Abstract
Background
The Paleolithic diet, a diet devoid of food-processing procedure, seems to produce a greater decrease in weight compared to healthy reference diets but its limited food choices make it difficult to implement in our modern times where refined food is dominant.
Objective
To evaluate the effects of a 2-year diet that excludes only six refined foodstuffs implicated in obesity. Professional contact was kept minimal to approximate the approach used by most dieters.
Design
Single-arm, open-label, exploratory study.
Setting
One academic medical center, outpatient setting.
Patients
One hundred and five subjects with a mean age of 50 (SD, 14 years) and mean body mass index of 30.5 kg/m2 (SD, 4 kg/m2). Thirty-nine percent had type 2 diabetes.
Intervention
An ad libitum diet that excludes six refined foodstuffs (margarine, vegetable oils, butter, cream, processed meat, and sugary drinks) called the “1,2,3 diet”.
Outcomes
Weight at 2 years was the primary outcome. Secondary outcomes included number of patients who lost more than 5% of initial body weight, glycated hemoglobin (HbA1c) level, and changes in dietary behavior.
Results
Average weight loss was 4.8 kg (p<0.001), representing 5.6% of their initial body weight. Among completers (51%), the average weight loss was 5.5 kg (p<0.001), and 56% had a reduction of at least 5% of their initial body weight. Among diabetics, weight loss was similar to nondiabetics, and mean HbA1c level decreased by 1% (p=0.001) without modification in glucose-lowering medications. A higher intake of bread, dairy products, chocolate, and fresh fruits was the typical trend in dietary changes reported by completers.
Conclusion
In this exploratory study, there was a significant long-term weight loss with the “1,2,3 diet” despite minimal professional contact. Given the lack of a control group and high attrition rate, further evaluation of this diet is warranted.
Introduction
High-fat or high-carbohydrate foods in societies where the western diet (WD) has prevailed seem to be among the primary driving forces behind the obesity epidemic.Citation1
WD is characterized by a consumption of highly palatable refined sugars and fats. Modern food transformation allowed for unprecedented levels of nutrient combinations and densities,Citation4 and the body of evidence supporting mechanisms by which such food produces obesity is constantly growing.Citation5,Citation6 By contrast, the diets of our Paleolithic ancestors (2.6 million to ∼10,000 years ago), before the advent of modern agriculture, differed considerably from current standards and obesity remains virtually absent among populations who retain an ancestral nutrition.Citation2
The “Paleolithic” diet is a modern dietary regimen that attempts to approximate the characteristics of such ancestral food. It primarily advocates the intake of lean meat, eggs, seafood, fruits, vegetables (including potatoes and other tubers) and nuts, and excludes cereal grains, dairy products, as well as all types of refined sugars or fats typically found in a WD.Citation7 Short-term randomized studies have suggested beneficial effects of a Paleolithic diet on weight, waist circumference, and metabolic balance, including insulin sensitivity, when administered ad libitum versus other types of healthy reference diets.Citation3,Citation8–Citation10 But a key finding is that weight loss was due to a spontaneous 20%–30% decrease in caloric intakeCitation11,Citation12 that would be consistent with a better activation of satiety signals.Citation5,Citation13 If this dietary pattern seems attractive, its feasibility on the long-term is questionable because Paleolithic dieters report difficulty adhering to the diet in a real-life setting.Citation12 This is in line with a 2015 US news survey ranking 35 diets with input from a panel of health experts, which placed the Paleolithic diet dead last, citing a great option but difficult to duplicate in modern times.Citation14 Potential explanation to the unsustainably of Paleolithic nutrition includes the radical departure from the observed industrial food choices of the average consumer.Citation15,Citation16 Taken together, data suggest that a diet devoid of significant food-processing procedure could be more satiating and produces a greater weight loss than guideline-based diets but is virtually impossible to implement in our modern times where refined food is dominant in the global food system.Citation16
As a result, we hypothesized that by excluding six of the most frequently reported refined foodstuffs associated with obesity in human and animal studies (margarine,Citation17,Citation18 vegetable oils,Citation18,Citation19 butter,Citation17,Citation20 cream,Citation21,Citation22 processed meat,Citation20 and sugary drinksCitation23), it was possible to overcome the limited food choices of the Paleolithic diet and induce a spontaneous decrease in food intake resulting in subsequent long-term weight loss.
