Abstract
The belief that obesity is protective against osteoporosis has recently come into question. The latest epidemiologic and clinical studies have shown that a high level of fat mass might be a risk factor for osteoporosis and fragility fractures. Further, increasing evidence seems to indicate that different components of the metabolic syndrome, ie, hypertension, increased triglycerides, reduced high-density lipoprotein cholesterol, are also potential risk factors for the development of low bone mineral density and osteoporosis. This review considers both the older and more recent data in the literature in order to evaluate further the relationship between fat tissue and bone tissue.
Introduction
Obesity and osteoporosis are two important global health problems with an increasing prevalence and a high impact on both mortality and morbidity.Citation1–Citation4 Interestingly, during recent decades, both diseases have become a major health threat worldwide.Citation2 Age and female gender increase the risk of developing both obesity and osteoporosis, which affect millions of women.Citation3,Citation5–Citation7 Age-related changes in body composition, metabolic factors, and hormonal levels after menopause, accompanied by a decline in physical activity, may all provide mechanisms for the propensity to gain weight and, in particular, for the increase in fat mass often characterized by replacement of lean mass by adipose tissue.Citation3,Citation4
Obesity is due to an imbalance in which energy intake exceeds energy expenditure over a prolonged period.Citation2 In healthy adults, body weight is tightly regulated despite day-to-day variations in food intake and energy expenditure. Several environmental, nutritional, and hormonal factors appear to influence body weight.Citation1–Citation3 For instance, postmenopausal women often show increased body weight, likely due to a decrease in basal metabolism, alteration of hormonal levels, and reduced physical activity.Citation8 Moreover, obese postmenopausal women are often affected by hypertension, dyslipidemia, diabetes mellitus, and cardiovascular disease, and have an increased risk of developing some cancers.Citation3,Citation9,Citation10 Interestingly, these women have always been considered protected against osteoporosis.Citation5,Citation6,Citation11
Osteoporosis is a metabolic bone disease characterized by excessive skeletal fragility (due to a reduction in both bone quantity and quality), leading to an increased risk of developing spontaneous and traumatic bone fractures.Citation7 More than 40% of postmeno-pausal women, on average, will suffer at least one osteoporosis fracture, often leading to permanent and severe disability, nursing home placement, and even death.Citation11,Citation12 The rate of bone loss in adults reflects the interaction between genetic and environmental factors, which also influences the extent of bone acquisition during growth, known as peak bone mass.Citation13
It is known that fractures in childhood have been associated with alterations in body composition, such as increased adiposity and bone structure, suggesting that these might be the earliest signs of skeletal insufficiency.Citation14 Soon after menopause, the process of bone loss begins in women, due to increased bone resorption by osteoclasts, which overcomes bone formation by osteoblasts.Citation13 Moreover, osteoblast function declines with aging, determining the imbalance between bone resorption and bone formation.Citation15 Traditionally, osteoporosis has been regarded as a disorder associated only with fracture and skeletal disability in old age, but recent studies demonstrate that bone mineral density appears to be a better long-term predictor of death than blood pressure or cholesterol.Citation12,Citation16 Further data published in recent decades indicate that low bone mineral density is a strong and independent predictor of all-cause mortality, including cardiovascular mortality.Citation12,Citation16
Body fat and lean mass are correlated with bone mineral density, with obesity apparently confering protection against bone loss after menopause.Citation5,Citation6,Citation17 The pathophysiological role of adipose tissue in skeletal homeostasis probably lies in the role that several adipokines play in bone remodeling via their effects on either bone formation or resorption. Since the demonstration that bone cells express several specific hormone receptors, the skeleton has come to be considered an endocrine target organ.Citation18–Citation21 Additionally, recent observations have shown that bone-derived factors, such as osteocalcin and osteopontin, may affect body weight control and glucose homeostasis,Citation22–Citation24 suggesting a possible role of bone tissue as an endocrine organ with the presence of a potential feedback mechanism between the skeleton and endocrine organs.Citation25 Thus, the cross-talk between fat and bone likely constitutes a homoeostatic feedback system in which adipokines and molecules secreted by osteoblasts and osteoclasts represent the link of an active bone-adipose axis. However, the mechanism(s) by which all these events occur remains unclear.
Fat and bone correlation: evidence-based observations
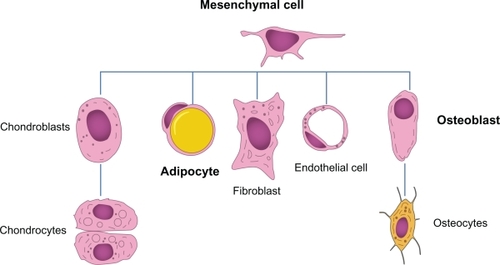
In the last three decades, the association between obesity and osteoporosis has been actively investigated from epidemiological, clinical, and basic research points of view, and common pathophysiological links have been proposed: both obesity and osteoporosis are influenced by genetic and environmental factors, or the interaction between them; aging is associated with both diseases and with a high incidence of bone loss and bone marrow adiposity; bone remodeling and adiposity are both regulated via a complex interplay of adipokines and hormones; and adipocytes and osteoblasts derive from a common progenitor, ie, the mesenchymal stem cell,Citation11 as shown in .
Figure 1 Several cell lines deriving from a common mesenchymal stem cell. The presence of different stimuli may induce differentiation of the progenitor into one cell line instead of another. However, this event might underscore the presence of a certain degree of plasticity among the cell lineages.
