Abstract
Purpose
This postmarketing surveillance survey was conducted to investigate the utility of the CONsistency in r-FSH Starting dOses for individualized tReatmenT (CONSORT) calculator for individualizing recombinant human follicle-stimulating hormone (r-hFSH) starting doses for controlled ovarian stimulation (COS) in routine clinical practice.
Methods
This was a 3-year, open-label, observational study evaluating data from women undergoing COS for assisted reproductive technology at 31 German fertility centers. Physicians stated their recommended r-hFSH starting dose, then generated a CONSORT-recommended r-hFSH starting dose. Physicians could prescribe any r-hFSH starting dose. The primary objective was to compare the r-hFSH starting dose recommended by the physician with the CONSORT-calculated dose and that prescribed. Statistical analyses were conducted post hoc.
Results
Data were collected from 2,579 patients; the mean (standard deviation [SD]) age was 30.5 (2.93) years (range: 19–40 years). The mean (SD) CONSORT-calculated r-hFSH starting dose was significantly lower than the physician-recommended dose (134.5 [38.0] IU versus 164.6 [47.1] IU; P<0.0001); the mean (SD) starting dose prescribed was 162.2 (48.4) IU. CONSORT-calculated doses were prescribed for 27.3% (number [n] =677) of patients, and non-CONSORT-calculated doses prescribed for 72.7% (n=1,800). The mean (SD) number of oocytes retrieved per patient was 10.6 (6.15) and 11.4 (6.66) in the CONSORT and non-CONSORT groups, respectively; the mean (SD) number of embryos transferred per patient was 1.98 (0.41) and 2.03 (0.45), respectively. Clinical pregnancy rates per COS cycle were 38.8% (CONSORT) and 34.8% (non-CONSORT) (P=0.142); clinical pregnancy rates per embryos transferred were 45.0% and 39.5%, respectively (P=0.049). Miscarriage occurred in 14.8% of all clinical pregnancies (CONSORT: 12.5%; non-CONSORT: 15.3%). The rate of grade 3 ovarian hyperstimulation syndrome (OHSS) was 0.3% (n=2) in the CONSORT group and 0.6% (n=11) in the non-CONSORT group. OHSS led to hospitalization in 0.81% (n=21) of cases (CONSORT group: 0.74% [n=5]; non-CONSORT group: 0.83% [n=15]).
Conclusion
Physician-recommended r-hFSH starting doses were generally higher than those calculated by CONSORT; most patients were prescribed a higher starting dose than that recommended by CONSORT.
Introduction
In women undergoing controlled ovarian stimulation (COS) during assisted reproductive technology (ART), daily doses of recombinant human follicle-stimulating hormone (r-hFSH) to induce multifollicular development typically range from 100–350 IU.Citation1 However, women differ greatly in their ovarian response to gonadotrophin stimulation, and there is currently no established consensus for determining the optimal follicle-stimulating hormone (FSH) dose, with starting doses often based on patient characteristics, such as age, combined with a physician’s clinical experience and judgment.Citation1 Various factors have been identified that may be associated with ovarian response to COS, including: patient age,Citation2–Citation5 body mass index (BMI),Citation6 estradiol,Citation7 basal FSH,Citation7–Citation9 inhibin-B,Citation7,Citation8 anti-Müllerian hormone,Citation2,Citation7 ovarian stromal blood flow,Citation10 and antral follicle count (AFC).Citation7,Citation8,Citation10,Citation11
The CONsistency in r-FSH Starting dOses for individualized tReatmenT (CONSORT) dosing algorithm was developed to determine the optimal starting dose of r-hFSH (follitropin alfa) in normo-ovulatory women aged 18–34 years undergoing COS in a long gonadotrophin-releasing hormone (GnRH) agonist protocol.Citation1,Citation12 Four baseline variables were included in the CONSORT calculator,Citation1 namely age, BMI, early follicular phase serum FSH level, and AFC. In a multinational pilot study to clinically validate the CONSORT calculator, 76.4% (123/161) of patients were allocated a lower dose than the physician would have prescribed in routine practice.Citation12 Despite achieving good clinical pregnancy rates (34.2% per started cycle), cycle cancellation owing to an inadequate response often occurred in the lowest starting dose group (75 IU).Citation12 The CONSORT algorithm was subsequently modified so that the lowest starting dose that could be recommended was 112.5 IU. A multinational, randomized study was then conducted to compare the ovarian response in normo-ovulatory women (number [n] =200) aged 18–34 years who received a daily dose of r-hFSH allocated by the CONSORT calculator versus a standard starting dose of 150 IU.Citation13 Mean daily and total doses of r-hFSH were significantly lower in the CONSORT group than in the standard-dosing group (P<0.001 for both). In the CONSORT group, fewer oocytes were retrieved when compared with the standard-dosing group (P=0.037; primary endpoint); however, the number of embryos transferred (ET) in each group (P=0.674) and the clinical pregnancy rates (CONSORT 36.0% versus standard dosing 35.5%; estimated difference [95% confidence interval {CI}]: 0.6 [−13.5, 14.6]) were similar.Citation13
The postmarketing surveillance study reported here evaluated the starting dose of r-hFSH recommended by the treating physician, the dose recommended by the CONSORT calculator, and the dose actually received by the patient in routine clinical practice in Germany.
