Abstract
Objectives
To determine the prevalence, types and severity of hearing loss and associated factors in a hospital population of adult Nigerians with diabetes mellitus.
Subjects and methods
This study was a prospective hospital-based study conducted at the Otorhinolaryngology and Diabetic Clinics of the University of Nigeria Teaching Hospital (UNTH) Ituku-Ozalla, Enugu, for a period of 12 months. Consecutively presenting eligible adult diabetics and their age- and sex-matched healthy controls were recruited. Each case and control participant had clinical and otologic examination, followed by pure tone audiometry. Data were analyzed using descriptive and comparative statistics.
Results
There were 224 patients and 192 control participants. The patients comprised 112 males and 112 females (sex ratio=1:1), whose mean age was 47.6 years (range: 26–80 years). The prevalence of hearing loss was 46.9%. This comprised 43.8% sensorineural and 3.1% conductive hearing losses. The distribution of hearing loss by severity was mild 25.0%, moderate 15.6% and severe 6.3%. The controls comprised 96 males and 96 females whose mean age was 44.6 years (range: 25–79 years). The prevalence of hearing loss was significantly higher overall and by type (sensorineural hearing loss, conductive hearing loss) in cases compared with controls.
Conclusion
The prevalence of hearing loss among diabetic adults at UNTH, Enugu, is comparatively high. Hearing loss is predominantly sensorineural and often mild to moderate in severity. Routine audiometric evaluation of all adult diabetics at UNTH is recommended.
Keywords:
Introduction
Diabetes mellitus (DM) is a disease characterized by hyperglycemia due to absolute or relative deficiency of insulin.Citation1 Sustained hyperglycemia is associated with multisystemic complications and multiple end-organ damage. Currently, there is a worldwide pandemic of DMCitation1–Citation3 and by extension, the inherent complications.
The auditory apparatus is one of the vulnerable end organs in DM due to ischemic cochlear damage resulting from diabetic microangiopathy.Citation4–Citation6 DM-related hearing loss is a major public health issue in both low- and middle-income countries and the developed economies. With the projected increase in the world’s diabetes burden due to increased longevity and changes in lifestyle, its prevalence is bound to increase. It impacts adversely on the patients’ quality of life and their capacity for independent living.
Previous studies investigating hearing thresholds in DM, in NigeriaCitation7–Citation9 and elsewhere,Citation10–Citation16 have been dominated by descriptive cross-sectional surveys, often with widely variable results. The reported prevalence of hearing loss ranges from 0.0% to 90.0%. Consequently, the investigators conducted a hospital-based case–control audiometric evaluation of adult Nigerians with and without DM to determine the prevalence, types and profile of hearing loss and the associated characteristics. In addition to provision of valid comparative data, the generated data will assist public health policymakers and implementers, and otologic care providers in optimizing the quality of life of persons living with DM.
Significance of the study
DM is a systemic disease with multiple systemic complications and end-organ damage. DM-related hearing loss and its adverse impact in the quality of life is a major public health issue across the globe. The study would provide valid comparative data that will assist public health policymakers and otologic care providers in optimizing the quality of life of persons living with DM.
Subjects and methods
Background
Established in 1970 and located in Enugu, southeastern Nigeria, the University of Nigeria Teaching Hospital (UNTH) is one of the first-generation public tertiary health care facilities in Nigeria. UNTH provides undergraduate and postgraduate medical training, outpatient/inpatient clinical care and undertakes research. At UNTH, the otorhinolaryngology (synonym: Ear Nose and Throat [ENT]) department provides medical, surgical and audiometric ENT care, while a dedicated Diabetic Unit, in the hospital’s Internal Medicine Department, provides inpatient and outpatient diabetic care. The UNTH’s feeder population comprises inhabitants of Nigeria’s southeast geopolitical zone and beyond.
The study, conducted for 1 year at the ENT and Diabetic Clinics of UNTH, was a prospective case–control study of eligible diabetic adults and their age- and sex-matched healthy controls.