We, therefore, implemented a nonrestricted calorie diet that fully excluded these six foodstuffs, named the “1,2,3” diet. Professional contact was kept minimal to approximate the approach used by most dieters.Citation24 The aim of this one-arm exploratory study was to assess the effect of such diet on long-term body-weight change in an overweight and obese population. The clinical trial protocol registration number is ISRCTN49630431.
Methods
Design
We performed a one-arm, open-label study conducted over 2 years with outcome assessment at baseline, 6, 12, and 24 months. It was estimated that a sample size of 60 would give the study 90% power at the 5% significance level to detect a difference of 2.6 kg between baseline and 2 years. Based on an anticipated 40% dropout rate, we aimed to recruit at least 100 participants.
Setting
Recruitment and data collection were completed at the Gastroenterology/Nutrition Department, Hospital Antoine Beclère, Clamart, France.
Participants
Consecutive overweight and obese patients who presented to the outpatient consultation with a chief complaint related to their excess weight were recruited for the study. Participants completed a comprehensive medical examination and routine blood tests. Participants were eligible if they were older than 18 years and had a body mass index ≥25 kg/m2. Criteria for exclusion were pregnancy, recent modifications to the usual diet, or recent serious hypoglycemic events for diabetics. The study was conducted from February 2011 to December 2015. The study protocol was reviewed and approved by the Hospital Antoine-Belcère human subject’s committee and was conducted according to the guidelines laid down in the Declaration of Helsinki. Participants gave written informed consent.
Intervention: the 1,2,3 diet
The purpose of the “1,2,3 diet” is to help restore early satiety among overweight western dieters by excluding processed food. However, in order to avoid large departure from available food choices of the average western dieter, participants were asked to fully exclude only six of the most frequently reported processed foodstuffs associated with weight gain: vegetable oils, margarine, butter, cream, processed meat, and sugary drinks. All other foods were allowed and could be consumed freely until satiety. The diet prescription handed to patients is described in more detail in . A practical example on how to implement the diet was given to each patient based on his 3-day food record performed at baseline. To approximate the approach used by most dieters in a real-life setting,Citation24 we intentionally implemented a low-intensity intervention: Patients were encouraged to come back for a first visit 1 month after the onset of the diet and every 2–3 months thereafter. However, patients were free to consult more often if needed. There were no electronically delivered counseling between visits, and no specific advice was given regarding meal frequency or physical activity. The consultation did not last >60 minutes.
Outcomes and measurements
Weight
Body weight was measured at baseline and at each visit (while patients wore light clothing and no shoes) to the nearest 0.5 kg on the same scale calibrated daily. Height was measured by a stadiometer at baseline. The primary outcome was weight at 2 years.
Glycated hemoglobin (HbA1c)
Participants who presented with type 2 diabetes mellitus (T2DM) were followed elsewhere for diabetes. Therapeutic regimens were individualized at the discretion of their doctors throughout the study. Medications used (alone or in combination) for glycemic control were metformin, glucagon-like peptide-1 analog (GLP-1), dipeptidyl peptidase 4 inhibitor, and sulfonylureas. HbA1c level was analyzed in our hospital’s laboratory (high-performance liquid chromatography method) at baseline and at 6, 12, 18, and 24 months. Exposure to glucose-lowering therapy, including insulin, was assessed at baseline and every 6 months thereafter as well.
Changes in dietary behavior
A 3-day food record (2 week days and 1 weekend day) was completed by the participants before initiating the diet (baseline), at each subsequent visit and at 2 years. Subjects were instructed to estimate the amount of food eaten by using colored food-portion photographs representing known weights and household measuring utensils (e.g., cup, spoon, grams). The food record was reviewed and completed in detail with the help of a study dietician. We assessed overall changes in dietary behavior by comparing the diet composition at baseline and at 2 years.
