Abstract
Background
Dalfampridine extended release tablets (dalfampridine-ER; prolonged-, modified, or sustained-release fampridine outside the US), 10 mg twice daily, was approved by the US Food and Drug Administration (FDA) in January 2010 to improve walking in people with multiple sclerosis, as determined by an increase in walking speed.
Objective
To provide a descriptive analysis of reported adverse events (AEs) for commercially available dalfampridine-ER from March 2010 through March 31, 2015.
Methods
Five-year postmarketing data for dalfampridine-ER were available from the exposure of approximately 107,000 patients in the US (103,700 patient-years). Commonly reported AEs (≥2% of all reported AEs) and serious AEs were determined. The incidence of reported seizures was determined and the events were further investigated.
Results
Among the 107,000 patients exposed to dalfampridine-ER (70% female; mean age 52.1), the most common AEs were dizziness (3.7%), insomnia (3.2%), balance disorder (3%), fall (2.4%), headache (2.4%), nausea (2.1%), and urinary tract infection (2%). Other common AEs were drug ineffectiveness (5.8%), gait disturbance (4.6%), and inappropriate dosing (3.1%). Serious AEs included rare anaphylactic reactions (five cases) and drug hypersensitivity reactions (eight cases). A total of 657 seizure cases were reported (6.3/1,000 patient-years); of these, 324 were medically confirmed (3.1/1,000 patient-years). Incidence of reported seizures was stable over time. Duration of treatment prior to a seizure ranged from a single dose to >4 years; 12% of the seizures occurred within a week of starting treatment.
Conclusion
The 5-year US postmarketing safety data of dalfampridine-ER is consistent with the safety profile observed in clinical trials. Incidence of reported seizures remained stable over time. Since commercial availability in March 2010, a warning regarding the risk of anaphylaxis and severe allergic reactions was added to the US prescribing information.
Introduction
Mobility impairment is common among patients with multiple sclerosis (MS), and walking difficulty is often considered the most challenging aspect of the disease.Citation1 Based on consistent changes in walking speed in clinical studies,Citation2,Citation3 dalfampridine extended release tablets (dalfampridine-ER; known as prolonged-release fampridine in Europe and as fampridine modified- or sustained-release elsewhere) 10 mg twice daily, were approved by the US Food and Drug Administration (FDA) to improve walking in people with MS.Citation4 Dalfampridine-ER is a broad spectrum blocker of voltage-gated potassium channels that enhances nerve conduction in demyelinated axons, which is the proposed mechanism of action for its clinical effects.Citation5
In the clinical trials, dalfampridine-ER at the recommended dosage of a 10 mg tablet taken twice daily, approximately 12 hours apart, had a tolerability profile with adverse events (AEs) that were generally of mild or moderate severity. The most commonly reported AEs were balance disorders, paresthesia, dizziness, anxiety, headache, insomnia, and confusion.Citation2,Citation3 Since people with MS have an approximately 3-fold higher risk of seizure relative to the general population,Citation6,Citation7 and because dalfampridine-ER has a dose-dependent risk of seizure,Citation8,Citation9 it was important to monitor for seizure-related events. In the double-blind clinical studies, seizures were reported in 0.2% of patients,Citation2,Citation3 and in the pooled extensions of these trials the seizure rate was 0.8% (corresponding to 4.1 per 1,000 patient-years) among those who had up to 5 years of dalfampridine-ER exposure.Citation10 These rates were generally similar to the 3.49 per 1,000 patient-years reported by Eriksson et alCitation11 for a first seizure in a Swedish MS population. Although dalfampridine-ER is contraindicated in patients with a history of seizures,Citation4 a Risk Evaluation and Mitigation Strategy was instituted to inform health care providers and patients about seizure risk with use of dalfampridine-ER. The Risk Evaluation and Mitigation Strategy was subsequently discontinued, since the program met its goals and was no longer required.Citation12 However, postmarketing surveillance for seizure events continues to be an important component of monitoring the safety of dalfampridine-ER in clinical practice.
A previous analysis evaluated the safety data of dalfampridine-ER in the US over the first year postmarketing.Citation13 The findings were consistent with the safety profile observed during clinical development, including a seizure incidence that was not substantially different from that observed in clinical trials. The current study was undertaken to extend this safety evaluation of dalfampridine-ER in the US based on AEs reported from 2010 through 2015, encompassing the first 5 years postmarketing. Furthermore, data from a post-approval program of enhanced surveillance and assessment were evaluated to further characterize the seizure risk potential.