Extensive data have shown that, in healthy pre-menopausal and postmenopausal women, total body fat is positively related to bone mineral density, an important and measurable determinant of fracture risk,Citation26–Citation27 that high body weight (or body mass index) is correlated with high bone mineral density, and that decreased body weight leads to bone loss.Citation28–Citation32 Furthermore, fat mass, the most important index of obesity, has been demonstrated to have a similarly beneficial effect, leading to an increase in bone mass,Citation17,Citation33 while a beneficial effect of fat mass on bone mineral density is confirmed in white women but not in white men.Citation34
Although these data indicate that obesity exerts a protective effect on bone tissue, more recent studies have described an opposite event. In particular, although cross-sectional and longitudinal studies have shown that bone mass is positively related to body weight and body mass index, there are controversial issues as to whether lean mass or fat mass might be the most important determinant of bone mineral density.Citation6 In particular, the evidence suggests an inverse relationship between obesity and osteoporosis depending on how obesity is defined. In the studies where obesity is defined on the basis of body mass index or body weight, obesity appears to act as a protective factor against bone loss and fractures; however, if obesity is considered as a percentage of body fat and distribution, as in the study published by Zhao et al in a Chinese population,Citation11 it becomes a risk factor for osteoporosis.
In particular, there are data indicating that women with a high body mass index (25–29.9 kg/m2) are protected from osteoporosis, but there is increasing evidence conflicting with this observation, suggesting that obesity (body mass index > 30) might actually interfere with bone health.Citation11
In accordance with the data reported by Zhao et al,Citation11 Hsu et al showed that matching of Chinese subjects by body mass index, across 5 kg strata of body weight, revealed a negative relationship between fat and bone mass, and the risk of osteoporosis and nonspinal fractures was significantly higher for subjects with a higher proportion of body fat, independent of body weight.Citation35 Our group has recently demonstrated that 37% of 395 obese adult subjects had significant skeletal changes. In particular, this subpopulation showed a lower bone mineral density at the lumbar spine than expected for both their young age and high body mass index.Citation36 Further characterization showed that different grades of adiposity could affect skeletal health status differently. In fact, stratification of the population into three different groups according to body mass index status, showed a slightly different bone mineral density pattern among the groups. Overweight subjects (body mass index 26–29) did not show any change in skeletal health, while obese and severely obese subjects (body mass index > 30) had significant alteration in their bone mineral density levels, with an increased number of individuals having a lower bone mass than would be expected for their age and body weight.Citation36 Evaluation of hormonal, metabolic, and lipid profiles did not show significant differences between the groups, athough more detailed analysis seemed to show higher inflammatory markers and lower levels of circulating vitamin D (Migliaccio et al, unpublished data). Indeed, data published by Blum et al from a cohort of 153 premenopausal women demonstrated that a high amount of fat mass is negatively associated with bone mass.Citation37 Thus, all these data suggest an important role for fat distribution as total fat mass itself. A recent study of 907 healthy postmenopausal women by Kim et al demonstrated that body weight was positively related to bone mineral density and vertebral fracture risk, whereas percentage of body fat and waist circumference were related to a low bone mineral density and to a higher risk for vertebral fractures.Citation38
Even racial differences appear to influence fat and bone interaction. Castro et al reported that obesity is negatively associated with bone mineral density in black women, but not in white women,Citation39 while Afghani and Goran reported an inverse correlation between subcutaneous abdominal adipose tissue and bone mineral density in whites, but not in blacks. In the same study, the authors reported an inverse association between visceral fat and bone mineral density in blacks, but not in whites.Citation40 These conflicting results suggest a complex effect of fat mass on bone tissue related to sample size, ethnicity, gender, study design, methods of statistical analysis, and population structure. Nevertheless, several lines of evidence from environmental and medical interventions support an inverse correlation between fat and bone mass, ie, physical exercise increases bone mass while reducing fat mass,Citation41 supplementation with calcium and vitamin D appears beneficial for the prevention of both osteoporosis and obesity,Citation42 and menopause is also associated with increased fat mass, increased bone loss, and decreased lean mass.Citation43 Estrogen replacement therapy in postmenopausal women improves both lean mass and bone mass, and reverses menopause-related weight gain.Citation44 Whereas estrogens reduce the risk of bone loss and obesity, other pharmacological interventions have been shown to increase both osteoporosis and obesity, such as treatment with gonadotropin-releasing hormone agonists and the use of glucocorticoids.Citation45–Citation48 Additionally, recent findings have indicated that some antidiabetic drugs, which interfere with peroxisome proliferator-activated receptor gamma (PPARγ) and thus with adipocyte differentiation, also appear to influence skeletal homeostasis and fracture risk significantly.
Thiazolidinedione and other selective PPARγ agonists, such as rosiglitazone and pioglitazone, play a prominent role in the treatment of type 2 diabetic patients. In vitro analyses demonstrate that various PPARγ ligands not only induce murine bone marrow stromal cell adipogenesis, but also inhibit osteogenesis,Citation49 and in vivo studies demonstrate that PPARγ agonists reduce bone mineral density and increase fracture rates, notably distal extremity fractures in female type 2 diabetic patients.Citation50
Fat and bone correlation: potential mechanisms of interaction
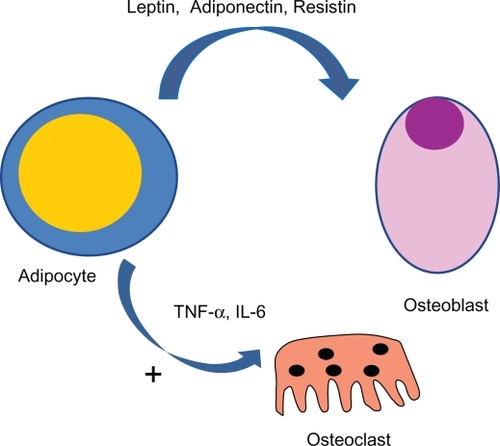
Several potential mechanisms have been proposed to explain the complex relationship between adipose tissue and bone tissue. Fat has long been viewed as a passive energy reservoir, but since the discovery of leptin and identification of other adipose tissue-derived hormones and serum mediators,Citation51–Citation53 fat has come to be considered as an active endocrine organ which modulates energy homeostasis. Adipose tissue also secretes various inflammatory cytokines, including interleukin (IL)-6 and tumor necrosis factor-alpha,Citation54 and altered production of these proinflammatory mediators is thought to have adverse metabolic and cardiovascular consequences. All these molecules, which include resistin, leptin, adiponectin, and IL-6, affect human energy homeostasis and may well be involved in bone metabolism, contributing to the complex relationship between adipose tissue and bone tissue ().Citation55
Figure 2 A complex link between adipocytes and bone cell exists. Several cytokines are secreted by fat tissue and act on bone cells. In particular, several proinflammatory cytokines (eg, IL-6 and TNF-α) act as osteoclastogenic factors with a potentially stimulating mechanism.