Methods
Study design
This 3-year, multicenter, open-label, observational, post-marketing surveillance study (ClinicalTrials.gov identifier: NCT01100333) evaluated data from women treated at 31 German ART centers. Standard practice in Germany for ART during the study period was followed; therefore, up to three embryos could be transferred and supernumerary two pronuclei oocytes could be cryopreserved for future use.
Primary objective
The primary objective of this noninterventional study was to compare the starting dose of r-hFSH recommended by the treating physician for COS with the starting dose selected by the CONSORT calculator and the dose actually prescribed to the patient.
Patients
The study collected data from women who were scheduled to undergo COS with follitropin alfa (GONAL-f®; Merck Serono SA, Geneva, Switzerland, a subsidiary of Merck KGaA, Darmstadt, Germany) to induce multifollicular development in an ART cycle. Key inclusion criteria included baseline age ≤35 years, BMI ≤30 kg/m2, and early follicular phase (cycle days 2–4) basal serum FSH levels ≤12 IU/L. Patients were excluded if they required combination treatment with clomiphene citrate, follitropin beta, urine-derived human FSH, human menopausal gonadotrophin, or luteinizing hormone during COS. One ART cycle per patient was included in this analysis; in 67.3% of patients, this was their first ART cycle and in 13.6%, this was their second ART cycle. For the remaining patients, this cycle number was three or above.
Treatment
Prior to the initiation of COS, physicians were asked to record their recommended starting dose of r-hFSH for each patient. Each patient’s age, BMI, early follicular phase serum FSH level, and AFC were then entered into the online CONSORT calculator, and the CONSORT-recommended starting dose of r-hFSH was recorded. The CONSORT calculator could select one of seven r-hFSH starting doses: 75 IU; 112.5 IU; 150 IU; 187.5 IU; 225 IU; 262.5 IU; or 300 IU. However, the treating physician could prescribe any starting dose of r-hFSH, and they were asked to recommend this before they were aware of the CONSORT-recommended dose. After this, they were free to follow the CONSORT-recommended dose, their original recommended dose, or a different prescribing dose. Dose reduction was possible if the treating physician considered a patient to be at risk of ovarian hyperstimulation syndrome (OHSS). Also, cycles could be cancelled in cases of inadequate or excessive ovarian response.
Data collection
The physician-planned starting dose of r-hFSH, four baseline factors, CONSORT-calculated starting dose, and the starting dose actually prescribed for the patient were recorded and faxed to ANFOMED GmbH (Möhrendorf, Germany). Additional routine clinical and laboratory data were entered prospectively by the physician into a standardized electronic data collection system (RecDateCitation14). All data were recorded anonymously.
Outcome measures
The primary endpoint was a comparison of the starting doses of r-hFSH recommended by the treating physician, selected by the CONSORT calculator, and those actually administered to the patient. Secondary endpoints were dose of r-hFSH on the last day of COS, total dose of r-hFSH, duration of COS, and the number of oocytes retrieved. Clinical pregnancy (ultrasound identification of an intrauterine gestational sac with fetal cardiac activity) rates were also reported.
Safety outcomes included the incidence of serious adverse events (SAEs). OHSS was considered to be an SAE if it resulted in hospitalization. Grade 1 OHSS was considered to be a normal response to COS.