Methods
AE reporting and database
In the US, AEs can be reported to the drug manufacturer by consumers as well as by health care professionals. The manufacturer is then required to send all reported AEs to the FDA.Citation14 The current analysis was prospectively designed to descriptively evaluate all AEs of dalfampridine-ER, regardless of causality, that were reported and recorded in the manufacturer’s product safety database from March 1, 2010 through March 31, 2015; the product safety database is an Argus (Oracle® Argus Safety 7.0; Redwood Shores, CA, USA) AE management system. All AEs in the database were classified using the Medical Dictionary for Regulatory Activities (MedDRA; version 18.0). As this research was conducted on deidentified data from electronic databases containing federally mandated AE information, no ethics committee approval was sought.
AEs
The proportion of AE reports was estimated by event, as classified at the MedDRA preferred term level. The most commonly reported AEs were those with a frequency ≥2% of all reported AEs.
Reports of seizures
Patients with events reported under the MedDRA High Level Group Term Seizures, including subtypes, were defined as seizure cases. Any seizure case reported or confirmed by a health care practitioner was considered as medically confirmed; whereas, those reported only by consumers were considered as potential seizure cases. The fact that dalfampridine-ER is only available for dispensing via specialty pharmacies in the US allowed accurate estimates of patient exposure. The incidence rate of seizure cases was calculated using the estimate of patient exposure of 103,700 person-years. All reports of confirmed and potential seizures were followed-up using a structured questionnaire designed to collect information on dose, regimen, usage, seizure history, concomitant medications, and any prior use of pharmacy-compounded formulations of dalfampridine. All cases of seizure were reviewed to ascertain patient demographics, time to event from treatment onset, and the presence of any additional seizure-related risk factors.
Results
Demographics
Surveillance data were available from approximately 107,000 patients who filled a prescription for dalfampridine-ER during the 5 years of product availability, representing an overall exposure of approximately 103,700 patient-years. Of these patients for whom demographic data were available (n=87,815), 70% were female, and the mean age was 52.1 years; the proportion of patients aged ≥65 years was 13%. Among the 107,000 patients exposed to dalfampridine-ER, 23.9% (25,526) reported at least one AE. Of the patients who reported AEs, 75% were female, and the mean age was 55.3; the proportion of patients aged ≥65 years was 19% ().
Table 1 Demographic characteristics of dalfampridine-ER users
AEs
A total of 60,949 AEs were reported that were generally consistent with those reported in double-blind clinical studies and comparable to those in the FDA-approved prescribing information for dalfampridine-ER. The most commonly reported AEs () included dizziness (3.7%), insomnia (3.2%), balance disorder (3%), fall (2.4%), headache (2.4%), nausea (2.1%), and symptoms of urinary tract infection (UTI; 2%). Other common AEs were drug ineffectiveness (5.8%), gait disturbance (4.6%), and inappropriate dosing (3.1%). Inappropriate dosing was reported as an AE when a patient failed to take the prescribed dose, including accidental overdose; in the majority of cases, this code represented 10 mg once daily dosing.
Table 2 Most commonly reported adverse events (≥2% of all reported events; N=60,949
Commonly reported serious AEs (SAEs) included dyspnea (n=48), suicidal ideation (n=32), and cerebrovascular events (n=37). Rare SAEs included anaphylactic reactions (n=5) and drug hypersensitivity reactions (n=8). At least two of the anaphylaxis events were considered positive dechallenge cases, ie, the events resolved after withdrawal of dalfampridine-ER. At least 4 non-serious positive rechallenge drug hypersensitivity cases were observed (ie, signs and symptoms of the event occurred again following reinitiation of the drug).
