Abstract
Aims
To investigate changes in glycated hemoglobin (HbA1c), body weight (BW), and systolic blood pressure (SBP) in type 2 diabetes (T2D) primary care patients initiating dapagliflozin treatment.
Methods
T2D patients who started dapagliflozin in 985 general and 32 diabetologist practices (Disease Analyzer, Germany: December 2012–October 2014) were analyzed (3- and 6-month follow-up). Multivariate linear regression analyses were used to identify clinical characteristics and comorbidity associated with changes in HbA1c, BW, and SBP.
Results
The study included 1,169 T2D patients (age: 62.5 years; men: 59.3%; diabetologist care: 23%) with newly initiated dapagliflozin therapy. At the 3-month stage, dapagliflozin significantly reduced HbA1c (−0.8%±1.4%) compared to the baseline (8.5%±1.5%) (P<0.001). Changes were maintained after 6 months (−0.8%±1.5%) (P<0.001). Patients with high baseline HbA1c values (>9%) showed greater reductions in HbA1c than the overall sample (3 months −1.8%, 6 months −1.8%; both P<0.05). BW and SBP also showed statistically significant reductions with dapagliflozin over 3 and 6 months (−2.2 kg, P<0.001; −2.2 mmHg, P=0.003 and −2.5 kg, P<0.001; −2.3 mmHg, P=0.011, respectively). After 3 months, 53% of patients achieved a reduction in both HbA1c and BW; the same holds true for 45% of patients at the 6-month mark. Similar results were observed both in general and diabetologist practices. In multivariate analyses, baseline HbA1c (parameter estimate: −0.6479) and diabetologist care (−0.2553) were independent predictors of HbA1c change (6 months) (all P<0.05).
Conclusion
T2D patients treated with dapagliflozin therapy achieved statistically significant reductions in HbA1c, BW, and SBP in a real-world primary and diabetologist care setting. The changes were comparable to the results of the dapagliflozin clinical trial program.
Introduction
Renal glucose production increases with insulin resistance, the hallmark of type 2 diabetes (T2D).Citation1 Approximately 40% of the increased endogenous glucose release in patients with T2D has been attributed to renal gluconeogenesis.Citation1 Furthermore, the capacity for renal glucose reabsorption is higher in patients with T2D, thus perpetuating hyperglycemia.Citation2 SGLT2 is located in the proximal tubule and is involved in the reabsorption of glucose (~90%) in the kidney.Citation3 As the action of SGLT2 is independent of insulin, its inhibition should not be influenced by the degree of insulin resistance or insulin secretion.Citation4 Therefore, SGLT2 inhibitors have the potential to be effective in reducing hyperglycemia by stimulating urinary glucose excretion at any stage and duration of T2D. In 2015, the American Diabetes Association and European Association for the Study of Diabetes issued an update to their joint position statement on the management of hyperglycemia in T2D.Citation5 SGLT2 inhibitors are mentioned among the options for second-line therapy after treatment with metformin and as alternative first-line options in patients with contraindications to metformin in an oral triple therapy or as add-on to insulin therapy.Citation5
Dapagliflozin was the first approved selective SGLT2 inhibitor in Europe that reduces hyperglycemia through the reduction of glucose reabsorption into the kidney.Citation6 Previous randomized clinical studies on dapagliflozin have demonstrated reductions in glycated hemoglobin (HbA1c) at all stages of T2D progression, favorable effects on body weight (BW), and a moderate lowering of blood pressure. These benefits have been observed when dapagliflozin was used as monotherapy or in combination with metformin (normal and extended release formulations), sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, or insulin.Citation7–Citation16 However, there is a paucity of data on the efficacy of dapagliflozin in a real-world setting, eg, in primary care patients with T2D. Furthermore, studies to determine baseline characteristics that could be used to predict which patients would benefit most from dapagliflozin treatment are lacking.
The first aim of this study was to investigate changes in treatment outcomes (HbA1c, BW, systolic blood pressure [SBP]) for T2D patients initiating dapagliflozin therapy using a large database representative of practices in Germany. The second aim was to evaluate baseline clinical predictors of changes in HbA1c, weight, and SBP after dapagliflozin initiation.