Statistics
The primary outcome was the change in weight over a period of 2 years and the secondary outcome was the number of patients who lost >5% of their initial weight. We used the intention-to-treat principle and analyzed all the participants who were assigned to the diet. For the primary analysis, missing weight was handled using the last observation carried forward. Analysis has been also performed on the participants who completed the study. All other analyses were performed on participants who provided measurements at the concerning time point. The evolution of weight and HbA1c at 2 years has been analyzed using the signed rank test. The Clopper–Pearson method was used to calculate the 95% CI of proportion. The Wilcoxon test was used to compare the change in weight between subgroups. Quantitative data were expressed as mean (SD) and qualitative data as number and percentages (percentages were calculated excluding missing data). All tests were two-sided with α risk at 5%. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Participants
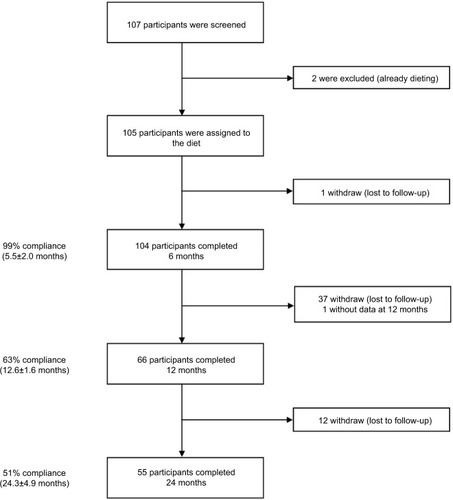
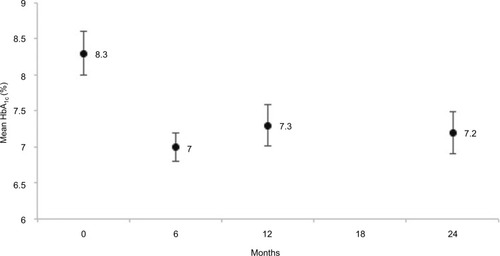
Of the 107 patients recruited, two were excluded because they were already dieting. A total of 105 adults (74 women and 31 men) with a mean age of 50 years (SD, 14 years) and a mean BMI of 31 kg/m2 (SD, 4 kg/m2) followed the “1,2,3 diet”. Most participants were white (55%), 34% were African, and 11% were Asian. Forty percent had T2DM with a mean HbA1c of 8.3% (SD, 2%), most (86%) were on average treated with 1.9 (SD, 1.2) oral antidiabetic drugs and 17% were on insulin. Fifty-four participants (51%) completed the study (i.e., provided measurements at 2 years; ). Baseline characteristics were similar among all participants and completers ().
Table 1 Baseline characteristics of the study participantsTable Footnotea
Body weight
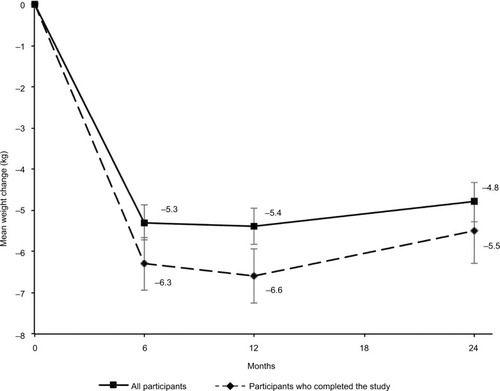
The mean weight loss from baseline to 2 years was 4.8±5.0 kg (p<0.001), which represented 5.6% of initial weight. Among completers mean weight loss was 5.5±5.8 kg (p<0.001) or 6.2% of initial body weight (). At 2 years, 56% (95% CI, 41–69) of the completers had lost >5% of their initial weight and 22% (95% CI, 12–36) had lost at least 10% of their initial weight. In subgroup analysis, the statistically significant decrease in weight was observed similarly in obese and overweight patients () and in patients with or without T2DM (). A multivariate analysis (adjusted for age, sex, BMI, and diabetes) was performed, but no independent predictive factor on weight loss was identified.
Figure 3 Weight changes over 2 years.
Figure 4 Weight changes according to initial BMI (A) and type 2 diabetes mellitus (B) at various time points.
Abbreviation: BMI, body mass index.