Seizures
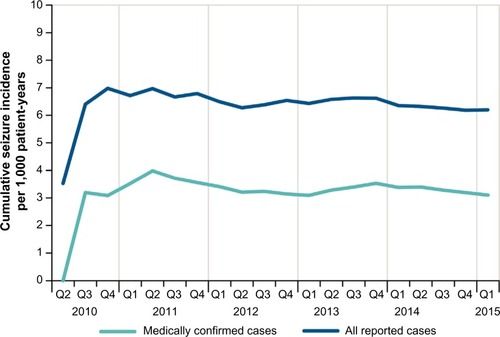
Of the 107,000 patients exposed to dalfampridine-ER, 657 cases reported seizures, representing a rate of approximately 6.3 per 1,000 patient-years of use (); a total of 685 seizure events were reported among these cases, indicating that some had more than one seizure. Of these, 324 cases were medically confirmed, yielding an estimated incidence rate of 3.1 per 1,000 patient-years of use (). The demographic characteristics of patients who reported seizures showed that approximately two thirds were women (449 women, 171 men, 37 unknown), and the mean patient age was 53 years; 168 cases were missing age information.
Table 3 Reported seizure cases in the first 5 years of postmarketing surveillance of dalfampridine-ER
Duration of dalfampridine-ER treatment prior to the earliest seizure event ranged from one dose to >4 years. A total of 79 seizures (12%) occurred within a week of starting treatment, and the incidence rate of reported seizure cases was approximately 5.7 per 1,000 patient-years of use after the first week ().
In addition to MS, other seizure-related risk factors that were present in these patients with reported seizures were a prior history of seizure, including febrile seizure (7%); concomitant use of bupropion or tramadol (7%); and a history of renal impairment (1%; severity not known) (). Inappropriate dosing was also a risk factor, as 5% of all reported seizure cases (n=32) had dosing intervals that were shorter than 8 hours. For both the total and medically confirmed seizure cases, the cumulative incidence was stable over time (), with only month-to-month fluctuations in reporting.
Discussion
This 5-year postmarketing safety evaluation of dalfampridine-ER shows that the reported AEs were generally consistent with expectations based on experience from the dalfampridine clinical development program. The findings were similar to those of previously reported 1- and 2-year US postmarketing surveillance periods,Citation13,Citation15 and comparable to dalfampridine postmarketing surveillance data in France, using the European Database for Multiple Sclerosis.Citation16
In the first 5 years of marketing, the incidence rate of medically confirmed seizures was 3.1 per 1,000 patient-years of use, and 11% of the cases occurred within the first week of exposure. The incidence of any reported seizure was higher than the incidence of medically confirmed seizure, 6.3 per 1,000 patient-years of use, with 12% of those events occurring within the first week of use. Considering that there are different symptoms that can be confused with a seizure disorder such as confusion, loss of consciousness, incoherence, muscle spasms, and syncope, the actual incidence of seizure associated with dalfampridine-ER is likely between the overall observed rate and that of medically confirmed cases. Further, the actual seizure rate in real-world users of dalfampridine-ER has been stable over time and comparable to the incidence rate from the long-term, open-label trials (4.1 per 1,000 patient-years).Citation10
A number of factors may have contributed to the reported seizures in this postmarketing patient population. In addition to the known seizure risk associated with MS, a notable proportion of patients who reported seizures had one or more other risk factors, including prior seizure history, concomitant use of medications that are known to lower seizure threshold, or renal impairment. The severity of renal impairment in this postmarketing population was not known. Since a history of seizure, or moderate or severe renal impairment are contraindications for dalfampridine-ER use,Citation4 these findings suggest that patients may be either inadequately evaluated for contraindications or treated despite the presence of contraindications. In this regard, we previously reported that 3% of dalfampridine-ER users had a history of seizures and 5% had some level of renal impairment at the time of prescription.Citation17 Another factor that may have contributed to seizures is the use of concomitant medications that are known to lower seizure threshold. Use of such medications is not a specific contraindication of dalfampridine. However, the fact that 7% of all patients who reported seizures had concomitant use of either bupropion or tramadol suggests the need for careful monitoring. Inappropriate dosing of dalfampridine-ER also led to seizures in some patients; there is a dose-dependent increase in the risk of seizures with dalfampridine-ER.Citation8,Citation9 Given that there is only one approved dose (10 mg, twice daily, approximately 12 hours apart), reports of inappropriate dosing are seemingly high, thus highlighting the importance of adhering to the prescribing guidelines as well as to the approved dosing schedule for dalfampridine-ER.