Fat tissue is one of the major sources of aromatase, an enzyme also expressed in the gonads, which synthesizes estrogens from androgen precursors. Estrogens are steroid hormones which play a pivotal role in the maintenance of skeletal homeostasis, protecting against osteoporosis by reducing bone resorption and stimulating bone formation. This extragonadal estrogen synthesis in fat tissue becomes the dominant estrogen source in postmenopausal women, due to the lack of ovarian function.Citation6 Additionally, in obese post-menopausal women, increased estrogen synthesis by adipose tissue has been suggested as one of the potential mechanisms for the protective effect of fat mass on bone.
On the other hand, studies in humans lacking aromatase and in estrogen receptor-α and receptor-β knockout mice indicate that estrogens protect against bone loss, and support the hypothesis that these hormones may inhibit the development of obesity,Citation56–Citation61 as suggested also by the prevention of menopause-induced fat mass gainCitation62–Citation64 and reduction of the incidence of osteoporotic fractures by estrogen replacement therapy.Citation65 In support of this hypothesis, decreased endogenous estrogen levels have been shown to be coupled with an increase in adipocyte numbers and decreased osteoblast counts in the bone marrow of postmenopausal women.Citation66
As mentioned above, several adipokines are involved in the fat-bone interaction. Leptin suppresses appetite, increases energy expenditure, and regulates bone remodeling, and is the most important adipocyte-derived hormone.Citation67–Citation70 The effect of leptin on bone is complex, and both negativeCitation37,Citation71 and positive actionsCitation72–Citation74 on bone mineral density have been reported in humans. Leptin-deficient ob/ob mice and leptin receptor-deficient db/db mice are extremely obese, with increased vertebral trabecular bone volume due to increased bone formation, despite hypogonadism and hypercortisolism.Citation69 Interestingly, intracerebroventricular infusion of leptin in both ob/ob and wild-type mice was shown to decrease vertebral trabecular bone mass.Citation69 In vivo studies indicate that the effect of leptin may depend on its site and mode of action,Citation75–Citation77 and it has been proposed that peripheral administration of leptin could increase bone mass by inhibiting bone resorptionCitation78 and increasing bone formation,Citation52,Citation79 while inhibiting bone formation through a central nervous system effect.Citation69 In vitro studies also found that leptin can act directly on bone marrow-derived mesenchymal stem cells to enhance their differentiation into osteoblasts and to inhibit their differentiation into adipocytes.Citation79,Citation80
Takeda et al expanded these observations further, demonstrating that the effects of intracerebroventricular leptin are mediated by the sympathetic nervous system, and that osteoblasts express β-adrenergic receptors via which it is probable that administration of β-adrenergic agonists decreases trabecular bone volume by inhibiting bone formation.Citation70 Noradrenaline also seems to increase bone resorption by promoting nuclear factor kappa-B ligand expression and inhibiting osteoblast proliferation.Citation81–Citation83 The effect of leptin on obesity is mediated via proopiomelanocortin neurons and neuropeptide Y.Citation84 Leptin reduces food intake and increases energy expenditure by stimulating proopiomelanocortin neurons to secrete α-melanocyte-stimulating hormone,Citation85 which, in addition to having an effect on obesity, might contribute to bone resorptionCitation82 but not bone formation.Citation70 Interestingly, type 4 α-melanocyte-stimulating hormone receptor knockout mice have high bone mass due to decreased bone resorption.Citation82
Leptin also inhibits expression of neuropeptide Y, a hypothalamus-derived peptide, essential for the regulation of food consumption, energy homeostasis, and bone remodeling.Citation86,Citation87 Hypothalamus-specific NPY knockout mice show a significant decrease in body weight, a significant increase in food intake, and a two-fold increase in trabecular bone volume compared with wild-type animals.Citation88,Citation89
Adiponectin is another adipocyte-derived hormone which has anti-inflammatory and antiatherogenic effects, regulating energy homeostasis and bone remodeling.Citation90–Citation94 In contrast with leptin, serum adiponectin levels are reduced in obese and diabetic subjectsCitation95 and increase after weight loss.Citation92 Human osteoblasts express adiponectin and its receptors,Citation92 but both negative and positive links between adiponectin and bone mineral density have been reported.Citation96,Citation97 Other in vivo and in vitro studies show that adiponectin increases bone mass by suppressing osteoclastogenesis and activating osteoblastogenesis,Citation92–Citation94 suggesting that a rise in adiponectin levels caused by fat reduction could have a beneficial effect on bone mineral density.
Thommesen et al showed that resistin may play a role in bone remodeling, indicating that it is expressed in mesenchymal stem cells, osteoblasts, and osteoclasts in bone marrow. Resistin increases osteoblast proliferation and cytokine release, as well as osteoclast differentiation,Citation98 so the effect of resistin on bone is still unclear, and further studies are needed to understand its role better.