Statistical analysis
No formal sample size calculation was performed, owing to the exploratory and observational nature of the study. All statistical analyses were unplanned, and therefore conducted post hoc. The differences between the physician-recommended, CONSORT-calculated, and prescribed starting doses of r-hFSH were compared using the two-sided Wilcoxon signed-rank test, with P-values of ≤0.05 considered to be significant. Secondary outcomes were compared between patients who received the CONSORT-calculated starting dose (CONSORT group) and those who did not receive the CONSORT-calculated dose (non-CONSORT group) using descriptive statistics. Clinical pregnancy rates per started cycle and per embryo transfer were also compared between groups using a two-sided chi-square test, with P-values of ≤0.05 considered to be significant. There was no imputation for missing data; therefore, for each variable, the number of cycles included differed.
Changes to planned analyses
The planned inclusion criteria restricted enrollment to patients aged ≤35 years, with a BMI ≤30 kg/m2 and who used a long GnRH agonist protocol; however, patients aged ≥35 years (n=87) with a BMI >30 kg/m2 (n=16), or who used a GnRH antagonist protocol (n=707), were included in the analyses, as data on the use of the CONSORT algorithm in these patient groups were considered to be of scientific value.
Subgroup analysis
Additional comparisons of pregnancy rates in each group were performed for patients aged <35 years and ≥35 years. A two-sided chi-square test was used to compare the clinical pregnancy rates of patients aged <35 years, while a two-sided Fisher’s exact test was used to compare clinical pregnancy rates in patients aged ≥35 years owing to the small sample size.
Results
Baseline characteristics
Evaluable data on 2,579 patients were matched with cycle data saved on the RecDate database for 2,579 cycles of ART between April 2008 and July 2011. Baseline patient characteristics are shown in .
Table 1 Baseline patient demographic and disease characteristics
A long GnRH agonist protocol was used in 70.0% (1,649/2,356) of cycles, while the remaining 30.0% (707/2,356) of cycles used a GnRH antagonist, or a short or ultralong GnRH agonist protocol. Details of the GnRH protocol used for downregulation were missing for 208 patients; 15 patients did not undergo downregulation.
Starting dose of r-hFSH
In patients with both the CONSORT-calculated and the prescribed starting doses available (n=2,477), 27.3% (677/2,477) received the CONSORT-calculated starting dose and 72.7% (1,800/2,477) received a non-CONSORT-calculated starting dose.
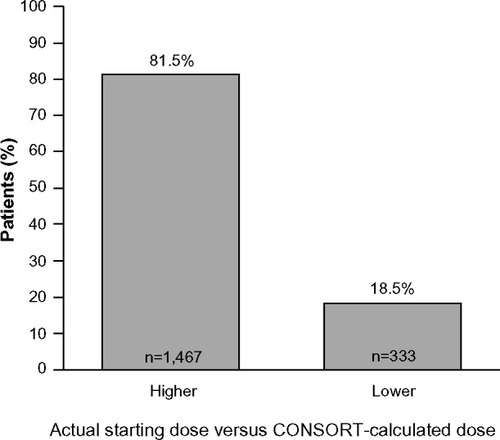
The proportion of cycles during which patients in the non-CONSORT group were prescribed r-hFSH starting doses that were higher or lower than their CONSORT-recommended dose are shown in .
Figure 1 Starting doses of r-hFSH received by patients in the non-CONSORT group (n=1,800) compared with their CONSORT-calculated dosea.
Abbreviations: n, sample number; CONSORT, CONsistency in r-FSH Starting dOses for individualized tReatmenT; r-hFSH, recombinant human follicle-stimulating hormone.
The mean doses recommended by the physician and CONSORT-calculated doses are shown in . The CONSORT-calculated mean dose was significantly lower than the physician-recommended mean dose (P<0.0001); however, no significant difference was found between the mean starting doses of r-hFSH recommended by the physician and the actual dose received ().
Table 2 Starting doses of r-hFSH
COS characteristics
The mean dose of r-hFSH on the last day of COS and the mean total dose of r-hFSH were both lower in the CONSORT group when compared with the non-CONSORT group; however, the need for dose adjustment and duration of COS were similar in both groups ().