Overall, the most frequently reported postmarketing AEs were those that had already been identified in the clinical trials as common events including dizziness, insomnia, balance disorder, headache, nausea, and UTI.Citation2,Citation3 People with MS have an increased risk for neurogenic bladder and UTI;Citation18 however, recent studies that used confirmatory laboratory analyses showed that dalfampridine-ER does not increase the risk of confirmed bacterial infections but only of urinary symptom reports.Citation18,Citation19 Although falls were commonly reported in this postmarketing population, the rate of falls was lower than expected for an MS population, in which the underlying pathology of gait disturbance, balance impairment, spasticity, and muscle weakness contributes to the fall frequency and a higher fall incidence than in the general population.Citation20,Citation21 For perspective, a meta-analysis that focused on a 3-month observation period reported falls in 56% of people with MS.Citation22 In clinical studies, the frequency of falls was not higher with dalfampridine-ER relative to placebo.Citation2,Citation3,Citation19 Similarly, while gait disturbance was frequently reported as an AE, this event was generally reported with a similar incidence in placebo-and dalfampridine-ER-treated patients in clinical trials.Citation2,Citation3,Citation19
Although the reporting of SAEs did not indicate additional safety signals, there were several clinically relevant safety issues that should be noted, including the occurrence of dyspnea in 48 patients. It is likely that these reports are related to disease rather than to treatment with dalfampridine-ER, as respiratory complications may occur in any stage of MS owing to respiratory muscle weakness, bulbar weak ness, impaired voluntary control and impaired automatic control.Citation23–Citation25 Similarly, cerebrovascular events were reported in some patients and these may also relate to the disease, as the prevalence of ischemic heart disease, congestive heart failure, and stroke are higher in MS relative to the general population.Citation26,Citation27 Suicidal behavior was also reported in the postmarketing population. It is likely these events are not related to treatment, as patients with MS have been reported to have a greater than 2-fold higher suicide rate relative to the general population.Citation28,Citation29
There were a few incidences of rare SAEs, such as hypersensitivity reactions and anaphylaxis reported in a small number of patients. Anaphylaxis is a clinical diagnosis based on systemic manifestations, often following acute exposure to a causative agent. The true incidence of anaphylaxis in people with MS is not known, but the prevalence for the US general population is at least 1.6% and is likely to be higher.Citation30 A warning regarding the risk of anaphylaxis and severe allergic reaction was added to the dalfampridine-ER prescribing information.Citation4 Although cases of serious liver injury or cases of AEs related to abuse potential were of special interest to the FDA at the time of dalfampridine-ER approval,Citation31 neither of these issues was identified as a safety concern during the postmarketing surveillance period.Citation32
Limitations of this analysis include that it is dependent on spontaneous reporting of AEs. Thus, it is possible that some events may have been underreported. On the other hand, AEs were evaluated regardless of causality, and some of the events may reflect the disease state and may not be specific to treatment with dalfampridine.
Conclusion
Findings from 5-year postmarketing surveillance suggest that the safety profile of dalfampridine-ER in real-world clinical practice is generally consistent with the safety profile established in the clinical trials. The seizure incidence in medically confirmed cases was similar to that of previous reports, and the overall incidence of reported seizures remained stable over time. Since product launch, a warning regarding the risk of anaphylaxis and severe allergic reactions has been added to the US prescribing information. While postmarketing surveillance safety data will continue to be collected, data collected to date do not indicate any new safety signals after 5 years on the market.
Acknowledgments
This study was funded by Acorda Therapeutics, Inc. Editorial assistance was provided by E Jay Bienen, PhD, of The Curry Rockefeller Group, LLC, and was funded by Acorda Therapeutics, Inc.
Disclosure
Authors are employees and stock holders of Acorda Therapeutics, Inc. The authors confirm that this paper is an accurate representation of the study results.