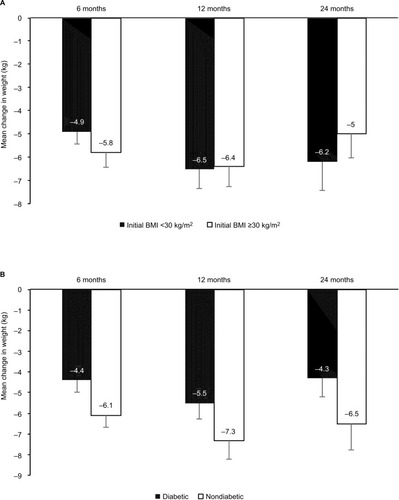
Note: Vertical bars indicate standard errors.

HbA1c
The mean HbA1c level was reduced at 6 months and this difference was still observed at 2 years (−1.1%±2.1%, p=0.001; ). On average, completers used 0.3±1.0 more oral medication to manage glycemia between baseline and 2 years; however, no statistical difference was observed (p=0.23). No new onset of T2DM was detected; however, among diabetics, seven patients were on insulin therapy at 2 years compared to four patients at baseline.
Changes in dietary behavior at 2 years
Reported food intake among completers showed excellent adherence to the “1,2,3 diet” because the consumption of all six prohibited foodstuffs was markedly lower (or became occasional). The typical trend in dietary changes reported by completers at 2 years is depicted in . Adherence to the diet was associated with a spontaneous higher intake of fresh bread, cheese, chocolate, and fresh fruits essentially, compared to their usual diet at baseline. Caloric intake was not assessed; however, a mean weight loss of 5.5 kg at 2 years corresponds to an average energy deficit of ∼70 kcal/day.
Table 2 Diet composition (g/day) as estimated from 3-day food record among completers (n=51) at baseline and at 2 years
Food preparations containing refined fat were easier to circumvent at home and in restaurants but difficult to avoid at company’s cafeterias and during friends or family gatherings. No side-effects were reported by completers.
Follow-up
Mean total number of visits per participant was 2.2±1.1, 5.2±1.7, and 9.7±2.9 at 6, 12, and 24 months, respectively, which is consistent with a low-intensity follow-up (one professional contact per month or less). For completers the mean number of visits per year was 4.9±1.5.
Discussion
Principal findings
This study suggests that moderately obese patients who avoid or markedly reduce oil, butter, margarine, mayonnaise, cream, processed meat, and sweet beverages might achieve successful weight loss at 2 years (5.6%) without counting calories or keeping up with an intensive professional follow-up. However, the high attrition rate suggests that it might be challenging for many to adhere to such diet on the long term. Glycemic levels improved significantly in diabetic patients, but the diet did not allow the reduction of glucose-lowering medication.
In the context of the current literature on weight-loss diets
Improvements in weight and HbA1c compared to baseline are in line with short-term Paleolithic diet studies.Citation41 However, on the long term, a 2013 AHA/ACC/TOS obesity guideline and a 2015 systematic review and meta-analysis concluded that a macronutrient composition to a diet is of minor importance to weight loss.Citation25,Citation26 Instead, long-term effect on body weight depends mainly on the intensity of the intervention. Low-intensity (less than one monthly session) to moderate-intensity (between one and two sessions per month) interventions do not produce any weight loss. Only high-intensity comprehensive lifestyle intervention programs (>14 sessions with trained interventionists in 6 months) will allow 35%–60% of overweight/obese adults to maintain a loss ≥5% of initial body weight at 2 years’ follow-up.Citation25,Citation26 Based on this comprehensive data, the “1,2,3 diet” is the first low-intensity diet to our knowledge that suggests a possible weight loss at 2 years. A 2-year Paleolithic diet study in postmenopausal women showed similar weight loss, but intervention was of moderate intensity.Citation3
Potential mechanism and explanations
Improved leptin sensitivity
Peripheral messengers such as leptin (a hormone essentially secreted by the adipose tissue) act strongly on the homeostatic central neural system (that mediates hunger/satiety feeling) to modulate food intake.Citation27 During caloric restriction, weight loss induces a decrease in leptin levels resulting in an upregulation of these central neurons encouraging the consumption of food despite the persistence of adequate fat stores.Citation28
In leptin-resistant states, in which leptin signaling is subdued,Citation29 weight loss increases further the odds of weight regain.Citation28 Added fats and oils, processed meat, and sugary drinks promote leptin resistance,Citation5,Citation21,Citation22,Citation30,Citation31 a process mediated by the interplay of intestinal microbial dysbiosis, intestinal permeability, and the immune system.Citation32 The exclusion of these foodstuffs in the “1,2,3” diet might have facilitated dietary restraint as witnessed by the meaningful long-term weight loss despite a low-intensity, ad libitum approach. This is consistent with an improved leptin sensitivityCitation5,Citation13 probably mediated by a more favorable microbiota and inflammatory profile. In parallel, the observed increase in fruits, dark chocolate, or fermented cheese consumption might have contributed as well to this enhanced satiety. Indeed, the regular consumption of those phenolic compound–rich foods may beneficially balance the gut microbiota,Citation42 induce the release of satiating gut hormones such as GLP-1 and decrease the orexigenic ghrelin expression on the long term.Citation43