IL-6 is a pluripotent inflammatory cytokine, released from adipocytes, adipose tissue matrix, osteoblast, and elsewhere in the body.Citation99 In particular, adipose tissue accounts for one-third of circulating levels of IL-6. Obese subjects have high circulating levels of this proinflammatory cytokine,Citation100,Citation101 and genetic polymorphism of IL-6 is associated with obesity.Citation102 Moreover, peripheral administration of IL-6 induces hyperlipidemia, hyperglycemia, and insulin resistance in rodents and humans.Citation103 In contrast, administration of IL-6 in the central nervous system increases energy expenditure and decreases body fat in rodents.Citation103 IL-6 is also a well recognized stimulator of osteoclastogenesis and bone resorptionCitation104,Citation105 but some data show that IL-6 mRNA is expressed in preosteoblasts and osteoblasts,Citation106 and that IL-6 stimulates osteoblast proliferation and differentiationCitation107 by controlling the production of local factors,Citation108 and it might play a role in bone formation in conditions of high bone turnover.Citation108,Citation109
In addition to adipocytes, adipose tissue contains various stromal and vascular cells, including fibroblasts, vascular endothelial cells, and inflammatory cells. Adipocytes were initially thought to be the major source of adipose-derived mediators, but recent studies have shown that macrophages infiltrate adipose tissue, and that these macrophages, along with other cells that reside in the stroma, also contribute to the production and secretion of humoral mediators, particularly inflammatory cytokines.Citation54 A paracrine loop involving free fatty acids and inflammatory cytokines has been postulated to establish a vicious cycle between adipocytes and macrophages, thereby propagating inflammation.Citation110,Citation111 Therefore, it is important to define interactions between adipocytes, osteoblasts, and stromal cells in obese subjects.
Adipocytes and osteoblasts: a common origin
Adipocytes and osteoblasts originate from a common progenitor, ie, a pluripotential mesenchymal stem cell,Citation112 which has an equal propensity for differentiation into adipocytes or osteoblasts (or other lines) under the influence of several cell-derived transcription factors. This process is complex, suggesting significant plasticity and multifaceted mechanism(s) of regulation within different cell lineages, among which are adipocytes and osteoblasts.Citation113,Citation114 Several studies have examined the function of adipocytes in bone marrow. Mesenchymal stem cells isolated from marrow in postmenopausal osteoporotic patients express more adipose differentiation markers than those from subjects with normal bone mass.Citation115
As mentioned earlier, adipocytes secrete endocrine and paracrine factors that strongly influence bone differentiation and remodeling. Estrogens are among these factors, explaining why increased body weight in postmenopausal women is associated with slower rates of bone loss.Citation113,Citation116,Citation117 However, the interaction between estrogens and fat appears to be complex. Martin and Zissimos showed pronounced fatty infiltration in the bone marrow of rats following oophorectomy, suggesting a pivotal role of estrogen in regulating adipocyte recruitment.Citation118 On the other hand, the presence of aromatase in fat cells allows higher intramarrow conversion of testosterone into estrogens which, in turn, can inhibit bone resorption.Citation113
The effect of estrogen on bone and adipose tissue formation has long been recognized in experimental animal models.Citation118,Citation119 In humans, changes in estrogen status due to advancing age and menopause have been correlated with increased levels of IL-6 and IL-11, which are both associated with bone loss.Citation120 It is interesting to speculate whether the increase in adipogenesis subsequent to menopause is due to a relief of repression or to an induction of the adipogenic phenotype, even though in vitro data suggest that the default “switch” might be adipogenesis, a process which might normally be inhibited in vivo prior to estrogen depletion.Citation24
Other members of the nuclear hormone receptor family contribute to control of adipogenesis and osteogenesis. PPARγ plays a central role in initiating adipogenesis.Citation113 Mutations of the PPARγ gene are associated with an altered balance between bone and fat formation in the bone marrow. The nuclear hormone receptor family of transcriptional regulatory proteins is activated by a range of ligands, including steroid hormones, naturally occurring metabolites, synthetic chemicals, and as yet unidentified endogenous compounds (orphan receptors).
Thiazolidinedione and other PPARγ ligands, such as rosiglitazone and pioglitazone, play a prominent role in the treatment of type 2 diabetes. However, in vitro analyses demonstrate that various PPARγ ligands not only induce murine bone marrow stromal cell adipogenesis but also inhibit osteogenesis.Citation49 In particular, PPARγ-2 is the dominant regulator of adipogenesis, and ligand activation of PPARγ-2 favors differentiation of mesenchymal stem cells into adipocytes rather than into osteoblasts.Citation116 Akune et al showed that PPARγ insufficiency led to increased osteoblastogenesis in vitro and higher trabecular bone volume in vivo, confirming the key role of mesenchymal stem cell lineage allocation in the skeleton.Citation112 Interestingly, aged mice exhibit fat infiltration into bone marrow and enhanced expression of PPARγ-2, along with reduced mRNA expression of bone differentiation factors.Citation121 Mice with premature aging (the SAM-P/6 model) show nearly identical patterns of adipocyte infiltration, with impaired osteoblastogenesis,Citation122 indicating that aging, or events that accelerate aging, result in significant bone marrow adiposity and a defect in osteoblastogenesis in mice.Citation123
The Wnt signaling pathway works in a coordinated manner with other transmembrane signals, including multiple ligands, antagonists, receptors, coreceptors, and transcriptional mediators, such as β-catenin.Citation124 Specific elements of the Wnt signaling pathway have been found to inhibit adipogenesisCitation125,Citation126 while promoting osteogenesis.Citation127–Citation131 Wnt inhibition of adipogenesis is mediated via β-catenin, which interferes with PPARγ transcriptional activation of downstream targets.Citation132 Following exposure to transforming growth factor beta, human bone marrow mesenchymal stem cells increase their expression of various Wnt receptors and ligands.Citation133
Members of the epidermal growth factor family, such as protein Pref-1, influence both adipogenesis and osteogenesis. In vitro analysis of human bone marrow mesenchymal stem cells has shown that Pref-1 overexpression blocks both adipogenesis and osteogenesis. This finding is consistent with the hypothesis that Pref-1 maintains mesenchymal stem cells in a multipotent state.Citation134
Further experimental tools, such as gene microarrays, are being used to document the relationship between classical steroid hormones and bone and fat formation in the marrow. One study has examined the skeletal phenotype of mice deficient in both thyroid receptors α and β. These mice showed increased mRNA levels for adipocyte-specific genes, increased numbers of bone marrow adipocytes, and reduced trabecular and total bone mineral density.Citation135 The inbred SAM-P/6 murine strain provides a model of accelerated senescence characterized by osteopenia and increased fat mass in bone marrow.Citation136 Recent studies have found that 1,25(OH)2 vitamin D treatment inhibits adipogenesis and enhances osteogenesis in SAM-P/6 mice, with a 50% reduction in PPARγ mRNA and protein levels.Citation123 Moreover, gene microarray analyses demonstrated coordinated induction of osteoblastogenic genes and a reduction of adipogenic genes after 1,25 (OH)2 vitamin D treatment, which stimulates not only bone formation but also bone resorption, according to circulating biomarkers of bone turnover.Citation137 Overall, these recent findings involving classical steroid receptors support the inverse relationship between adipogenic and osteogenic differentiation in the bone marrow microenvironment. This is mediated, in part, by cross-talk between the pathways activated by steroid receptors, PPARs, and other cytokines and paracrine factors.