Table 3 COS characteristics in the CONSORT and non-CONSORT starting dose groupsTable Footnotea
For patients who received an r-hFSH starting dose that was higher or lower than the CONSORT-calculated dose, the mean (standard deviation [SD]) doses of r-hFSH on the last day of COS and of total r-hFSH were higher than those in the “CONSORT-calculated dose” group ().
Table 4 COS characteristics in the higher than, actual, and lower than CONSORT-calculated dose groups
Treatment outcomes
Treatment outcomes for the CONSORT and non-CONSORT starting dose groups are shown in . Similar numbers of oocytes were retrieved and similar numbers of embryos per patient were transferred in the two groups.
Table 5 Treatment outcomes in the CONSORT and non-CONSORT starting dose groups
The clinical pregnancy rate per ET was higher in the CONSORT group than in the non-CONSORT group (P=0.049), but there was no difference in the clinical pregnancy rates per COS cycle started between the two groups (P=0.142).
In women aged <35 years (96.6%; n=2,491), clinical pregnancy rates per ET were higher in the CONSORT than in the non-CONSORT group (P=0.044), but clinical pregnancy rates per started cycle were similar in the two groups (P=0.144; ). For women aged ≥35 years (3.4%; n=87), clinical pregnancy rates per ET (P=0.273) and per COS cycle (P=0.296) were slighter lower in the CONSORT group versus the non-CONSORT group, but these differences were not significant ().
Table 6 Clinical pregnancy rates in the CONSORT and non-CONSORT starting dose groups for patients aged <35 years and ≥35 years
Safety
Symptoms of OHSS were reported in 11.3% (291/2,579) of cycles (OHSS data were missing for 34.6% [892/2,579] of cycles); 3.0% (78/2,579) were grade 2 (CONSORT: 2.8% [19/677]; non-CONSORT: 2.9% [53/1,800]) and 0.5% (14/2,579) were grade 3 (CONSORT: 0.3% [2/677]; non-CONSORT: 0.6% [11/1,800]). OHSS was considered an SAE (ie, required hospitalization) in 0.81% (21/2,579) of cases (CONSORT group: 0.74% [5/677]; non-CONSORT group: 0.83% [15/1,800]).
Miscarriage occurred in 14.8% (132/889) of clinical pregnancies (CONSORT: 12.5% [33/263]; non-CONSORT: 15.3% [96/626]). Singleton births occurred in 55.2% (363/658) of patients, twins in 24.3% (160/658), and triplets in 0.46% (3/658). No births were reported in 20.1% (132/658) of cycles. Data were missing for 265 cycles.
Discussion
This postmarketing surveillance study compared the starting dose of r-hFSH recommended by the CONSORT calculator with that planned by the treating physician and the starting dose actually received by the patient. Overall, approximately one-third of patients were prescribed the CONSORT-calculated starting dose of r-hFSH. Most of the patients in the non-CONSORT group (81.5%) received a starting dose of r-hFSH that was higher than the CONSORT-calculated dose. Indeed, the mean CONSORT-calculated starting dose (134.5 IU) was lower than both the starting dose recommended by the physician (164.6 IU) and the actual starting dose (162.2 IU). Although similar proportions of patients in the CONSORT and non-CONSORT groups required dose adjustments during COS, the mean r-hFSH dose on the last day of COS and mean total r-hFSH dose were higher in the non-CONSORT group.