Shifting from addictive obesogenic food to addictive nonobesogenic food
Another biologic process that facilitates weight regain after a diet is related to the addictive properties of processed food.Citation28,Citation33 Humans trying to cut back on such food during weight loss report craving and anxiety that ultimately drive food intake.Citation28 However, not all addictive foods are equally implicated in weight gain. For instance, rats binging on sugar do not experience weight gain, whereas binging on refined fat-enriched food is associated with an increase in body weight.Citation34,Citation35 In the “1,2,3” diet patients compensated their low intake of processed fats by a higher consumption of fresh bread, cheese, and chocolate. These food items described as addictiveCitation33,Citation36 seem, however, not to be associated with obesity or inflammatory microbiota.Citation20,Citation37,Citation38 For instance, a recent human studyCitation39 indicates that an increase in cheese consumption can beneficially modify the gut microbiota to increase short-chain fatty acid (SCFA) levels when compared to diet containing butter. The evidence for a potential role of SCFA to counteract obesity by stimulating the secretion of satiety hormones and energy expenditure is increasing. Therefore, we can speculate that the long-term adherence to the “1,2,3” diet might be due to a spontaneous shift to highly rewarding, yet not obesogenic, food. These hypotheses remain speculative until further studies can be conducted that include a thorough assessment of dietary changes, microbiota, and leptin profiles.
Limitations of the study
The main strength of this exploratory study is the relatively long duration and a high heterogeneity of patients, making the results easier to generalize to other free-living populations. However, it has several limitations. This was a single-arm, open-label investigation. Although lacking a control group it suggests that this approach to weight loss might be helpful to motivate overweight or mildly obese patients not willing to engage in high-intensity programs. Further studies should include a control group to further test the efficacy of the “1,2,3” diet. The open-label design may introduce a bias. When participants know that they are being provided with an experimental diet, their compliance may rise and could lead to better outcomes.Citation44 However, such bias is less relevant in long-term studies because diminished compliance after the first few months is typical in weight-loss trials.Citation45
Another limitation is related to our primary analysis where missing weight was handled using the last observation carried forward. This statistical approach probably understates weight regain of lost-to-follow-up patients. Only completers’ data might, therefore, be relevant for assessment. Fifty-one percent of patients completed the study, making the attrition rate higher than our expected 40% (however, the power attained with our sample size remains above 90%). This high attrition rate at 2 years underscores the difficulty of long-term follow-up with diets. However, attrition rates attributable to lifestyle modification programs are higher than 35% in one-third of clinical trials and appear to be strongly correlated with treatment duration and inversely correlated to intensity of interventions.Citation40 This reminds us that, because many studies did not control for high attrition rates, the reported results in the literature are probably a best-case scenario.
Implications
This 2-year preliminary study suggests that most mildly obese patients were able to successfully adopt an ad libitum diet devoid of only six refined foods even with minimal professional contact. Adherence to such diet revealed significant sustained weight loss and improved glycemic profile among this population. These long-term data suggest that the “1,2,3” diet could be a viable option for some obese and diabetic patients not willing to engage in constraining lifestyle intervention programs. Further research is necessary to confirm these data and assess the long-term safety of the “1,2,3” diet.