Finally, other factors, such as total caloric intake, type of nutrients, alcohol consumption, oxygen tension, and cellular oxidation-reduction pathways influence bone marrow adipogenesis despite osteoblastogenesis,Citation24 showing that the bone marrow mesenchymal stem cell may consider multiple differentiation pathways during its lifetime and, indeed, may dedifferentiate and transdifferentiate in response to changes in the microenvironment.
Conclusion
Obesity and osteoporosis are two major global health problems with an increasing prevalence and a high impact on mortality and morbidity. Menopause is characterized by the cessation of ovarian estrogen production and is associated with increased bone loss, increased fat mass, and decreased lean mass. Age-related changes in hormone levels, in association with changes in body composition, metabolic factors, and decreased physical activity, probably provide the mechanisms for the propensity to postmenopausal gain of fat mass, and thus increased body weight. Estrogen synthesis in adipose tissue, through aromatase, becomes the dominant estrogen source in postmenopausal women. In particular, in obese postmenopausal women, increased estrogen synthesis by fat tissue has been suggested as one of the potential mechanisms for the protective effect of fat mass on bone.
Even though there are data indicating that women with high body mass index are protected from osteoporosis, increasing evidence seems to show conflicting results regarding this issue, suggesting that obesity might actually interfere with bone health. In particular, the relationship between obesity and osteoporosis depends on how obesity is defined. If obesity is defined on the basis of body mass index or body weight, it appears to protect against bone loss and fractures. However, if obesity is based on the percentage of body fat, it may be a risk factor for osteoporosis.
The existence of a cross-talk between fat and the skeleton suggests a homoeostatic feedback system in which adipokines and bone-derived molecules form part of an active bone-adipose axis. However, the mechanism(s) by which all these events occur remains unclear. Of course, further basic science research and epidemiological studies with large sample sizes, robust study design, and careful data analysis will be needed to show the true effect of fat mass on bone.
The relationship between fat mass and bone is confounded by complex genetic backgrounds and by interactions between metabolic factors and regulatory pathways influencing both obesity and osteoporosis. Although the evidence that adipose tissue exerts a protective effect against bone loss is greater than that showing a negative association, the recent increasing data on this issue suggest that both obese and nonobese postmenopausal women should be considered at risk for alteration in bone mineral density and osteoporosis. Specific and careful characterization of skeletal metabolism and further studies evaluating skeleton changes may be useful in obese women, because aging itself might also increase their risk of developing fractures later in life.
Disclosure
The authors report no conflicts of interest in this work.
References
- Kado DM Huang MH Karlamangla AS Barrett-Connor E Greendale GA Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study J Am Geriatr Soc 2004 52 1662 1667 15450042
- Rossner S Obesity: the disease of the twenty-first century Int J Obes Relat Metab Disord 2002 26 Suppl 4 S2 S4 12457290
- Hu FB Overweight and obesity in women: health risks and consequences J Women Health (Larchmt) 2003 12 2 163 172
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation World Health Organ Tech Rep Ser 2000 894 1 253
- Albala C Yanez M Devoto E Sostin C Zeballos L Santos JL Obesity as a protective factor for postmenopausal osteoporosis Int J Obes Relat Metab Disord 1996 20 1027 1032 8923160
- Reid IR Relationships among body mass, its components, and bone Bone 2002 31 547 555 12477567
- NIH Consensus development panel on osteoporosis JAMA 2001 285 785 795 11176917
- Cagnacci A Zanin R Cannoletta M Generali M Caretto S Volpe A Menopause, estrogens, progestin, or their combination on body weight and anthropometric measurements Fertil Steril 2007 88 6 1603 1608 17481628
- Lebovitz HE The relationship of obesity to the metabolic syndrome Int J Clin Pract Suppl 2003 134 18 27 12793594
- Sowers JR Obesity as a cardiovascular risk factor Am J Med 2003 8 37S 41S 14678864
- Zhao LJ Jiang H Papasian CJ Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis J Bone Miner Res 2008 23 17 29 17784844
- Johansson C Black D Johnell O Oden A Mellstrom D Bone mineral density is a predictor of survival Calcif Tissue Int 1998 63 190 196 9701621
- Brown S Rosen CJ Osteoporosis Med Clin North Am 2003 87 1039 1063 14621330
- Goulding A Jones IE Taylor RW Williams SM Manning PJ Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy X-ray absorptiometry study J Pediatr 2001 139 509 515 11598596
- Kveiborg M Flyvbjerg A Rattan SI Kassem M Changes in the insulin-lik growth factor-system may contribute to in vitro agerelate impaired osteoblast functions Exp Geronto 2000 35 1061 1074
- Von der Recke P Hansen MA Hassager C The association between low bone mass at the menopause and cardiovascular mortality Am J Med 1999 106 273 278 10190374
- Reid IR Plank LD Evans MC Fat mass is an important determinant of whole body bone density in premenopausal women but not in men J Clin Endoc Metab 1992 75 779 782
- Eriksen EF Colvard DS Berg NJ Evidence of estrogen receptors in normal human osteoblast-like cells Science 1988 1 241 4861 84 86 3388021
- Kim HJ New understanding of glucocorticoid action in bone cells BMB Rep 2010 43 8 524 529 20797313