Methods
The Disease Analyzer database (IMS Health GmbH & Co. OHG) collects drug prescriptions, diagnoses, and basic medical and demographic data directly obtained from the computer system of a representative sample of general practitioners and internal medicine practices throughout Germany.Citation17 For such studies based on anonymous data in Germany no special ethic approval or patient consent is required and hence it was not sought for this study. The analyzed database period for the current study was from December 2012 to October 2014 (985 general and 32 diabetologist practices). Patients with T2D who were initiated on dapagliflozin therapy during the study period (index date) were included. Two cohorts with ≥3- or ≥6-month follow-up after index date were analyzed, respectively. The practice visit records were used to determine baseline demographic characteristics 6 months prior to the index date.
Macrovascular complications were determined based on primary care diagnoses (ICD-10 codes) for coronary heart disease (I24, I25), myocardial infarction (I21, I22, I23, I25.2), stroke (I63, I64, G45), and peripheral vascular disease (I739, E105, E115, E145). Microvascular complications included retinopathy (E113, E143, H360), neuropathy (E114, E144), and nephropathy (N18, N19, E112, E142, Z49, Z992). Treatment with antidiabetic drugs prior to the index date was also assessed. Finally, the recorded HbA1c values and the documented BW and SBP before and after the index date were included in the analyses.
Descriptive statistics were provided and changes in HbA1c, BW, and blood pressure were assessed using paired t-tests. Two-sided tests were used and a P-value of <0.05 was considered as statistically significant. Scatter plots were used to visualize changes in BW and changes in HbA1c. Multivariate linear regression model was fitted to investigate the associations between clinical variables or comorbidity and changes in HbA1c, BW, and SBP, respectively. All analyses were carried out in accordance with the Good Practice of Secondary Data Analysis (GPS): guidelines and recommendations of the German SocietyCitation18 using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Upon patient selection, 1,169 new users of dapagliflozin (age: 62.5 years; male: 59.3%) were included. The baseline clinical characteristics are shown in . Approximately 25% of the T2D patients who received dapagliflozin were treated by diabetologists. There was a high prevalence of privately insured patients. Approximately 10% of the patients received dapagliflozin monotherapy, whereas biguanides were the antidiabetic drug most frequently prescribed in combination with dapagliflozin, followed by insulin ().
Table 1 Baseline characteristics of type 2 diabetes patients who received newly prescribed dapagliflozin in primary care practices in Germany (Disease Analyzer)
Macrovascular complications and related risk factors were frequently found in the T2D patients initiating dapagliflozin therapy (). Coronary heart disease, peripheral vascular disease, history of myocardial infarction, or stroke was diagnosed in 47% of the patients prior to onset of dapagliflozin treatment. Microvascular diabetes complications were observed in 22% of the study population ().
At 3 months, a statistically significant reduction in mean HbA1c from baseline (8.5%) was observed with dapagliflozin (−0.8%) (P<0.001) (). The reduction in HbA1c with dapagliflozin treatment was maintained at 6 months (−0.8%: standard deviation [SD]: 1.4) (P<0.001) (). Greater HbA1c decreases were observed with dapagliflozin in 466 patients with higher baseline HbA1c >8.0% (−1.3%; SD: 1.5) (P<0.001) and 251 patients with >9.0% (−1.8%; SD: 1.7) (P<0.001) at 3 months, and these greater reductions were still evident at 6 months (data not shown).
Table 2 Three-month (after the ID) changes in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy in primary care practices in Germany
Table 3 Six-month (after the ID) changes in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy in primary care practices in Germany
A statistically significant reduction in mean BW was observed in patients treated with dapagliflozin at 3 months (−2.3 kg) () and was maintained through 6 months (−2.5 kg) () (both P<0.001). The mean reduction in SBP was statistically significant at 3 months (−2.2 mmHg) and was also maintained at 6 months (−2.3 mm Hg) (both P<0.05) ( and ). The effects of dapagliflozin on HbA1c and BW were largely comparable in both T2D patients treated in general practices and diabetologist care ( and ). The reductions in SBP at both 3 and 6 months were somewhat greater in patients treated by diabetologists compared to patients in general practices.
Scatter plots representing the relationship between reductions in HbA1c and BW after 3 and 6 months of treatment are shown in and , respectively. Approximately 50% of patients receiving dapagliflozin responded simultaneously to the two-item endpoint of combined HbA1c (>0% HbA1c%) and BW (>0 kg) reductions at 3 months (). The combined endpoint reductions were achieved in 45% of new dapagliflozin patients after 6 months (). Furthermore, ~19% of patients treated with dapagliflozin achieved an HbA1c reduction at 3 months despite an increase in BW (). At 6 months, this proportion increased to 25% (). After 3 months of dapagliflozin treatment, only 8.5% failed to exhibit reductions in both HbA1c and BW. After 6 months, the corresponding proportion was 12.4%.
Figure 1 Scatter plot representing the relationship between the change in HbA1c (%) and body weight (kg) after 3 months in type 2 diabetes patients initiating dapagliflozin treatment in primary care practices.
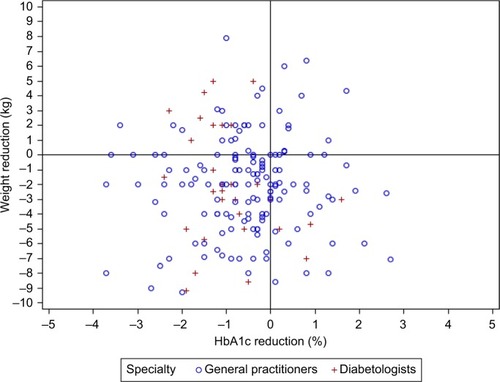
Figure 2 Scatter plot representing the relationship between the change in HbA1c (%) and weight (kg) after 6 months in type 2 diabetes patients initiating dapagliflozin treatment in primary care practices.
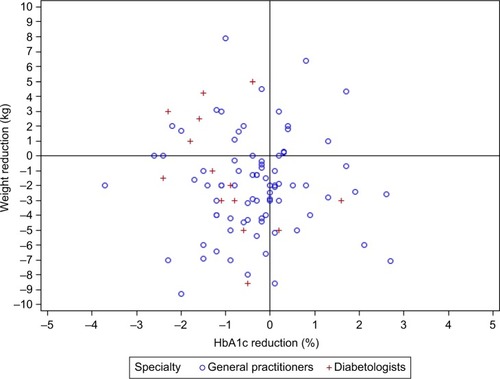
The correlations of baseline clinical variables with changes in HbA1c after onset of dapagliflozin treatment were investigated using linear regression. Only baseline HbA1c was statistically significantly inversely related to change in HbA1c after both 3 and 6 months, indicating that higher HbA1c values were related to a greater decrease after onset of dapagliflozin treatment (P<0.0001). Furthermore, age was positively correlated with the HbA1c decrease observed at 3 months, indicating that HbA1c increased with age after the index date (). Finally, diabetologist treatment was related to a significantly greater decrease in HbA1c at 6 months compared to treatment in general practices.
Table 4 Variables associated with HbA1c (%) change after 3 and 6 months in type 2 diabetes patients initiating dapagliflozin in primary care practices: multivariate linear regression models
Baseline BW was inversely related to weight change (kg) both at 3 and 6 months, indicating that higher BW was related to a greater change after onset of dapagliflozin treatment (). Furthermore, diabetologist care was related to a greater weight change at 3 months after onset of dapagliflozin therapy compared to treatment in general practices.
Table 5 Variables associated with body weight change (kg) after 3 and 6 months in type 2 diabetes patients initiating dapagliflozin in primary care practices: multivariate linear regression model
With respect to changes in SBP at 3 months, only baseline systolic values (negative) and age (positive) were statistically significantly related (data not shown). Thus, higher baseline SBP was related to a greater decrease. At 6 months, only baseline SBP was negatively related to its change; this change was statistically significant.
Changes in HbA1c, BW, and SBP in various subgroups of patients treated with antidiabetic combination therapies, including dapagliflozin, are shown in Tables S1 and S2. After 3 months of treatment, both dapagliflozin monotherapy and combination therapy with metformin, insulin, or DPP-4 inhibitors yielded statistically significant decreases of HbA1c and BW (Table S1). A statistically significant reduction of SBP was also observed in patients treated with dapagliflozin and DPP-4 inhibitors. After 6 months of treatment, similar statistically significant reductions of HbA1c and BW were found for dapagliflozin monotherapy (Tables S2). Moreover, a statistically significant decrease in HbA1c and BW was observed for dapagliflozin combination therapy with insulin or DPP-4 inhibitors. A statistically significant HbA1c reduction was maintained in patients with dual treatment with metformin, whereas a borderline statistical significance was found for BW (P=0.06). With respect to SBP, no statistically significant decrease was observed in all four treatment subgroups after 6 months of treatment. However, patient numbers are small in several of these groups, thus limiting statistical power.
Discussion
For the first time, a real-world study evaluated the short-term changes in HbA1c, BW, and SBP in T2D patients initiating dapagliflozin treatment in primary care and diabetologist practices. Overall, T2D patients treated with dapagliflozin exhibited statistically significant reductions in HbA1c (−0.8%), BW (−2.5 kg), and SBP (−2.3 mmHg) after 6 months of treatment. In addition, HbA1c reductions were greater in patients with poor glycemic control. These real-world treatment effects are comparable to results of dapagliflozin randomized clinical trials.Citation7–Citation15,Citation18–Citation21,Citation26
Changes in HbA1c
In the dapagliflozin clinical trial program, monotherapy yielded a statistically significant HbA1c reduction of 0.66% after 24 weeks when compared to placebo (−0.89% vs −0.23%, P<0.0001).Citation7 As an add-on to metformin, dapagliflozin consistently improved HbA1c (placebo-corrected) by 0.54% after 24 weeks (−0.84% vs −0.30%, P<0.0001).Citation8 In third-line therapy, when dapagliflozin was used concomitant with the DPP-4 inhibitors sitagliptin or saxagliptin, HbA1c reductions of −0.5% (−0.5% vs 0.0%, P<0.0001) and −0.72% (−0.82% vs 0.10%, P<0.0001), respectively, were observed compared with placebo after 24 weeks.Citation10,Citation16 In third-line therapy with metformin and sulfonylureas, dapagliflozin led to placebo-corrected HbA1c reductions of −0.69% (P<0.0001).Citation15 Moreover, addition of dapagliflozin in T2D patients receiving high doses of insulin (≥30 IU/day) and up to two other antidiabetic agents effectively reduced HbA1c by 0.57% compared with placebo after 24 weeks (−0.96 vs −0.39%, P<0.001)Citation11 and by 0.35% over a period of 2 years (−0.78% vs −0.43%, P<0.0007) with stable insulin doses over the entire study period and a net increase of placebo by 19.2 IU at 2 years when compared to the study drug.Citation19
In the present study, the HbA1c reduction of 0.8% achieved with dapagliflozin after 3 and 6 months was commensurate with most HbA1c reductions over 24 weeks found in the dapagliflozin arm of clinical trials, rather than their placebo-corrected margin.
Similar results were obtained for the second largest cohort of patients using dapagliflozin as an add-on to insulin (33%). This cohort exhibited an HbA1c reduction of 0.8% after 6 months. Identical numerical reduction of HbA1c at 6 months was shown for the oral triple therapy adding dapagliflozin to a preexisting treatment with metformin and a DPP-4 inhibitor. This observation in real-life yielded reductions similar to the recently published clinical trial using dapagliflozin as a third-line drug add-on to metformin and saxagliptin.Citation16
This analysis also indicated clinical variables that are independently related to change in HbA1c after initiation of dapagliflozin treatment. Most important, the baseline HbA1c was inversely related to both the changes in HbA1c at 3 and 6 months. This result is in accordance with a recent clinical trial with statistically significantly greater HbA1c reductions observed with dapagliflozin treatment in patients with baseline HbA1c ≥8.0% (−0.56%) and ≥9.0% (−0.99%) at 6 months compared to the overall sample (−0.46%).Citation20
In this real-world study, a greater HbA1c reduction was found in patients treated in diabetologist practices. This outcome most likely reflects the fact that diabetologists are more familiar with the relatively new dapagliflozin therapy. The positive correlation with age found in the present study ostensibly indicates that older patients have a more advanced stage of the disease along with declining kidney function, which makes it more difficult to achieve a reduction in HbA1c using dapagliflozin.
Taken together, dapagliflozin usage in real life favorably reflected the HbA1c reductions previously measured in the dapagliflozin clinical trial program. Higher baseline HbA1c values led to greater reductions and if higher age is associated with decreasing kidney function, smaller reductions are well explainable due to dapagliflozin’s mechanism of action.