Data sharing statement
Please contact author for data requests.
Author contributions
Designed research (RC, SN and HT), conducted research (RC and SN), analyzed data (HT, MG, PL, SN, ID and BH), wrote paper (RC, HT), had primary responsibility for final content (ID, SN, and RC). All the authors (RC, MG, PL, BH, SN, ID, and HT) contributed to revising, editing, and approving the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Chaput JP Perusse L Despres JP Tremblay A Bouchard C Findings from the Quebec Family Study on the Etiology of Obesity: genetics and environmental highlights Curr Obes Rep 2014 3 54 66 24533236
- Lindeberg S Berntorp E Nilsson-Ehle P Terent A Vessby B Age relations of cardiovascular risk factors in a traditional Melanesian society: the Kitava Study Am J Clin Nutr 1997 66 4 845 852 9322559
- Mellberg C Sandberg S Ryberg M Long term effet of a Paleolithic-type diet in obese postmenopausal women: two-year randomized trial Eur J Clin Nutr 2014 68 3 350 357 24473459
- Cordain L Eaton SB Sebastian A Origins and evolution of the Western diet: health implications for the 21th century Am J Clin Nutr 2005 81 2 341 354 15699220
- Spreadbury I Comparison with ancestral diets suggests dense acellular carbohydrates promote an inflammatory microbiota, and may be the primary dietary cause of leptin resistance and obesity Diabetes Metab Syndr Obes 2012 5 175 189 22826636
- de Lartigue G Ronveaux CC Raybould HE Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity Mol Metab 2014 3 6 595 607 25161883
- Eaton SB Eaton SB3rd Paleolithic vs. modern diets-selected pathophysiological implications Eur J Nutr 2000 39 2 67 70 10918987
- Lindeberg S Jönsson T Granfeldt Y A Palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease Diabetologia 2007 50 9 1795 1807 17583796
- Jonsson T Granfeldt Y Ahrén B Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study Cardiovasc Diabetol 2009 8 35 19604407
- Boers I Muskiet FA Berkelaar E Favourable effects of consuming a Palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study Lipids Health Dis 2014 13 160 25304296
- Jonsson T Granfeldt Y Erlanson-Albertsson C Ahren B Lindeberg S A paleolithic diet is more satiating per calorie than a mediterranean-like diet in individuals with ischemic heart disease Nutr Metab (Lond) 2010 7 85 21118562
- Jonsson T Granfeldt Y Lindeberg S Hallberg AC Subjective satiety and other experiences of a Paleolithic diet compared to a diabetes diet in patients with type 2 diabetes Nutr J 2013 12 105 23890471
- Huo L Maeng L Bjorbaek C Grill HJ Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin Endocrinology 2007 148 5 2189 2197 17317774
- US [webpage on the Internet] News Best Diet Rankings 2015 Available from: http://health.usnews.com/best-diet
- Metzgar M Rideout TC Fontes-Villalba M Kuipers RS The feasibility of a Paleolithic diet for low-income consumers Nutr Res 2011 31 6 444 451 21745626
- Monteiro CA Moubarac JC Cannon G Ng SW Popkin B Ultra-processed products are becoming dominant in the global food system Obes Rev 2013 14 Suppl 2 21 28 24102801
- Keita H Ramirez-San Juan E Paniagua-Castro N Garduno-Siciliano L Quevedo L The long-term ingestion of a diet high in extra virgin olive oil produces obesity and insulin resistance but protects endothelial function in rats: a preliminary study Diabetol Metab Syndr 2013 5 1 53 24330822
- Dong D Bilger M van Dam RM Finkelstein EA Consumption of specific foods and beverages and excess weight gain among children and adolescents Health Aff (Millwood) 2015 34 11 1940 1948 26526253
- Deol P Evans JR Dhahbi J Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver PLoS One 2015 10 7 e0132672 26200659
- Mozaffarian D Hao T Rimm EB Willett WC Hu FB Changes in diet and lifestyle and long-term weight gain in women and men N Engl J Med 2011 364 25 2392 2404 21696306