Ethics
Prior to commencement of the study, ethics clearance was obtained from the Medical and Health Research Ethics Committee (Institutional Review Board) of UNTH, Enugu, compliant with the tenets of the 1964 Helsinki Declaration on research involving human subjects. Additionally, written informed consent was obtained from each participant, case, and control, before recruitment into the study.
Eligibility cases
Adults aged 19 years or older diagnosed with types 1 or 2 DM for 5 years or longer, showing absence of congenital anomalies of, or infective/inflammatory/neoplastic lesions of the outer, middle or inner ear were included in the study. Also excluded were potential participants who had coexisting tuberculosis, syphilis, sickle cell disease, hypertension, Human Immune Deficiency Virus infection, neoplasia; or with past history of head injury, acoustic trauma, ear surgery, familial deafness and use of ototoxic drugs 1 month prior to recruitment.
Controls
Age- and sex-matched healthy nondiabetic adults without any of the above conditions contraindicating enrollment were recruited from the hospital community.
Sample size and sampling technique
A minimum sample size for the study was calculated using Fisher’s formula.Citation17 Consecutively presenting patients who met the inclusion criteria were recruited into the study.
Study instrument
This was a pretested investigator-administered questionnaire/proforma specifically designed for the study. It contained subsections on participants’ demographic and clinical characteristics, and findings of audiometric assessment.
Study procedures
A peripheral venous blood sample was obtained for fasting blood sugar determination using AccuCheck™ (Roche Diagnostic GmbH, Mannheim, Germany) and human immune deficiency virus 1 and 2 screening test with Determme (Alere)™ (Alere Medical Co. Ltd, Chiba, Japan) from each case and control participant. A midstream urine sample was also obtained for urinalysis.
Subsequently, each subject (case and control) had a general and systemic examination, and otologic examination using Led Head light (Tiger Head Battery Group Co., Ltd, Guangzhou, People’s Republic of China) and a battery-powered otoscope (Welch Allyn Inc., Skaneateles Falls, NY, USA). Any impacted wax in the external auditory canal was removed either with wax hook (Downs Surgical, Sheffield, UK) or by instilling wax softening solution – Cerumol (Thornton & Ross Ltd, Huddersfield, UK) before syringing with Higginson’s syringe (Downs Surgical) filled with normal saline (Juhel Pharmaceuticals, Awka, Nigeria) at body temperature. This was followed by a re-examination of the ears to confirm that the external auditory canal was clear. Pure tone audiometry, in a sound-treated room, using MEDIMATE 602 audiometer (Madsen Electronics, Taastrup, Denmark) calibrated to ISO standard (9002) was performed on each subject to determine the hearing threshold for octave frequencies 250–8000 Hz. The level of hearing for each subject was determined based on pure tone audiometric finding. The average for each frequency considered was determined, and the degree of hearing loss for each patient was determined based on the World Health Organization standard classificationCitation18 ().
Table 1 WHO grading of hearing impairment as modified in 1991
Data analysis
Data were entered into and analyzed using the Statistical package for Social Sciences for Windows, version 18 (SPSS Inc., Chicago, IL, USA). Descriptive statistics yielded frequencies, percentages and proportions. Comparative statistical tests for significance of observed intergroup differences were performed using Pearson’s chi-square test or Fisher’s exact test for categorical variables and Student’s t-test for continuous/metric variables. In all comparisons, a p value <0.05, at one degree of freedom, was considered statistically significant.
Results
Two hundred and thirty cases were recruited in the study; however, six cases with incomplete data were excluded from the analysis.
The participants (N=416) comprised 224 cases and 192 controls. The cases consisted of 112 (50.0%) males, while their control counterparts consisted of 96 (50.0%) males. The cases were aged 47.6±10.9 standard deviation (SD) years (range: 26–80 years), while the controls were aged 44.6±12.8 SD years (range: 25–79 years; ). Both groups differed significantly in age (diabetics vs controls: 47.5±10.9 vs 44.6±12.8, t=2.5927, p<0.0099). The mean fasting blood sugar of the cases was significantly higher than that of controls (175.3±75.6 vs 76.59±10.77 mg/dL, t=7.31, p<0.05).
