Abstract
Background
The prevalence of obesity is growing rapidly and has become a global problem that increases the risk for many diseases. It is influenced by many factors, including consumption of the Western-style diet, characterized as a high-fat diet. Within the central nervous system, the hypothalamus is a critical site in maintaining energy homeostasis and sensing nutrient status, including palmitate, the major component of high-fat-diet.
Methods
In the present study, we conducted a variety of studies to investigate the specific role of salubrinal on palmitate-induced hypothalamic cell death, leptin signaling, and ER stress in an embryonic hypothalamic cell line. Experiments were also performed to identify the underlying mechanisms of the protective effect of salubrinal.
Results
Our results indicate that salubrinal protects hypothalamic cells against PA-induced ER stress and improves hypothalamic leptin sensitivity.
Conclusion
Taken together, our findings conclusively reveal that salubrinal abrogates palmitate-induced hypothalamic leptin resistance and ER stress via NF-κB pathway.
Introduction
The prevalence of obesity is growing rapidly worldwide and has become a severe problem that increases the risk for various chronic diseases, including heart disease, type 2 diabetes, certain types of cancer, and obstructive sleep apnea.Citation1,Citation2 In the last two decades, there has been a growing interest in studies involving the interrelated physiological and pathological factors affecting obesity including genetics, physical activities, and diet. Among all these factors, high-fat diet (HFD) has become of particular interest as it is a major contributor to the increased prevalence of obesity. HFD-induced obese mice display increased free fatty acid levels, including palmitate (PA).Citation3–Citation5 PA is the main saturated free fatty acids in adipose tissue and circulation. It is often used to mimic HFD in experiments in vitro and will downregulate nicotinamide phosphoribosyltransferase activity and nicotinamide adenine dinucleotide levels in human hepatocytes, which are critical for liver functions.Citation6,Citation7
Moreover, HFD-susceptible mice develop increased circulating leptin concentrations but fail to regulate food intake and energy expenditure, hallmarks of leptin resistance. Leptin is an essential hormone secreted by adipocytes. It mainly binds to leptin receptors in the arcuate nucleus of the hypothalamus and activates the Janus-activated kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling. Leptin acts to inhibit orexigenic agouti-related peptide (AgRP) neurons and promote anorexigenic proopiomelanocortin neurons.Citation8–Citation10
Leptin resistance is a major feature of obesity. Impaired leptin signaling and oxidative stress occurring within the hypothalamus are identified as important factors related to leptin resistance and obesity. Although the precise neuronal basis of leptin resistance remains unknown, some defects have been proposed to be predominant underlying causes, including endoplasmic reticulum (ER) stress.Citation11 ER stress occurs when there is an accumulation of misfolded proteins and can, in turn, activate various biological processes including apoptosis.Citation12,Citation13
Salubrinal, a well-known inhibitor of eukaryotic initiation factor 2 subunit ALPHA phosphatase, counteracts ER stress and protects various cell types against ER stress-related cellular damage and cell death.Citation14–Citation16 However, the effects of salubrinal on PA-induced cellular damage in the hypothalamus have yet to be determined, particularly under the context of cell death, leptin resistance, and ER stress.
In the present study, we performed a series of studies to determine the influence of PA in hypothalamic cells and to examine the protective effect of salubrinal on PA-induced hypothalamic cell death. In addition, we also verify the signaling pathways underlying this protection. Our results indicate that salubrinal protects hypothalamic cells against PA-induced ER stress and improves hypothalamic leptin sensitivity.
Materials and methods
Chemicals and antibodies
Recombinant mouse leptin was purchased from R&D Systems (Minneapolis, MN, USA). Salubrinal was purchased from Calbiochem (La Jolla, CA, USA). Antibodies used in these experiments include anti-phospho-JAK2 Tyr1007/1008 (1:1,000, #3771), anti-phospho-Stat3 Tyr705 (1:1,000, #9145), anti-Stat3 (1:1,000, #8768), anti-phospho-PERK Thr980 (1:1,000, #3179), anti-PERK (1:1,000, #5683), anti-CHOP (1:1,000, #2895), anti-NF-κB p65 (1:1,000, #8242), anti-Lamin B1 (1:1,000, #13435), anti-phospho-IκBα (1:1,000, #2859), anti-IκBα (1:1,000,#9242), and anti-β-Actin (1:5,000, #3700) from Cell Signaling Technology (Beverly, MA, USA). PA was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). PA was solubilized in pre-heated 0.1 N NaOH and diluted in pre-warmed 10% fatty acid free-BSA solution to give a final concentration of 5 mM. