one published paper on human participants, while the other four reports were meeting abstracts. One meeting abstract reported original data from a clinical trial while the others reiterated the data from the published paper.
Figure 2 Flow diagram of systematic literature search for articles concerning autism (including Fragile X Syndrome) and arbaclofen or R-baclofen.
Abbreviation: ASD, autism spectrum disorder.
Evidence from animal models
The Fmr1-knockout mouse model shows similar neuropathology to that found in the human brain of FXS boys and is a model of ASD (as described in the Introduction). To determine if STX209 could provide improvement in both behavioral and neuropathological characteristics of the Fmr1-knockout mouse, age-matched male wild-type and Fmr1-knockout littermates with and without STX209 treatment where compared.Citation27 Neuropathological measurements included protein synthesis and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking in hippocampal neurons, polysome profiling in brain tissue, and spine density in pyramidal neurons from the binocular visual cortex. Behavioral measures included the incidence of audiogenic seizures, marble burying (a measure of repetitive behavior) and the open field traveling test (an index of general motor activity).
Overall, this study demonstrated that STX209 reversed neuropathological and behavioral abnormalities in the Fmr1-knockout mouse. Treatment with STX209 decreased protein synthesis and AMPA receptor internalization in hippocampal neurons, increased the 80S monosome to polysome ratio (an indication of decreased protein translation), and corrected the excess dendritic spine density characteristic of the Fmr1-knockout mouse. STX209 treatment also corrected behavioral abnormalities associated with the Fmr1-knockout mouse. STX209 significantly reduced the incidence of audiogenic seizures in the Fmr1-knockout mouse, with a significantly lower minimum effective dose as compared to racemic baclofen. STX209 also significantly reduced marble burying, a measure of repetitive behavior, in the Fmr1-knockout mouse. However, since STX209 also reduced locomotion in the open field traveling test, the authors indicated that such reduction in locomotion could have accounted for some or all of the effect of the marble burying test. With this positive behavioral and physiological data, clinical trials were initiated for STX209.
It should be mentioned that a previous study demonstrated that R-baclofen significantly reduced audiogenic seizures in the Fmr1-knockout mouse with a single dose of 1 mg/kg, but not when provided subchronically for 6 days.Citation28 In this study, R-baclofen was also found to reduce audiogenic seizures in the Fmr1-knockout mouse when provided at a lower dose (0.5 mg/kg) along with the mGluR5 inverse agonist 2-methyl-6-(phenylethynyl) pyridine.Citation28 This study also demonstrated that R-baclofen did not significantly change the expression of GABAB receptors.Citation28
Evidence from human studies
Human studies have been conducted with STX209 on both ASD and FXS populations. For the FXS population, one multisite randomized DBPC crossover study with washout was performed in the US over 15 months on 63 adults (up to 39 years old) and children (as young as 6 years old).Citation29 Dosing was flexible, with tapering up of the drug every 3–4 days to a maximum of 10 mg twice a day for children and 10 mg three times per day for adults until the optimal tolerated dose was established. The participants were evaluated at 2 and 4 weeks after starting the treatment and then tapered off the treatment over 1–2 weeks. A 7-day washout period separated the two treatments (placebo and STX209). Assessment measures included the Clinical Global Impression Scale (CGIS), the Aberrant Behavior Checklist (ABC), Vineland Adaptive Behavior Scales (Second Edition; VABS), the Social Responsiveness Scale (SRS), the Repetitive Behavior Scale-Revised, the Child and Adolescent Symptom Inventory Anxiety Scale, the Attention Deficit Hyperactivity Disorder Rating Scale-IV, a measure of vocabulary and short-term and working memory, and a Visual Analog Scale (VAS) of the child’s three most problematic behaviors. Overall, the primary endpoint, the irritability subscale of the ABC, was not significantly different between active treatment and placebo portions of the trial but a secondary endpoint, the VAS problem behavior rating, was significantly better with the active treatment as compared to the placebo. Another secondary measure, the social avoidance ABC subscale, was found to significantly improve more during active treatment as compared to placebo. In addition, when the FXS participants with the worst social impairments were analyzed separately, multiple outcome measures, including the VABS socialization subscale, the CGIS Severity and Improvement, clinical and parent treatment preference, and percentage of responders, were significantly better during active treatment as compared to placebo. Thus, overall, this study supports the notion that STX209 improved social function, particularly in FXS individuals with the most significant social impairments. This study did suffer from some significant limitations, including participant heterogeneity and the relatively short exposure to active treatment, especially considering the fact that the drug dose was being optimized during the relatively short treatment period.
Two clinical studies, one open-label and one DBPC,Citation30,Citation31 have been conducted in individuals with ASD using STX209. In an 8-week, open-label trial, 32 children and adolescents with ASD and an ABC irritability score ≥17 were titrated up to optimal tolerated dose, increasing the dose every 3–4 days to a maximum dose of 10 mg twice a day for participants aged 6–11 years of age or 10 mg thrice a day for participants aged 12–17 years of age.Citation30 Outcome measures including the ABC, the CGIS Severity and Improvement, the SRS, the Children’s Yale–Brown Obsessive Compulsive Scale – modified for Pervasive Development Disorder, the Child and Adolescent Symptom Inventory-4 Anxiety scale, the Attention Deficit Hyperactivity Disorder Rating Scale-IV, the VABS, and the Leiter-R Brief intellectual quotient, occurred every 2 weeks. Significant improvement occurred on all primary and secondary outcomes measures except the VABS, the Leiter-R Brief intellectual quotient, and the ABC inappropriate speech subscale. Overall, 41% were rated as responders using a very rigorous criterion. This initial open-label study confirmed the tolerability of STX209 in the ASD population and demonstrated possible efficacy for a broad range of outcome measures, suggesting that further controlled studies were warranted.
To further investigate the efficacy of STX209 in the ASD population, a 12-week DBPC trial was conducted on 150 children, adolescents, and young adults with ASD and reduced social function (as defined by an ABC lethargy/social withdrawal subscale ≥8).Citation31 Treatment was titrated over 4 weeks to a maximum of 10 mg thrice a day for participants aged 5–11 years of age or 15 mg thrice a day for participants aged 12–21 years of age, with 130 completing the study. Outcome measures included the ABC, the CGIS Severity and Improvement, and the Socialization and Communication subscales of the VABS. The primary outcome measure, the ABC lethargy/social withdrawal subscale, was not significantly different between the STX209 and placebo groups. For the secondary outcomes measures, CGIS Severity significantly improved more in the STX209 group as compared to the placebo group, but other secondary endpoints did not demonstrate greater improvements in the STX209 group as compared to the placebo group. However, a per protocol analysis demonstrated a significant improvement in the VABS socialization subscale in the STX209 as compared to the placebo group. The improvement in the VABS socialization subscale was also more evident in higher functioning participants. This study demonstrated that STX209 improved a measure of social function in some children with ASD but that future studies are needed to define the precise characteristics of the individuals with ASD that will optimally respond to STX209. Since this study has only been reported in abstract form, it is difficult to assess some of the potential limitations and the significance of the subgroup analyses.
Grade of recommendation
For FXS, there are two bench research studiesCitation27,Citation28 (Level 5) and one high-quality randomized controlled studyCitation29 (Level 1b), resulting in a Grade of Recommendation of B for improving social function in individuals with FXS. This needs to be tempered by the fact that the evidence supports the effect of STX209 for a subgroup of FXS patients with poor social function. For the ASD population, there is one open-label trialCitation30 (Level 2b) and one high-quality randomized DBPC trialCitation31 (Level 1b), resulting in a Grade of Recommendation for individuals with ASD of B for improving social function. This recommendation does, however, need to be tempered by the fact that STX209 appears to have an effect on the subgroup of ASD children who are high-functioning and that the participants in the clinical trials were preselected to be irritable in one studyCitation30 (the open-label study) and to have poor social function in anotherCitation31 (the DBPC study).
Safety and tolerability
Overall, the clinical studies suggest that STX209 is well-tolerated. In the FXS DBPC trial, the incidence of adverse events was not significantly different between the active treatment and placebo arms of the study, although the one serious adverse event that occurred was in the active treatment arm. The individual was hospitalized for increased irritability; the individual had a history of previous hospitalizations for similar behavioral issues.Citation29 In the open-label ASD trial, STX209 was well-tolerated, with the most common adverse events being agitation, irritability, fatigue, hyperactivity, insomnia, and diarrhea, with the majority of the adverse events rated as mild and resolving without changing the dose.Citation30 In the DBPC study on individuals with ASD, approximately 11% of participants on STX209 discontinued because of adverse events, with the majority of the adverse events being behavioral.Citation31 This is compared to approximately 3% of participants on placebo. There was only one serious adverse event on STX209, suicidal ideation, which also occurred in one placebo participant. Thus, in the three STX209 clinical studies conducted on individuals with ASD or FXS, STX209 was well-tolerated with a low incidence of adverse events.
Clinical studiesCitation22–Citation25 in other disease populations have used arbaclofen placarbil, a non-STX209 form of arbaclofen. Although this form of arbaclofen has slightly different pharmacodynamics than STX209, some safety data can be obtained from these other clinical studies. In three DBPC trials for gastoesophegeal reflux, arbaclofen placarbil was found to be generally well-tolerated without a significant increase in adverse events in the treatment groups related to the medication.Citation22–Citation24 The most common adverse events in these studies were nausea, somnolence, dizziness, and headache, with one study reporting a dose-related increase in these adverse events.
Current product development
One of the primary goals of Seaside Therapeutics, Inc., was to develop targeted treatments for developmental disorders such as FXS and ASD based on modulation of the excitatory–inhibitory imbalance in neuronal pathways. As part of this venture, Seaside Therapeutics, Inc. developed several compounds, including STX209 and STX107 which is a selective mGlu5 antagonist. STX209 was the flagship compound that was used in several clinical trials in FXS and ASD patients. In June 2012, Seaside Therapeutics, Inc. announced a partnership with the giant pharmaceutical company Roche to further develop its compound. However, when the primary clinical outcome endpoint of the recent STX209 clinical trials did not attain statistical significance Roche backed out of its partnership with Seaside Therapeutics, Inc. Coincident with this announcement, Seaside Therapeutics, Inc. prematurely closed STX209 open-label extension studies for patients with FXS and ASD, citing limited resources to continue clinical trials. The former vice president of Seaside Therapeutics, Inc., Dr Paul Wang, has been quoted several times emphasizing that STX209 did demonstrate significance at several secondary endpoints in the clinical studies. Despite this positive outlook, Dr Wang left the company and is now the Senior Vice President and Head of Medical Research at Autism Speaks. STX209 does not appear to be available to patients on a clinical or research basis, even for compassionate use.
Future research
Clearly, preliminary clinical studies support the notion that arbaclofen in the form of STX209 has promise for improving social function in the ASD population. However, it is clear that it is more effective in particular subpopulations of individuals with ASD. Although the limited studies have provided some mixed results, it is clear that they point to social function as the target outcome measure and indicate that arbaclofen may be most effective for individuals with ASD that have particular characteristics. Thus, future clinical studies will need to carefully select participants or be designed to account for the known heterogeneity in the ASD population. Given the lack of treatment for children with ASD and FXS, hopefully a commercial or research organization will obtain funding to further investigate arbaclofen.
Disclosure
The author reports no conflicts of interest in this work.