Table 2 Age distribution of diabetes mellitus patients and controls
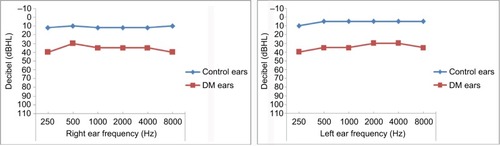
The prevalence of hearing loss was 46.9% among cases and 15.6% in controls. The patient–control age match with hearing threshold is shown in . Of the cases, normal hearing was present in 119 (53.1%), sensorineural hearing loss (SNHL) in 98 (43.8%) and conductive hearing loss (CHL; air–bone gap ≥15 dB) in 7 (3.1%); among the controls, hearing was normal in 162 (84.4%), SNHL was present in 30 (15.6%) and CHL in 0 (0.0%). None (0.0%) of the case or control participants had mixed hearing loss. The profile of hearing loss is shown in .
Table 3 Patient–control age match with hearing threshold
Table 4 Frequency distribution of pure tone audiogram among study participants
The diabetic patients consistently had significantly higher mean threshold values at all frequencies in both ears (). The prevalence of hearing loss was significantly higher overall (p<0.0001) and by type (SNHL, p<0.0001; CHL, p=0.0168) in diabetics compared with controls. shows the audiogram of the mean hearing thresholds of DM patients and the controls.
Table 5 Comparison of hearing thresholds between cases and controls at all frequencies
Discussion
There was equal sex dominance among the case and control participants, which comprised adult participants. This is similar to the demographic profile in the study of Ologe and Okoro,Citation8 but differs from the report by Lasisi et alCitation7 who had both children and adults as their study participants. The marginal age discrepancy between case and control participants, despite measures to achieve perfect age match, suggests that the age-determined influences on hearing threshold might partly account for the higher prevalence of hearing loss among diabetics. To eliminate the potential cofounding influence of age, related future studies should aim at perfect age match between cases and controls.
In this study, the prevalence of hearing loss, predominantly SNHL and CHL, was significantly higher among cases (46.9%) than controls (15.6%). There has been a wide variation in the prevalence of SNHL found in studies involving diabetic patients. Ranges such as 0%–93%Citation19,Citation20 have been quoted. There has not been any satisfactory explanation for this wide variation. The present finding is consistent with reports elsewhere (0%–93.0%),Citation19,Citation20 but far higher than that reported by Lasisi et alCitation7 (17.0%) in Ibadan, Nigeria. Between-survey similarities and differences in inclusion criteria, participants’ demographics and clinical profile might explain these observations. While the participants in this study were adults who have had diabetes for 5 years or longer, the IbadanCitation7 report included pediatric subjects with <5-year history of diabetes. To enable valid comparisons between the survey results, the authors suggest the standardization and adoption of a standard recruitment procedure during future surveys. The high prevalence of hearing loss among diabetics emphasizes the constant necessity for routine periodic audiometric evaluation among adult diabetics. Future longitudinal studies are needed to assess the temporal profile of diabetes-related hearing loss and inform the frequency of audiometric assessments. The hearing thresholds of the cases compared with controls showed significant difference at all frequencies in both right and left ears, with the cases showing higher thresholds. This is consistent with the findings of Ologe and OkoroCitation8 and underscores the constant necessity for periodic otologic screening in all adult diabetics.
The severity of hearing loss among the cases was frequently mild (25.0%) and moderate (15.6%); severe (6.3%) hearing loss was relatively infrequent. Thus, 21.9% of patients had moderate to severe hearing impairment, which will add to the burden of their systemic morbidity and adversely impact on their quality of life and performance.Citation18
The type-specific prevalence of hearing loss showed a predominance of SNHL over CHL. Microangiopathy and peripheral neuropathy, common in diabetics, might explain this finding. Although a case–control study, the conclusions drawn from this study and the extrapolation of its findings are limited by its hospital-based cross-sectional design, strict age criterion for enrollment and the potential influence of age-related hearing loss. Therefore, the results cannot be generalized to the entire population and do not provide information on the temporal trend. Population-based surveys across all ages, preferably of longitudinal design, are warranted.