The mean number of oocytes retrieved (11.4 oocytes versus 10.6 oocytes, respectively) and clinical pregnancy rates per started cycle (34.8% versus 38.8%, respectively [P=0.142]) were similar in the non-CONSORT and CONSORT groups. However, clinical pregnancy rates per ET were lower in the non-CONSORT group (39.5%) than in the CONSORT group (45.0% [P=0.049]). This may have been due to differences in procedures across the 31 centers. Furthermore, this study was not powered to analyze secondary endpoints. Nevertheless, the clinical pregnancy rates per ET in our study compare favorably with those reported from the German IVF Registry, which includes women aged >35 years using in vitro fertilization (29.5%–30.0%) and 28.4% using intracytoplasmic sperm injection.Citation15,Citation16
Severe OHSS (grade 3) was reported in 0.5% of cycles, with a lower frequency in the CONSORT group than in the non-CONSORT group (0.3% versus 0.6%, respectively). Miscarriage occurred in 14.8% of clinical pregnancies, with a numerically lower rate in the CONSORT group than in the non-CONSORT group (12.5% versus 15.3%, respectively). The rates of miscarriage per clinical pregnancy observed in this study were lower than those calculated using data (fresh cycles) from the 2008 and 2010 reports of the German IVF Registry (18.9% and 18.8% per clinical pregnancy, respectively).Citation15,Citation16
We acknowledge the limitations associated with the observational nature of this postmarketing surveillance study, which include a lack of randomization, potential patient selection bias, and the reliance on the accurate reporting of events. One of the main criticisms of observational data is the selection bias that may arise during assignment of a treatment by the physician and the lack of matched patient populations in treatment groups.Citation17 In this study, the treating physicians may have based their choice of r-hFSH starting dose on nonstudied covariates, or they may have been influenced by the CONSORT calculator, altering their opinion of an appropriate recommended dose for consecutive patients treated. In addition, patients at greater risk of poor or excessive ovarian response were more likely to have cycles cancelled; the withdrawal of these patients could bias the results, for example, when calculating clinical pregnancy rates per ET. Also, owing to reliance on the accurate reporting of events, the numbers of cycles for which evaluable data were available for outcomes differed and, therefore, these results may not be generalizable to all populations of women undergoing ART. Furthermore, since the primary population in this study comprised women aged ≤35 years with a BMI of ≤30 kg/m2 – a population of women less commonly seen in United States clinical practice, for example – these data may not be applicable to all populations of women. Although SAEs have been described here, the reporting of adverse events is not required in German postmarketing surveillance studies. Finally, it should also be noted that all statistical analyses were carried out post hoc, so any conclusions from these data should be made with caution.
In the present study, the CONSORT calculator only selected 75 IU as the starting dose in three patients. Interestingly, the CONSORT calculator pilot study found an inadequate response using starting doses of 75 IU, and this dose was subsequently excluded from the CONSORT randomized controlled study.Citation13
Despite the original CONSORT algorithm being developed for women aged <35 years (undergoing COS using a long GnRH agonist protocol),Citation1,Citation12 no significant difference was found in clinical pregnancy rates (per ET or per started cycle) for the CONSORT and non-CONSORT groups for the subset of older (≥35 years) women in this study. However, owing to the small patient number, these findings should be treated with caution. Also, short or ultralong GnRH agonist or antagonist protocols were used for some patients, again representing scenarios that the CONSORT calculator was not developed for.
Conclusion
Dosing models for use in COS represent an important step toward individualization of ART protocols. In this large observational study, the starting doses of r-hFSH for COS recommended by physicians in routine clinical practice were generally higher than the CONSORT-calculated doses. In addition, most patients received an actual starting dose of r-hFSH that was higher than the CONSORT-calculated dose, suggesting a lack of trust by the physician in the CONSORT-calculated dose. Further research to evaluate the full clinical impact of dosing algorithms on safety and efficacy during COS is warranted.
Author contributions
Olaf GJ Naether contributed to the study design, data analysis, manuscript drafting, and critical discussion. Andreas Tandler-Schneider contributed to the study design, data analysis, manuscript drafting, and critical discussion. Wilma Bilger contributed to the study design, study administration, data analysis, manuscript drafting, and critical discussion.
Acknowledgments
We thank the German RecDate Study Group for providing the data. Data management and analysis were conducted by ANFOMED GmbH, Möhrendorf, Germany (funded by Merck KGaA, Darmstadt, Germany). We thank Dr Elmar Beck (of ANFOMED GmbH) for his statistical expertise.
We also thank Laura McDonagh, Hannah Wills, and Catherine Kidd of Caudex Medical (funded by Merck KGaA, Darmstadt, Germany) for their assistance in the preparation of the manuscript.