References
- APADiagnostic and Statistical Manual of Mental Disorders4th edWashington, DCAmerican Psychiatric Association1994
- VolkmarFRMcPartlandJCFrom Kanner to DSM-5: autism as an evolving diagnostic conceptAnnu Rev Clin Psychol Epub1292013
- KulageKMSmaldoneAMCohnEGHow will DSM-5 affect autism diagnosis? A systematic literature review and meta-analysisJ Autism Dev Disord Epub2162014
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal InvestigatorsPrevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010MWR Surveill Sum20142863Suppl 2121
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal InvestigatorsCenters for Disease Control and PreventionPrevalence of autism spectrum disorders – autism and developmental disabilities monitoring network, 14 sites, United States, 2002MMWR Surveill Summ200756122817287715
- SchaeferGBMendelsohnNJProfessional Practice and Guidelines CommitteeClinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisionsGenet Med201315539940723519317
- RossignolDAGenuisSJFryeREEnvironmental toxicants and autism spectrum disorders: a systematic reviewTransl Psychiatry20144e36024518398
- HallmayerJClevelandSTorresAGenetic heritability and shared environmental factors among twin pairs with autismArch Gen Psychiatry201168111095110221727249
- RossignolDAFryeREA review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposuresMol Psychiatry201217438940122143005
- CoghlanSHorderJInksterBMendezMAMurphyDGNuttDJGABA system dysfunction in autism and related disorders: from synapse to symptomsNeurosci Biobehav Rev20123692044205522841562
- ChadmanKKGuarigliaSRYooJHNew directions in the treatment of autism spectrum disorders from animal model researchExpert Opin Drug Discov20127540741622494457
- StafstromCEHagermanPJPessahINPathophysiology of Epilepsy in Autism Spectrum DisordersNoebelsJLAvoliMRogawskiMAOlsenRWDelgado-EscuetaAVJasper’s Basic Mechanisms of the Epilepsies4th edBethesda, MDNational Center for Biotechnology Information2012
- TranfagliaMRThe psychiatric presentation of fragile x: evolution of the diagnosis and treatment of the psychiatric comorbidities of fragile X syndromeDev Neurosci201133533734821893938
- CliffordSDissanayakeCBuiQMHugginsRTaylorAKLoeschDZAutism spectrum phenotype in males and females with fragile X full mutation and premutationJ Autism Dev Disord20073747384717031449
- OsterweilEKKruegerDDReinholdKBearMFHypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndromeJ Neurosci20103046156161562721084617
- YanQJRammalMTranfagliaMBauchwitzRPSuppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEPNeuropharmacology20054971053106616054174
- DolenGOsterweilERaoBSCorrection of fragile X syndrome in miceNeuron200756695596218093519
- CarlsonGCGlutamate receptor dysfunction and drug targets across models of autism spectrum disordersPharmacol Biochem Behav2012100485085421315104
- BraatSKooyRFFragile X syndrome neurobiology translates into rational therapyDrug Discov Today Epub272014
- Sanchez-PonceRWangLQLuWvon HehnJCherubiniMRushRMetabolic and pharmacokinetic differentiation of STX209 and racemic baclofen in humansMetabolites20122596613
- LalRSukbuntherngJTaiEHArbaclofen placarbil, a novel R-baclofen prodrug: improved absorption, distribution, metabolism, and elimination properties compared with R-baclofenJ Pharmacol Exp Ther2009330391192119502531
- VakilNBHuffFJBianAJonesDSStamlerDArbaclofen placarbil in GERD: a randomized, double-blind, placebo-controlled studyAm J Gastroenterol201110681427143821519360
- GersonLBHuffFJHilaAArbaclofen placarbil decreases postprandial reflux in patients with gastroesophageal reflux diseaseAm J Gastroenterol201010561266127520040914
- VakilNBHuffFJCundyKCRandomised clinical trial: arbaclofen placarbil in gastro-oesophageal reflux disease – insights into study design for transient lower sphincter relaxation inhibitorsAliment Pharmacol Ther201338210711723721547
- NancePWHuffFJMartinez-ArizalaAEfficacy and safety study of arbaclofen placarbil in patients with spasticity due to spinal cord injurySpinal Cord20114999749021577221
- HowickJChalmersIGlasziouPThe Oxford 2011 Levels of Evidence: Oxford Centre for Evidence-Based Medicine2011 [cited September 3, 2011]. Available from: http://www.cebm.net/index.aspx?o=5653Accessed March 21, 2014
- HendersonCWijetungeLKinoshitaMNReversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofenSci Transl Med20124152152ra28
- PaceyLKTharmalingamSHampsonDRSubchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in fragile X syndromeJ Pharmacol Exp Ther2011338389790521636656
- Berry-KravisEMHesslDRathmellBEffects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trialSci Transl Med20124152152ra27
- EricksonCAVeenstra-VanderweeleJMMelmedRDSTX209 (Arbaclofen) for autism spectrum disorders: an 8-week open-label studyJ Autism Dev Disord201344495896424272415
- DelahuntyCWalton-BowenKKuriyamaNRandomized, Controlled, Phase 2 Trial of STX209 (Arbaclofen) for Social Function in ASDOrlando, FLAmerican Acadamy of Pediatrics2013