- Komm BS Terpening CM Benz DJ Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells Science 1988 241 4861 81 84 3164526
- Migliaccio S Davis VL Gibson MK Gray TK Korach KS Estrogens modulate the responsiveness of osteoblast-like cells (ROS 17/2.8) stably transfected with estrogen receptor Endocrinology 1992 130 5 2617 2624 1572285
- Gomez-Ambrosi J Rodriguez A Catalan V Fruhbeck G The bone-adipose axis in obesity and weight loss Obes Surg 2008 18 1134 1143 18563500
- Takeda S Effect of obesity on bone metabolism Clin Calcium 2008 18 632 637 18445881
- Gimble JM Zvonic S Floyd ZE Kassem M Nuttall ME Playing with bone and fat J Cell Biochem 2006 98 251 266 16479589
- Fukumoto S Martrin TJ Bone as an endocrine organ Trends Endocrinol Metab 2009 20 5 230 236 19546009
- Cummings SR Black DM Nevitt MC Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group Lancet 1993 341 72 75 8093403
- Melton LJIII Atkinson EJ O’Fallon WM Wahner HW Riggs BL Long-term fracture prediction by bone mineral assessed at different skeletal sites J Bone Miner Res 1993 8 1227 1233 8256660
- Mazess RB Barden HS Ettinger M Spine and femur density using dual-photon absorptiometry in US white women Bone Miner Res 1987 2 211 219
- Felson DT Zhang Y Hannan MT Anderson JJ Effects of weight and body mass index on bone mineral density in men and women: The Framingham study J Bone Miner Res 1993 8 567 573 8511983
- Marcus R Greendale G Blunt BA Correlates of bone mineral density in the postmenopausal estrogen/progestin interventions trial J Bone Miner Res 1994 9 1467 1476 7817832
- Reid IR Ames R Evans MC Determinants of total body and regional bone mineral density in normal postmenopausal women – a key role for fat mass J Clin Endocrinol Metab 1992 75 45 51 1619030
- Ravn P Cizza G Bjarnason NH Thompson D Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group J Bone Miner Res 1999 14 1622 1627 10469292
- Khosla S Atkinson EJ Riggs BL Melton LJIII Relationship between body composition and bone mass in women J Bone Miner Res 1996 11 857 863 8725184
- Pluijm SM Visser M Smit JH Popp-Snijders C Roos JC Lips P Determinants of bone mineral density in older men and women: Body composition as mediator J Bone Miner Res 2001 16 2142 2151 11697812
- Hsu YH Venners SA Terwedow HA Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women Am J Clin Nutr 2006 83 146 154 16400063
- Greco EA Fornari R Rossi F Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index Int J Clin Pract 2010 64 6 817 820 20518955
- Blum M Harris SS Must A Leptin, body composition and bone mineral density in premenopausal women Calcif Tissue Int 2003 73 27 32 14506951
- Kim KC Shin DH Lee SY Relation between Obesity and Bone Mineral Density and Vertebral Fractures in Korean Postmenopausal Women Yonsei Med J 2010 51 6 857 863 20879051
- Castro JP Joseph LA Shin JJ Differential effect of obesity on bone mineral density in White, Hispanic and African American women: A cross sectional study Nutr Metab (Lond) 2005 2 9 15817133
- Afghani A Goran MI Racial differences in the association of subcutaneous and visceral fat on bone mineral content in prepubertal children Calcif Tissue Int 2006 79 383 388 17115240
- Reid IR Legge M Stapleton JP Evans MC Grey AB Regular exercise dissociates fat mass and bone density in premenopausal women J Clin Endocrinol Metab 1995 80 1764 1768 7775619
- Reid IR Therapy of osteoporosis: Calcium, vitamin D, and exercise Am J Med Sci 1996 312 278 286 8969617
- Manson JE Martin KA Postmenopausal hormonereplacement therapy N Engl J Med 2001 345 34 40 11439947
- Sorensen MB Rosenfalck AM Hojgaard L Ottesen B Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy Obes Res 2001 9 622 626 11595778
- De Gregorio LH Lacativa PG Melazzi AC Russo LA Glucocorticoid-induced osteoporosis Arq Bras Endocrinol Metabol 2006 50 793 801 17117304
- Steinbuch M Youket TE Cohen S Oral glucocorticoid use is associated with an increased risk of fracture Osteoporos Int 2004 15 323 328 14762652
- Gaillard D Wabitsch M Pipy B Negrel R Control of terminal differentiation of adipose precursor cells by glucocorticoids J Lipid Res 1991 32 569 579 1649886
- Livingstone DE Jones GC Smith K Understanding the role of glucocorticoids in obesity: Tissue-specific alterations of corticosterone metabolism in obese Zucker rats Endocrinology 2000 141 560 563 10650936
- Lecka-Czernik B Moerman EJ Grant DF Lehmann JM Manolagas SC Jilka RL Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation Endocrinology 2002 143 2376 2384 12021203
- Riche DM Travis King S Bone loss and fractures risk associated with thiazolidinedione therapy Endocrinology 2007 148 6 2669 2680 17332064
- Kadowaki T Yamauchi T Adiponectin and adiponectin receptors Endocr Rev 2005 26 439 451 15897298
- Steppan CM Crawford DT Chidsey-Frink KL Ke H Swick AG Leptin is a potent stimulator of bone growth in ob/ob mice Regul Pept 2000 92 73 78 11024568
- Vendrell J Broch M Vilarrasa N Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity Obes Res 2004 12 962 971 15229336
- Tilg H Moschen AR Inflammatory mechanisms in the regulation of insulin resistance Mol Med 2008 14 3–4 222 231 18235842
- Magni P Dozio E Galliera E Ruscica M Corsi MM Molecular Aspects of Adipokine-Bone Interactions Curr Mol Med 2010 10 6 522 532 20642443
- Wade GN Gray JM Bartness TJ Gonadal influences on adiposity Int J Obes Relat Metab Disord 1985 9 Suppl 1 83 92
- Smith EP Boyd J Frank GR Estrogen resistance caused by a mutation in the estrogen receptor gene in a man N Engl J Med 1994 331 1056 1061 8090165
- Morishima A Grumbach MM Simpson ER Fisher C Qin K Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens J Clin Endocrinol Metab 1995 80 3689 3698 8530621
