Abstract
Background
Topical corticosteroid (TCS) treatment is widely prescribed for atopic dermatitis (AD). However, TCS treatment is associated with tachyphylaxis, and discontinuation after long-term use may cause exacerbation of symptoms. Some AD patients are reluctant to use TCS.
Objective
To evaluate patient-reported short- and long-term outcomes after discontinuation of TCS treatment for AD.
Methods
Questionnaires were distributed to adult AD patients (n=1,812) of doctors who did not recommend TCS as first-line therapy for patients who preferred to avoid TCS. Data collected included current TCS use, duration of TCS use, past discontinuation of TCS use, exacerbation of symptoms after discontinuation of TCS use, and limitations to daily activities because of AD.
Results
Of 918 respondents, 97.7% had used TCS, of whom 92.3% had experienced discontinuation of TCS use. After discontinuation, 63.9% experienced their most severe AD symptoms ever. The severity of exacerbation of symptoms was significantly correlated with the length of TCS use (P<0.001). Although most respondents who experienced severe exacerbation after TCS discontinuation were not current TCS users, they generally had fewer current limitations to activities than when AD symptoms were at their worst.
Conclusion
Adult Japanese AD patients who experience severe exacerbation of symptoms immediately after discontinuation of TCS use generally improve over time. We suggest caution regarding long-term TCS treatment in AD patients.
Introduction
The prevalence of atopic dermatitis (AD) has dramatically increased over recent decades.Citation1 In Japan, the average reported age of patients with AD has increased, and some patients experience severe and refractory symptoms.Citation2 Topical corticosteroid (TCS) treatment is widely prescribed for AD, but may cause skin atrophy, tachyphylaxis, and exacerbation of symptoms after discontinuation of long-term use.Citation3–Citation5
The Guidelines for the Management of Atopic Dermatitis in 2000 published by the Japanese Dermatological Association recommend TCS treatment as first-line therapy for AD, combined with secondary treatments such as skin hydration, elimination of aggravating factors, and immunosuppressant medications.Citation6 Although the current Japanese treatment guidelines discourage long-term TCS use, especially on the face,Citation7 discontinuation may be difficult. Severe exacerbation of symptoms such as rosacea-like dermatitis has been reported after long-term use and discontinuation of TCS in Japanese patients,Citation8–Citation10 and in other populations.Citation11–Citation14 However, no systematic review is reported for TCS use in AD patients in the Cochrane Library, and durations of randomized placebo-controlled trials for TCS use in AD patients are all short (mostly up to 20 weeks; 44 weeks at the longest)Citation15 and do not focus on withdrawal outcome (including exacerbation). Under such circumstances, some AD patients have refused to apply TCS,Citation16,Citation17 and they tend to be considered as having corticosteroid phobia in medical practice.Citation18 Only a few studies have focused on patient-reported outcomes in AD patients who experienced exacerbation of symptoms after discontinuation of TCS use.Citation16,Citation17
This study aimed to conduct a large-scale questionnaire survey targeting adult AD patients who had difficulty using TCS or preferred to avoid TCS, to evaluate short- and long-term outcomes after discontinuation of TCS use.
Subjects and methods
A mail survey was conducted from April 2006 to March 2007, targeting AD patients aged ≥16 years who had difficulty using TCS or preferred to avoid TCS. The questionnaires were distributed by 24 doctors who did not recommend TCS as first-line therapy for patients who preferred to avoid TCS. Questionnaires were distributed to 1,812 patients, who were asked to return the questionnaires by mail.
The questionnaire (see Figure S1) was constructed by Takahashi-Ando, together with medical doctors and AD patients. The following data were collected for each patient: sex, age, past TCS use, duration of TCS use, current TCS use, past and current tacrolimus ointment use, past discontinuation of TCS use, exacerbation of symptoms after discontinuation of TCS use, limitations to daily activities because of AD at the time of the worst symptoms and at the time of the survey, what improved the symptoms of AD if they were under control, and difficult experiences with medical treatment and consulting medical experts (see Figure S1).
Stata/SE (v13.0 for Windows; StataCorp LP, College Station, TX, USA) was used for statistical analysis. Binary logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for binary response variables measured in the survey. Ordinal logistic regression was used to estimate OR and 95% CI for ordinal response variables. Wald tests were used to estimate P-values to test the null hypothesis of no association between response and explanatory variables. Contrasts were used to compare the effects of the five categories of duration of TCS use on severity of exacerbation. The contrasts were based on comparing each category with the observation-weighted mean of subsequent categories. The proportional odds assumption for ordinal regression was tested using a likelihood ratio test.