References
- LaRoccaNGImpact of walking impairment in multiple sclerosis: perspectives of patients and care partnersPatient20114318920121766914
- GoodmanADBrownTRKruppLBSustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trialLancet2009373966573273819249634
- GoodmanADBrownTREdwardsKRA phase 3 trial of extended release oral dalfampridine in multiple sclerosisAnn Neurol201068449450220976768
- Ampyra® (dalfampridine) extended release (ER) tablets [prescribing information]New YorkAcorda Therapeutics, Inc.2014
- DunnJBlightADalfampridine: a brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosisCurr Med Res Opin20112771415142321595605
- NicolettiASofiaVBiondiREpilepsy and multiple sclerosis in Sicily: a population-based studyEpilepsia200344111445144814636354
- PoserCMBrinarVVEpilepsy and multiple sclerosisEpilepsy Behav20034161212609222
- HautSRBienenEJMillerAClinical overview of the seizure risk of dalfampridineExpert Opin Drug Saf201211465165722703551
- CornblathDRBienenEJBlightARThe safety profile of dalfampridine extended release in multiple sclerosis clinical trialsClin Ther20123451056106922497693
- GoodmanADBethouxFBrownTRLong-term safety and efficacy of dalfampridine for walking impairment in patients with multiple sclerosis: results of open-label extensions of two Phase 3 clinical trialsMult Scler201521101322133125583832
- ErikssonMBen-MenachemEAndersenOEpileptic seizures, cranial neuralgias and paroxysmal symptoms in remitting and progressive multiple sclerosisMult Scler20028649549912474990
- Food and Drug AdministrationSupplemental Approval, Release REMS Requirement, REMS Assessment AcknowledgmentSilver Spring, MDFood and Drug Administration2013 http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2013/022250Orig1s008ltr.pdfAccessed November 4, 2015
- JaraMBarkerGHenneyHR3rdDalfampridine extended-release tablets: one year of post-marketing safety experience in the United StatesNeuropsychiatr Dis Treat2013936537023662056
- US Food and Drug Administration [homepage on the Internet]FDA Adverse Event Reporting System (FAERS) Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/Accessed November 4, 2015
- JaraMAderaMAdedejiAHenneyHCarrazanaEDalfampridine Extended Release Tablets: Safety Profile After 2 Years of Post-marketing Experience in the United States [abstract]Mult Scler
- BertheCOngagnaJCCourtoisSProlonged-release Fampridine post-marketing experience in Alsace, France [abstract]Neurology201584SupplP1.126
- JaraMSidovarMFHenneyHR3rdPrescriber utilization of dalfampridine extended release tablets in multiple sclerosis: a retrospective pharmacy and medical claims analysisTher Clin Risk Manag2014111725565851
- KantorDChancellorMBSnellCWHenneyHR3rdRabinowiczALAssessment of confirmed urinary tract infection in patients treated with dalfampridine for multiple sclerosisPostgrad Med2015127221822225560174
- HuppertsRLyckeJShortCProlonged-release fampridine and walking and balance in multiple sclerosis: randomized MOBILE studyMult Scler Epub2015428
- MatsudaPNShumway-CookABamerAMJohnsonSLAmtmannDKraftGHFalls in multiple sclerosisPM R20113762463221777861
- GunnHJNewellPHaasBMarsdenJFFreemanJAIdentification of risk factors for falls in multiple sclerosis: a systematic review and meta-analysisPhys Ther201393450451323237970
- NilsagardYGunnHFreemanJFalls in people with MS-an individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United StatesMult Scler20152119210024948687
- HowardRSWilesCMHirschNPLohLSpencerGTNewsom-DavisJRespiratory involvement in multiple sclerosisBrain1992115Pt 24794941606478
- van KlaverenRBuyseTVan De GaerLMeekersJRochetteFDemedtsMMicturitional disturbances are associated with impaired breathing control in multiple sclerosisChest199911561539154510378546
- FarhatMRLoringSHRiskindPWeinhouseGDisturbance of respiratory muscle control in a patient with early-stage multiple sclerosisEur Respir J20134161454145623728406
- MarrieRAReiderNCohenJA systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosisMult Scler201521331833125533300
- MarrieRARudickRHorwitzRVascular comorbidity is associated with more rapid disability progression in multiple sclerosisNeurology201074131041104720350978
- SiegertRJAbernethyDADepression in multiple sclerosis: a reviewJ Neurol Neurosurg Psychiatry200576446947515774430
- ManouchehriniaATanasescuRTenchCRConstantinescuCSMortality in multiple sclerosis: meta-analysis of standardised mortality ratiosJ Neurol Neurosurg Psychiatry Epub201552
- WoodRACamargoCAJrLiebermanPAnaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United StatesJ Allergy Clin Immunol2014133246146724144575
- Food and Drug AdministrationNDA ApprovalSilver Spring, MDFood and Drug Administration2010 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2010/022250s000ltr.pdfAccessed November 4, 2015
- JaraMGauvinDVCaggianoAOWalterBParryTJHenneyHEvaluation of the Abuse Potential of Dalfampridine Extended Release Tablets (P565)28th Congress of the European Committee for Treatment and Research in Multiple SclerosisOctober 10–13; 2012Lyon, France