Merck KGaA, Darmstadt, Germany provided funding for the data documentation and statistical analysis of this postmarketing surveillance study and for medical writing support for the preparation of this manuscript. Except as otherwise stated, the authors confirm independence from the funding source. Except as expressly stated, the funding source did not participate or intervene in any way in the collection and/or interpretation of data and/or in the writing of this article. This article expresses the views and opinions of the authors. The funding provided by Merck KGaA is not conditioned in any way on any pre-existing or future business relationships between the company and the authors or on any business or other decisions the authors may have made in the past or may make in the future relating to the company or its products. The funding source and the authors expressly recognize that this manuscript is the result of an observational (noninterventional), postmarketing surveillance study, and as such it reflects current clinical practice in a specific country. In doing so, it may refer to pharmaceutical products, therapeutics, or indications not yet registered or approved in a given country. The funding source expressly declares that it does not promote, endorse, or advocate any potential uses of its products outside of the approved indications foreseen in the registered label in a given country. Any such uses remain a medical decision to be taken only by a suitably qualified health care professional.
Disclosure
Olaf GJ Naether and Andreas Tandler-Schneider have nothing to disclose. Wilma Bilger is an employee of Merck Serono GmbH, Germany.
References
- HowlesCMSaundersHAlamVEngrandPFSH Treatment Guidelines Clinical PanelPredictive factors and a corresponding treatment algorithm for controlled ovarian stimulation in patients treated with recombinant human follicle stimulating hormone (follitropin alfa) during assisted reproduction technology (ART) procedures. An analysis of 1378 patientsCurr Med Res Opin200622590791816709312
- FlemingRDeshpandeNTraynorIYatesRWDynamics of FSH-induced follicular growth in subfertile women: relationship with age, insulin resistance, oocyte yield and anti-Mullerian hormoneHum Reprod20062161436144116439501
- TempletonAMorrisJKParslowWFactors that affect outcome of in-vitro fertilisation treatmentLancet19963489039140214068937279
- van Noord-ZaadstraBMLoomanCWAlsbachHHabbemaJDte VeldeERKarbaatJDelaying childbearing: effect of age on fecundity and outcome of pregnancyBMJ19913026789136113652059713
- WrightVCSchieveLAReynoldsMAJengGKissinDAssisted reproductive technology surveillance – United States, 2001MMWR Surveill Summ200453112015123982
- VerbergMFEijkemansMJMacklonNSHeijnenEMFauserBCBroekmansFJPredictors of low response to mild ovarian stimulation initiated on cycle day 5 for IVFHum Reprod20072271919192417485438
- BroekmansFJKweeJHendriksDJMolBWLambalkCBA systematic review of tests predicting ovarian reserve and IVF outcomeHum Reprod Update200612668571816891297
- BancsiLFBroekmansFJEijkemansMJde JongFHHabbemaJDte VeldeERPredictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserveFertil Steril200277232833611821092
- BancsiLFBroekmansFJMolBWHabbemaJDte VeldeERPerformance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysisFertil Steril20037951091110012738501
- Popovic-TodorovicBLoftALindhardABangsbøllSAnderssonAMAndersenANA prospective study of predictive factors of ovarian response in ‘standard’ IVF/ICSI patients treated with recombinant FSH. A suggestion for a recombinant FSH dosage normogramHum Reprod200318478178712660271
- BancsiLFBroekmansFJLoomanCWHabbemaJDte VeldeERImpact of repeated antral follicle counts on the prediction of poor ovarian response in women undergoing in vitro fertilizationFertil Steril2004811354114711542
- OlivennesFHowlesCMBoriniACONSORT study groupIndividualizing FSH dose for assisted reproduction using a novel algorithm: the CONSORT studyReprod Biomed Online200918219520419192339
- OlivennesFTrewGBoriniARandomized, controlled, open-label, non-inferiority study of the CONSORT algorithm for individualized dosing of follitropin alfaReprod Biomed Online201530324825725596910
- PakSJWarlichJvan RooijTNRecDate – an IT-solution for the documentation and quality management of reproductive medicineZentralbl Gynakol20011238482486 German11562816
- BühlerKBals-PratschMBlumenauerVDIR Annual 2010 – German IVF-registryJournal für Reproduktionsmedizin und Endokrinologie201184253280
- KupkaMSBühlerKDahnckeWWendelkenMBals-PratschMSummary of the 2008 annual report of the German IVF registryReproduktionsmedizin und Endokrinologie2010713438
- KrishnanEFriesJFMeasuring effectiveness of drugs in observational databanks: promises and perilsArthritis Res Ther200462414415059263