Results
Completed questionnaires were received from 918 respondents (393 males, 523 females, two unknown). The age of distribution of respondents was as follows: 16–20 years, 8.4%; 21–30 years, 41.5%; 31–40 years, 38.8%; 41–50 years, 8.4%; ≥51 years, 2.4%; and unknown, 0.5%.
TCS use at any time was reported by 97.7% of the respondents (897/918). Duration of TCS use was as follows: <1 year, 7.7%; 1–5 years, 23.5%; 5–10 years, 22.7%; 10–20 years, 25.8%; >20 years, 14.6%; unknown, 5.7%. Among respondents who reported TCS use, 92.3% (828/897) had experienced discontinuation of TCS use, 83.2% (746/897) were not current users of TCS, 53.4% (479/897) had ever used tacrolimus ointment, and 10.1% (91/897) were current users of tacrolimus ointment.
Among respondents who had experienced discontinuation of TCS use, 63.9% (529/828) reported experiencing their most severe AD symptoms ever immediately after discontinuation (). shows that a longer duration of TCS use was associated with greater exacerbation of symptoms after discontinuation. Statistical analysis showed a 27% increased odds of reporting more severe exacerbation of symptoms by increasing duration of TCS use (OR: 1.27, 95% CI: 1.12–1.44, P<0.001). There was no evidence of non-proportionality of odds across the categories of the response (P=0.28). We also investigated whether the effect of duration of TCS use on severity of exacerbation after discontinuation is confounded by age in a multivariable ordinal logistic regression. Results showed that the duration of TCS use is not confounded by age (OR changed minimally from 1.27 to 1.24) and that age is not an independent predictor of severity of exacerbation (P=0.12).
Figure 1 Duration of use of TCS and severity of worsening at withdrawal of TCS.
Abbreviations: TCS, topical corticosteroids; y, years.
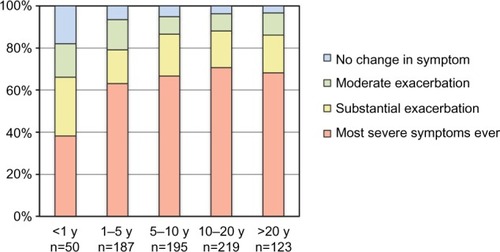
Table 1 Current use of TCS according to the severity of exacerbation of symptoms after discontinuation of TCS use n=828
In an additional analysis, we investigated further the relationship between duration of TCS use and severity of exacerbation after discontinuation by performing contrasts between the five categories of duration. Results showed that <1 year duration is statistically different from durations of >1 year (P<0.0001); 1–5 years duration may be different from durations >5 years (P=0.058); however, there was no evidence of a difference between durations longer than 5 years (P>0.4).
Patients who experienced more severe exacerbation after discontinuation of TCS use were significantly less likely to be current users of TCS and tacrolimus ointment than those who experienced less severe exacerbation ( and S1). Statistical analysis showed that increasing severity of exacerbation of symptoms immediately after discontinuation of TCS use was associated with a 47% decreased odds of current use of TCS (OR: 0.53, 95% CI: 0.44–0.65, P<0.001) and a 23% decreased odds of current use of tacrolimus (OR: 0.77, 95% CI: 0.60–0.98, P=0.035; Table S1).
Among respondents who reported their most severe AD symptoms ever immediately after discontinuation of TCS use, 86.2% (456/529) were not current users of either TCS or tacrolimus ointment. In these subjects, limitations to daily activities because of AD were evaluated (). At the time of their worst symptoms (immediately after discontinuation of TCS use), 37.3% reported difficulty with indoor movement and only 5.0% reported no limitations to any of the activities surveyed. At the time of the survey, 48.5% reported no limitations to any of the activities.