Changes in body weight
The weight loss observed in the present study was consistent with the weight loss observed across RCTs in clinical trials.Citation21 Twelve RCTs, including 2,005 participants in the intervention groups and 2,003 participants in the control groups with follow-up durations ranging from 12 to 104 weeks, were included in a meta-analysis.Citation21 BW decreases ranged from −3.33 to −1.54 kg after treatment with dapagliflozin.Citation21 The overall mean difference between the intervention and control groups was −2.10 kg (P<0.001).Citation21 In another network meta-analysis of RCTs, the mean change in weight associated with dapagliflozin was statistically significant compared with the other antidiabetic agents: −2.74 kg relative to DPP-4 inhibitors, and −4.67 kg relative to sulfonylureas.Citation22 The results from the present real-world study demonstrate that similar reductions in BW (−2.5 kg at 6 months) can be achieved in primary care patients. This weight loss has been attributed to the ~200–300 cal of glucose excreted per day as a result of treatment with dapagliflozin.Citation23 Although the impact of this amount of weight loss on mortality and cardiovascular events is debatable, even small weight decreases in T2D have been shown to improve treatment satisfaction and quality of life.Citation24,Citation25
Similar to HbA1c, baseline BW was negatively correlated with weight change at 3 months. The BW reduction observed in clinical trials for dapagliflozin was primarily caused by a reduction in body fat mass, as shown by dual energy X-ray absorptiometry and calorie loss.Citation26 A subgroup analysis using magnetic resonance imaging showed a reduction of visceral adipose tissue mass greater than 9% compared to the baseline during treatment with dapagliflozin. Furthermore, consistent with the changes in HbA1c, diabetologist care was associated with a greater change in BW at 3 months. This most likely reflects more intensive care and/or a more motivated patient population.
Changes in systolic blood pressure
A recent meta-analysis of 27 RCTs involving dapagliflozin (n=12) reported that SGLT2 inhibitor use was associated with a statistically significant reduction in SBP from baseline (−4.0 mmHg).Citation27 This real-world study also showed statistically significant reductions in SBP (−2.3 mmHg) at 6 months. The impact of the blood pressure reductions achieved with dapagliflozin on cardiovascular outcomes requires further investigation. The underlying mechanisms of a dapagliflozin-specific reduction of SBP must be identified. In the present investigation, baseline blood pressure (inverse association) was the only relevant variable associated with changes in SBP.
Strength and limitations of the study
The present study provides an illustration of prescriptions and diagnoses in primary care and diabetologist practices in Germany. The strength of the study is the use of a large nationwide database and the unbiased assessment of prescriptions and outcomes. As this study used primary care records, a number of limitations should be mentioned: First, no valid information regarding onset of diabetes was provided. Additionally, assessment of comorbidities solely relied on ICD codes filled in by physicians. Moreover, measurements of HbA1c and body mass index values were not standardized. Finally, data on socioeconomic status and lifestyle-related risk factors were also unavailable. It must also be mentioned that, in this study, the adherence to the dapagliflozin administration was not assessed. No investigation was also performed about the change of other antihyperglycemic drugs during the study period. Both adherence and therapy change might affect the findings.
Conclusion
This is the first report of real-world outcomes concerning the effect of dapagliflozin on HbA1c, BW, and systolic blood pressure in T2D patients in primary care and diabetologist practices. The absolute reductions in HbA1c (−0.8%), BW (−2.5 kg), and SBP (−2.3 mmHg) observed in patients inadequately controlled with other antidiabetic agents and insulin were similar to results from the dapagliflozin clinical trial program.
Acknowledgments
The study was supported by AstraZeneca GmbH, Germany.
Supplementary materials
Table S1 Three-month changes (after the ID) in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy in primary care practices in Germany
Table S2 Six-month changes (after the ID) in HbA1c, body weight (kg), and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy in primary care practices in Germany
Disclosure
MF Scheerer, R Rist, and O Proske are employees of Astra-Zeneca GmbH, Germany. Karel Kostev and Annika Meng are employees of IMS Health GmbH & Co. OHG. The authors report no other conflicts of interest in this work.