- Deopurkar R Ghanim H Friedman J Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3 Diabetes Care 2010 33 5 991 997 20067961
- Vasselli JR Scarpace PJ Harris RB Banks WA Dietary components in the development of leptin resistance Adv Nutr 2013 4 2 164 175 23493533
- Hu FB Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases Obes Rev 2013 14 8 606 619 23763695
- Foster GD Wyatt HR Hill JO A randomized trial of a low-carbohydrate diet for obesity N Engl J Med 2003 348 21 2082 2090 12761365
- Jensen MD Ryan DH Apovian CM 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society J Am Coll Cardiol 2014 63 2985 3023 24239920
- Tobias DK Chen M Manson JE Ludwig DS Willett W Hu FB Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis Lancet Diabetes Endocrinol 2015 3 12 968 979 26527511
- Ochner CN Tsai AG Kushner RF Wadden TA Treating obesity seriously: when recommendations for lifestyle change confront biological adaptations Lancet. Diabetes Endocrinol 2015 3 4 232 234 25682354
- Ochner CN Barrios DM Lee CD Pi-Sunyer FX Biological mechanisms that promote weight regain following weight loss in obese humans Physiol Behav 2013 120 106 113 23911805
- Zhang F Chen Y Heiman M Dimarchi R Leptin: structure, function and biology Vitam Horm 2005 71 345 372 16112274
- la Fleur SE van Rozen AJ Luijendijk MC Groeneweg F Adan RA A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia Int J Obes (Lond) 2010 34 3 537 546 20029382
- Kratz M von Eckardstein A Fobker M The impact of dietary fat composition on serum leptin concentrations in healthy nonobese men and women J Clin Endocrinol Metab 2002 87 11 5008 5014 12414865
- Cox AJ West NP Cripps AW Obesity, inflammation, and the gut microbiota Lancet Diabetes Endocrinol 2015 3 3 207 215 25066177
- Schulte EM Avena NM Gearhardt AN Which foods may be addictive? The roles of processing, fat content, and glycemic load PLoS One 2015 10 2 e0117959 25692302
- Avena NM The study of food addiction using animal models of binge eating Appetite 2010 55 3 734 737 20849896
- Avena NM Rada P Hoebel BG Sugar and fat bingeing have notable differences in addictive-like behavior J Nutr 2009 139 3 623 628 19176748
- Keser A Yüksel A Yeşiltepe-Mutlu G Bayhan A Özsu E Hatun Ş A new insight into food addiction in childhood obesity Turk J Pediatr 2015 57 3 219 224 26701938
- Cuenca-Garcia M Ruiz JR Ortega FB Castillo MJ HELENA Study Group Association between chocolate consumption and fatness in European adolescents Nutrition 2014 30 2 236 239 24139727
- Serra-Majem L Bautista-Castano I Relationship between bread and obesity Br J Nutr 2015 113 Suppl 2 S29 S35 26148919
- Zheng H Yde CC Clausen MR Metabolomics investigation to shed light on cheese as a possible piece in the French paradox puzzle J Agric Food Chem 2015 63 10 2830 2839 25727903
- Turk MW Yang K Hravnak M Sereika SM Ewing LJ Burke LE Randomized clinical trials of weight loss maintenance: a review J Cardiovasc Nurs 2009 24 1 58 80 19114803
- Manheim EW van Zuuren EJ Fedorowicz Z Pijl H Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis Am J Clin Nutr 2015 102 4 922 932 26269362
- Ozdal T Sela DA Xiao J Boyacioglu D Chen F Capanoglu E The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility Nutrients 2016 8 2 78 26861391
- Serrano J Casanova-Martí À Depoortere I Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols Mol Nutr Food Res 2016 60 12 2554 2564 27417519
- Kawamura A Kajiya K Kishi H Effects of the DASH-JUMP dietary intervention in Japanese participants with high-normal blood pressure and stage 1 hypertension: an open-label single-arm trial Hypertens Res 2016 39 11 777 785 27412796
- Saks FM Bray GA Carey VJ Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates N Engl J Med 2009 360 9 859 873 19246357