Table 2 Limitations to daily activities because of AD among AD patients who reported their most severe AD symptoms after TCS discontinuation and no current use of TCS nor tacrolimus ointment n=456
Among those whose symptoms were under control (n=466), the following answers were obtained for the reasons of improvement (multiple choice): spontaneous recovery (n=64), change of environment (n=57), change of diet (n=180), exercise (n=61), use of TCS or tacrolimus ointment (n=19), avoidance of TCS use (n=310), herbal treatments or other alternative remedies (n=151), and other (n=107). There was an association between ‘avoidance of TCS use’ and AD symptoms immediately after discontinuation, with 79.0% (226/286) of those who experienced their worst symptoms ever after discontinuation, 68.4% (54/79) of those who experienced substantial exacerbation, 51.2% (21/41) of those who experienced moderate exacerbation, and 15.0% (3/20) of those who experienced no change, considering ‘avoidance of TCS use’ to be a reason for improvement of their symptoms. Statistical analysis showed that increasing severity of exacerbation of symptoms immediately after discontinuation of TCS use is associated with a more than two-fold increased odds of reporting ‘avoidance of TCS’ as one of the reasons for improvement of symptoms (OR: 2.2, 95% CI: 1.7–2.8, P<0.001).
Many patients listed difficulties they had experienced in consulting medical experts and using medical treatments, including: long waiting time prior to consultation, short consultation time, no explanation of how to use medications, medical experts unwilling to listen to patients’ experiences, and unwanted treatments being recommended.
Discussion
This is the first large-scale study focusing on self-reported short- and long-term outcomes in adult AD patients after discontinuation of TCS use. The severity of exacerbation of symptoms was significantly correlated with the length of TCS use. Our findings are consistent with those of a previous study that reported a correlation between duration of TCS use and exacerbation of facial AD after discontinuation.Citation19 Interestingly, the effect of discontinuing TCS use on severity of exacerbation increased with increasing durations of use over the first 5 years but then appeared to plateau, suggesting at least a partial explanation as to why some patients experienced TCS-induced withdrawal exacerbation, while others did not.
In Japan, it is believed that major reasons for stopping TCS are exaggerated information of adverse effects made by the media and sellers of unproven alternatives to TCS,Citation20 and they were simply taken as corticosteroid phobia. However, this study found that those who experienced more severe aggravation after discontinuation were significantly less likely to be using TCS at the time of the survey (P<0.001), indicating that reluctance to use TCS may be associated with the patient’s own experience of TCS use. Thus, when a patient is reluctant to use TCS, physicians should consider the possibility that he/she has experienced TCS-induced adverse effects.
According to comments in the free description space on the questionnaire, many patients experienced lower effectiveness of TCS after continuous use, which induced them to discontinue TCS. Symptoms such as intense pruritus, redness, burning, and edema after discontinuation of TCS use were severe enough to induce suicidal thoughts in some subjects. Respondents reported symptoms consistent with the previously reported ‘red skin syndromes’Citation11 or rosacea-like dermatitis.Citation13,Citation14 Many patients experienced severe exacerbation not only on their face but also other areas of their body, and in some cases, symptoms spread to areas where patients had never applied TCS, suggesting that their symptoms were not simple rosacea-like dermatitis, which typically localizes on the face. Dermatitis occurring on areas where patients never applied TCS could possibly be attributed to corticosteroids being absorbed and systemically distributed. Such cases of dermatitis have also been reported by patients using inhaled corticosteroidsCitation21 or orally administered corticosteroids.Citation22
Many of these patients recovered eventually, and believed that continuing to avoid TCS use was necessary for recovery. Many also expressed frustration that conventional doctors pressured them to continue TCS use. Such patients may stop consulting conventional doctors, and may therefore be underrepresented in some clinical studies. The majority of these patients were not using tacrolimus ointment to control their symptoms.
A limitation of this study is potential selection bias. The response rate was 51%, which could induce selection bias, although the rate is typical for a mail survey. As this study focused on adult AD patients who had difficulty using TCS or preferred to avoid TCS, the population surveyed was not a representative sample of AD patients in Japan and they may have been more liable to experience TCS-induced withdrawal exacerbation than general AD patients. However, data from a multicenter retrospective analysis of 1,271 general AD patients (210 infants, 546 children, and 515 adolescents and adults) by Furue et al,Citation23 showed that after 6 months of TCS treatment, 3% had worse symptoms and 58% showed no improvement. In the subgroup of adult cases, the proportion of those with worse symptoms and no improvement was higher than those in the subgroups of children and infants. These results suggest that TCS treatment may not be beneficial for some general AD patients, especially adult cases. Furthermore, it may lead to worsening symptoms and long-term use could potentially result in TCS-induced adverse effects.