References
- Gerich JE Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications Diabet Med 2010 27 2 136 142 20546255
- Rahmoune H Thompson PW Ward JM Smith CD Hong G Brown J Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes Diabetes 2005 54 12 3427 3434 16306358
- Wright EM Loo DD Hirayama BA Biology of human sodium glucose transporters Physiol Rev 2011 91 2 733 794 21527736
- Brunton SA The potential role of sodium glucose co-transporter 2 inhibitors in the early treatment of type 2 diabetes mellitus Int J Clin Pract 2015 69 10 1071 1087 26147213
- Inzucchi SE Bergenstal RM Buse JB Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes Diabetologia 2015 58 3 429 442 25583541
- Hinnen D Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes Ther Adv Endocrinol Metab 2015 6 3 92 102 26137213
- Ferrannini E Ramos SJ Salsali A Tang W List JF Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial Diabetes Care 2010 33 10 2217 2224 20566676
- Bailey CJ Gross JL Pieters A Bastien A List JF Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial Lancet 2010 375 9733 2223 2233 20609968
- Strojek K Yoon KH Hruba V Elze M Langkilde AM Parikh S Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial Diabetes Obes Metab 2011 13 10 928 938 21672123
- Jabbour SA Hardy E Sugg J Parikh S Study 10 Group Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study Diabetes Care 2014 37 3 740 750 24144654
- Wilding JP Woo V Soler NG Pahor A Sugg J Rohwedder K Parikh S Dapagliflozin 006 Study Group Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial Ann Intern Med 2012 156 6 405 415 22431673
- Nauck MA Del Prato S Meier JJ Durán-García S Rohwedder K Elze M Parikh SJ Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial Diabetes Care 2011 34 9 2015 2022 21816980
- Henry RR Murray AV Marmolejo MH Hennicken D Ptaszynska A List JF Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial Int J Clin Pract 2012 66 5 446 456 22413962
- Matthaei S Catrinoiu D Celiński A A randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes Diabetes Care 2015 38 11 2018 2024 26324329
- Matthaei S Bowering K Rohwedder K Grohl A Parikh S Study 05 Group Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: a 24-week randomized, double-blind clinical trial Diabetes Care 2015 38 3 365 372 25592197
- Becher H Kostev K Schröder-Bernhardi D Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies Int J Clin Pharmacol Ther 2009 47 10 617 626 19825325
- Swart E Gothe H Geyer S Jaunzeme J Maier B Grobe TG Ihle P Good Practice of Secondary Data Analysis (GPS): guidelines and recommendations Gesundheitswesen 2015 77 2 120 126 German 25622207
- Mathieu C Ranetti AE Li D Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes Diabetes Care 2015 38 11 2009 2017 26246458
- Wilding JP Woo V Rohwedder K Sugg J Parikh S Dapagliflozin 006 Study Group Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years Diabetes Obes Metab 2014 16 2 124 136 23911013
- Cefalu WT Leiter LA de Bruin TW Gause-Nilsson I Sugg J Parikh SJ Dapagliflozin’s effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension Diabetes Care 2015 38 7 1218 1227 25852208
- Sun YN Zhou Y Chen X Che WS Leung SW The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: meta-analysis of randomised controlled trials BMJ Open 2014 4 4 e004619
- Goring S Hawkins N Wygant G Roudaut M Townsend R Wood I Barnett AH Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis Diabetes Obes Metab 2014 16 5 433 442 24237939
- Whaley JM Tirmenstein M Reilly TP Poucher SM Saye J Parikh S List JF Targeting the kidney and glucose excretion with dapagliflozin: preclinical and clinical evidence for SGLT2 inhibition as a new option for treatment of type 2 diabetes mellitus Diabetes Metab Syndr Obes 2012 5 135 148 22923998
- Grandy S Fox KM Bazata DD Association of self-reported weight change and quality of life, and exercise and weight management behaviors among adults with type 2 diabetes mellitus: the SHIELD study Cardiol Res Pract 2012 2012 892564 22645696
- Pi-Sunyer FX The impact of weight gain on motivation, compliance, and metabolic control in patients with type 2 diabetes mellitus Postgrad Med 2009 121 15 94 107 19820278
- Bolinder J Ljunggren Ö Johansson L Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin Diabetes Obes Metab 2014 16 2 159 169 23906445
- Baker WL Smyth LR Riche DM Bourret EM Chamberlin KW White WB Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis J Am Soc Hypertens 2014 8 4 262 275 24602971