Conclusion
There is high prevalence of hearing loss, predominantly of the sensorineural type, among adult diabetics at UNTH, Enugu. This might have adverse implications for their quality of life. Routine periodic audiometric assessment of adult diabetics is recommended to ensure early detection and timely otologic care.
Disclosure
The authors report no conflicts of interest in this work.
References
- Ginter E Simko V Type 2 diabetes mellitus, pandemic in 21st century Adv Exp Med Biol 2012 771 42 50 23393670
- Seidell JC Obesity, insulin resistance and diabetes–a worldwide epidemic Br J Nutr 2000 83 Suppl 1 S5 S8 10889785
- Sierra GN The Global pandemic of diabetes Afr J Diabetes Med 2009 4 8
- Makishima K Tanaka AK Pathological changes of the inner ear and central auditory pathway in diabetes Ann Otol Rhinol Laryngol 1971 80 2 218 228 5550775
- Jorgensen MB Buch NH Studies on inner ear and cranial nerves in diadetes Acta Otolaryngol 1961 107 179 182
- Smith TL Raynor E Prazma J Insulin-dependent diabetic micro-angiopathy in the inner ear J Laryngol Otol 1995 105 236 240
- Lasisi OA Nwaorgu OGB Bella AF Cochleo-vestibular complications of diabetes mellitus in Ibadan, Nigeria. Proceedings of the 17th World Congress of the International Federation of Otorhinolaryngological Societies. Elsevier 2003 International Congress Series 2003 1240 1325 1328
- Ologe FE Okoro EO Type 2 diabetes and hearing loss in Black Africans Diabet Med 2005 22 5 661 667 15842527
- Ologe FE Okoro EO Oyejola BA Hearing function in Nigerian children with a family history of type 2 diabetes Int J Pediatr Otorhinolaryngol 2005 69 387 391 15733599
- Ma F Gomez-Marin O Lee DJ Diabetes and hearing impairment in Mexican American adults: a population based study J Laryngol Otol 1998 112 9 835 839 9876372
- Cullen JR Cinnammond MJ Hearing Loss in diabetes J Laryngol Otol 1993 107 3 179 182 8509689
- Virtaniemi J Laakso M Nuutinen JN Karjalainen S Vartiainen E Hearing thresholds in insulin – dependent diabetic patients J Laryngol Otol 1994 108 10 837 841 7989828
- Austin DF Konrad-Martin D Griest S McMillan GP Fausti S Diabetes-related changes in hearing Laryngoscope 2009 119 9 1788 1796 19593813
- Bainbridge KE Hoffman HJ Cowie CC Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004 Ann of Intern Med 2008 149 1 1 10 18559825
- Diaz de Leon-Morales LV Jauregui-Renaud K Gray-Sevilla ME Hernández-Prado J Malacara-Hernández JM Auditory impairment in patients with type 2 diabetes mellitus Arch Med Res 2005 36 5 507 510 16099330
- Hirose K Hearing loss and diabetes: you may not know what you are missing Ann Intern Med 2008 149 1 54 55 18559823
- Araoye MO Research methodology with statistics for health and social sciences Nigeria Nathadex Ilorin 2004 115 129
- WHO Report of the Informal Working Group on the Prevention of Deafness and Hearing Impairment Program Planning WHO/PDH/91 Geneva 1991 Grade of Hearing Impairment 1-24 Available from: http://apps.who.int/iris/bitstream/10665/58839/1/WHO_PDH_91.1.pdf Accessed June 12, 2014
- Taylor IG Irwin J Some audiological aspects of diabetes mellitus J Laryngol Otol 1978 92 2 99 112 627773
- Axelsson A Sigroth K Vertes D Hearing in diabetes Acta Otolaryngol Suppl 1978 356 1 23