In conclusion, the findings of this study show that a substantial number of adult AD patients experience exacerbation of symptoms after discontinuation of TCS use, especially after long-term use, and many patients who experience such an exacerbation subsequently improve. Physicians are urged to be cautious of recommending long-term TCS use, and to be aware of the possibilities of TCS-induced adverse effects when a patient has become reluctant to use TCS. Since TCS is the first-line therapy worldwide, the adverse effects shown in our study could occur in general AD patients; thus, studies that investigate the outcome of TCS withdrawal in general AD patients are urgently needed.
Acknowledgments
We greatly appreciate the cooperation of the dermatologists, physicians, AD patients, and supporting help groups. This study was supported by the Takagi Fund of Citizen Science. Writing assistance was provided by Edanz Group Global Ltd (Wanchai, Hong Kong).
Supplementary materials
Table S1 Current use of tacrolimus according to the severity of exacerbation of symptoms after discontinuation of TCS use n=828.
Disclosure
The authors report no conflicts of interest in this work.
References
- WilliamsHRobertsonCStewartAWorldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in ChildhoodJ Allergy Clin Immunol19991031 Pt 11251389893196
- NishiokaKAtopic eczema of adult type in JapanAustralas J Dermatol199637Suppl 1S7S98713020
- KligmanAMFroschPJSteroid addictionInt J Dermatol19791812331153891
- ZhengPSLavkerRMLehmannPKligmanAMMorphologic investigations on the rebound phenomenon after corticosteroid-induced atrophy in human skinJ Invest Dermatol19848243453526368701
- HenggeURRuzickaTSchwartzRACorkMJAdverse effects of topical glucocorticosteroidsJ Am Acad Dermatol2006541115 quiz 16–1816384751
- KawashimaMTakigawaMNakagawaHGuidelines for therapy for atopic dermatitisJpn J Dermatoallergol2000110710991104 [Japanese with English abstract]
- SaekiHFurueMFurukawaFGuidelines for management of atopic dermatitisJ Dermatol2009361056357719785716
- FukayaMImprovement of atopic dermatitis after discontinuation of topical corticosteroid treatmentArch Dermatol2000136567968010815874
- TamakiAOhashiAIshidaTNakamuraMTreatment without steroid ointment for adult-type atopic dermatitisJpn J Dermatoallergol19931230234 [Japanese with English abstract]
- FukayaMSatoKSatoMTopical steroid addiction in atopic dermatitisDrug Healthc Patient Saf2014613113825378953
- RapaportMRapaportVThe red skin syndromes: corticosteroid addiction and withdrawalExpert Rev Dermatol200614547561
- ChenAYZirwasMJSteroid-induced rosacealike dermatitis: case report and review of the literatureCutis200983419820419445310
- RathiSKKumrahLTopical corticosteroid-induced rosacea-like dermatitis: a clinical study of 110 casesIndian J Dermatol Venereol Leprol2011771424621220878
- BhatYJManzoorSQayoomSSteroid-induced rosacea: a clinical study of 200 patientsIndian J Dermatol2011561303221572787
- HanifinJGuptaAKRajagopalanRIntermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patientsBr J Dermatol2002147352853712207596
- FukayaMWhy do patients with atopic dermatitis refuse to apply topical corticosteroids?Dermatology2000201324224511096196
- AndoNTestimony of One Thousand Patients of Atopic DermatitisTokyo, JapanKodomono Miraisya2008 [Japanese]
- Aubert-WastiauxHMoretLLe RhunATopical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequencyBr J Dermatol2011165480881421671892
- KatayamaIYokoyamaAMatsunagaTYokozekiHNishiokaKNon-steroid therapy for refractory facial dermatitis in adult atopic dermatitisJpn J Dermatol1994104875880 [Japanese with English abstract]
- TakeharaKAtopy BusinessTokyo, JapanBungeishunju Ltd2000 [Japanese]
- TashkinDPMurrayHESkeansMMurrayRPSkin manifestations of inhaled corticosteroids in COPD patients: results from Lung Health Study IIChest200412641123113315486373
- TomitaYTagamiHSteroid-withdrawal rosacea-like dermatitisJ Dermatol19891643353372532223
- FurueMTeraoHRikihisaWClinical dose and adverse effects of topical steroids in daily management of atopic dermatitisBr J Dermatol2003148112813312534606