- Heine PA Taylor JA Iwamoto GA Lubahn DB Cooke PS Increased adipose tissue in male and female estrogen receptor-alpha knockout mice Proc Natl Acad Sci U S A 2000 97 12729 12734 11070086
- Cooke PS Heine PA Taylor JA Lubahn DB The role of estrogen and estrogen receptor-alpha in male adipose tissue Mol Cell Endocrinol 2001 178 147 154 11403904
- Maffei L Murata Y Rochira V Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: Effects of testosterone, alendronate, and estradiol treatment J Clin Endocrinol Metab 2004 89 61 70 14715828
- Tchernof A Calles-Escandon J Sites CK Poehlman ET Menopause, central body fatness, and insulin resistance: Effects of hormone-replacement therapy Coron Artery Dis 1998 9 503 511 9847982
- Gambacciani M Ciaponi M Cappagli B De Simone L Orlandi R Genazzani AR Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy Maturitas 2001 39 125 132 11514111
- Jensen LB Vestergaard P Hermann AP Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: A randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study J Bone Miner Res 2003 18 333 342 12568411
- Rossouw JE Anderson GL Prentice RL Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial JAMA 2002 288 321 333 12117397
- Justesen J Stenderup K Ebbesen EN Mosekilde L Steiniche T Kassem M Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis Biogerontology 2001 2 165 171 11708718
- Zhang Y Proenca R Maffei M Barone M Leopold L Friedman JM Positional cloning of the mouse obese gene and its human homologue Nature 1994 372 425 432 7984236
- Mantzoros CS The role of leptin in human obesity and disease: A review of current evidence Ann Intern Med 1999 130 671 680 10215564
- Ducy P Amling M Takeda S Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass Cell 2000 100 197 207 10660043
- Takeda S Elefteriou F Levasseur R Leptin regulates bone formation via the sympathetic nervous system Cell 2002 111 305 317 12419242
- Kontogianni MD Dafni UG Routsias JG Skopouli FN Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women J Bone Miner Res 2004 19 546 551 15005840
- Goulding A Taylor RW Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women Calcif Tissue Int 1998 63 456 458 9817937
- Yamauchi M Sugimoto T Yamaguchi T Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women Clin Endocrinol (Oxf) 2001 55 341 347 11589677
- Pasco JA Henry MJ Kotowicz MA Serum leptin levels are associated with bone mass in nonobese women J Clin Endocrinol Metab 2001 86 1884 1887 11344177
- Thomas T Burguera B Is leptin the link between fat and bone mass? J Bone Miner Res 2002 17 1563 1569 12211425
- Thomas T Leptin: A potential mediator for protective effects of fat mass on bone tissue Joint Bone Spine 2003 70 18 21 12639613
- Thomas T The complex effects of leptin on bone metabolism through multiple pathways Curr Opin Pharmacol 2004 4 295 300 15140423
- Martin A de Vittoris R David V Leptin modulate both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats Endocrinology 2005 146 8 3652 3659 15845621
- Cornish J Callon KE Bava U Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo J Endocrinol 2001 175 405 415 12429038
- Thomas T Gori F Khosla S Jensen MD Burguera B Riggs BL Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes Endocrinology 1999 140 1630 1638 10098497
- Fu L Patel MS Bradley A Wagner EF Karsenty G The molecular clock mediates leptin-regulated bone formation Cell 2005 122 803 815 16143109
- Elefteriou F Ahn JD Takeda S Leptin regulation of bone resorption by the sympathetic nervous system and CART Nature 2005 434 514 520 15724149
- Karsenty G Convergence between bone and energy homeostases: Leptin regulation of bone mass Cell Metab 2006 4 341 348 17084709
- Friedman JM Halaas JL Leptin and the regulation of body weight in mammals Nature 1998 395 763 770 9796811
- Wisse BE Schwartz MW Role of melanocortins in control of obesity Lancet 2001 358 857 859 11567697
- Herzog H Neuropeptide Y and energy homeostasis: Insights from Y receptor knockout models Eur J Pharmacol 2003 480 21 29 14623347
- Liu YJ Araujo S Recker RR Deng HW Molecular and genetic mechanisms of obesity: Implications for future management Curr Mol Med 2003 3 325 340 12776988
- Sainsbury A Schwarzer C Couzens M Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice Proc Natl Acad Sci U S A 2002 99 8938 8943 12072562
- Baldock PA Sainsbury A Couzens M Hypothalamic Y2 receptors regulate bone formation J Clin Invest 2002 109 915 921 11927618
- Combs TP Berg AH Obici S Scherer PE Rossetti L Endogenous glucose production is inhibited by the adiposederived protein Acrp30 J Clin Invest 2001 108 1875 1881 11748271
- Berg AH Combs TP Du X Brownlee M Scherer PE The adipocyte-secreted protein Acrp30 enhances hepatic insulin action Nat Med 2001 7 947 953 11479628
- Berner HS Lyngstadaas SP Spahr A Adiponectin and its receptors are expressed in bone-forming cells Bone 2004 35 842 849 15454091
- Oshima K Nampei A Matsuda M Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast Biochem Biophys Res Commun 2005 331 520 526 15850790
- Luo XH Guo LJ Yuan LQ Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway Exp Cell Res 2005 309 99 109 15963981
- Weyer C Funahashi T Tanaka S Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia J Clin Endocrinol Metab 2001 86 1930 1935 11344187
- Lenchik L Register TC Hsu FC Adiponectin as a novel determinant of bone mineral density and visceral fat Bone 2003 33 646 651 14555270
- Jurimae J Rembel K Jurimae T Rehand M Adiponectin is associated with bone mineral density in perimenopausal women Horm Metab Res 2005 37 297 302 15971153
- Thommesen L Stunes AK Monjo M Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism J Cell Biochem 2006 99 3 824 834 16721825
- Fain JN Madan AK Hiler ML Cheema P Bahouth SW Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans Endocrinology 2004 145 2273 2282 14726444
- Fernandez-Real JM Ricart W Insulin resistance and chronic cardiovascular inflammatory syndrome Endocr Rev 2003 24 278 301 12788800
- Das UN Is obesity an inflammatory condition? Nutrition 2001 17 953 966 11744348
- Berthier MT Paradis AM Tchernof A The interleukin 6-174G/C polymorphism is associated with indices of obesity in men J Hum Genet 2003 48 14 19 12560873
- Kershaw EE Flier JS Adipose tissue as an endocrine organ J Clin Endocrinol Metab 2004 89 2548 2556 15181022
- Richards CD Langdon C Deschamps P Pennica D Shaughnessy SG Stimulation of osteoclast differentiation in vitro by mouse oncostatin M, leukaemia inhibitory factor, cardiotrophin-1 and interleukin 6: Synergy with dexamethasone Cytokine 2000 12 613 621 10843736
- Rodan GA Introduction to bone biology Bone 1992 13 Suppl 1 S3 S6 1581117
- Dodds RA Merry K Littlewood A Gowen M Expression of mRNA for IL1 beta, IL6 and TGF beta 1 in developing human bone and cartilage J Histochem Cytochem 1994 42 733 744 8189035
- Taguchi Y Yamamoto M Yamate T Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage Proc Assoc Am Physicians 1998 110 559 574 9824538
- Franchimont N Wertz S Malaise M 2005 Interleukin-6: An osteotropic factor influencing bone formation Bone 37 601 606 16112634 Sims NA Jenkins BJ Quinn JM Nakamura A Glatt M Gillespie MT Ernst M Martin TJ 2004 Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways J Clin Invest 113 379 389 14755335
- Sims NA Jenkins BJ Quinn JM Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways J Clin Invest 2004 113 379 389 14755335
- Suganami T Nishida J Ogawa Y A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor α Arterioscler Thromb Vasc Biol 2005 25 10 2062 2068 16123319
- Nishimura S Manabe I Nagai R Adipose tissue inflammation in obesity and metabolic syndrome Discov Med 2009 8 8 41 55 60 19788868
- Akune T Ohba S Kamekura S PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors J Clin Invest 2004 113 846 855 15067317
- Gimble JM Robinson CE Wu X Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells Mol Pharmacol 1996 50 1087 1094 8913339
- Rodriguez JP Montecinos L Rios S Reyes P Martinez J Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation J Cell Biochem 2000 79 557 565 10996846
- Sekiya I Larson BL Vuoristo JT Cui JG Prockop DJ Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs) J Bone Miner Res 2004 19 256 264 14969395
- Aubin JE Bone stem cells J Cell Biochem 1998 Suppl 30–31 73 82 19594448
- Cohen PG Aromatase, adiposity, aging and disease. The hypogonadal-metabolic-atherogenicdisease and aging connection Med Hypotheses 2001 56 702 708 11399122
- Martin RB Zissimos SL Relationships between marrow fat and bone turnover in ovariectomized and intact rats Bone 1991 12 123 131 2064840
- Martin RB Chow BD Lucas PA Bone marrow fat content in relation to bone remodeling and serum chemistry in intact and ovariectomized dogs Calcif Tissue Int 1990 46 189 194 2106378
- Cheleuitte D Mizuno S Glowacki J In vitro secretion of cytokines by human bone marrow: Effects of age and estrogen status J Clin Endocrinol Metab 1998 83 2043 2051 9626137
- Moerman EJ Teng K Lipschitz DA Lecka-Czernik B Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways Aging Cell 2004 3 379 389 15569355
- Kajkenova O Lecka-Czernik B Gubrij I Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6: a murine model of defective osteoblastogenesis and low turnover osteopenia J Bone Miner Res 1997 12 1772 1779 9383681
- Duque G Macoritto M Kremer R Vitamin D treatment of senescence accelerated mice (SAM-P/6) induces several regulators of stromal cell plasticity Biogerontology 2004 5 421 429 15609106
- Logan CY Nusse R The Wnt signaling pathway in development and disease Annu Rev Cell Dev Biol 2004 20 781 810 15473860
- Ross SE Erickson RL Gerin I Microarray analyses during adipogenesis: Understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor {alpha} in adipocyte metabolism Mol Cell Biol 2002 22 5989 5999 12138207
- Bennett CN Ross SE Longo KA Regulation of Wnt signaling during adipogenesis J Biol Chem 2002 277 30998 31004 12055200
- Gong Y Slee RB Fukai N LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development Cell 2001 107 513 523 11719191
- Boyden LM Mao J Belsky J High bone density due to a mutation in LDL-receptor-related protein 5 N Engl J Med 2002 346 1513 1521 12015390
- Kato M Patel MS Levasseur R Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor J Cell Biol 2002 157 303 314 11956231
- Little RD Carulli JP Del Mastro RG A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait Am J Hum Genet 2002 70 11 19 11741193
- Bennett CN Longo KA Wright WS Regulation of osteoblastogenesis and bone mass by Wnt10b Proc Natl Acad Sci U S A 2005 102 3324 3329 15728361
- Liu J Farmer SR Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes J Biol Chem 2004 279 45020 45027 15308623
- Zhou S Eid K Glowacki J Cooperation between TGFbeta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells J Bone Miner Res 2004 19 463 470 15040835
- Abdallah BM Jensen CH Gutierrez G Leslie RG Jensen TG Kassem M Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1 J Bone Miner Res 2004 19 841 852 15068508
- Kindblom JM Gevers EF Skrtic SM Increased adipogenesis in bone marrow but decreased bone mineral density in mice devoid of thyroid hormone receptors Bone 2005 36 607 616 15780976
- Kajkenova O Lecka-Czernik B Gubrij I Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia J Bone Miner Res 1997 12 1772 1779 9383681
- Duque G Macoritto M Dion N Ste-Marie LG Kremer R 1,25(OH)2D3 acts as a bone-forming agent in the hormone-independent senescence-accelerated mouse (SAM-P/6) Am J Physiol Endocrinol Metab 2005 288 E723 E730 15572658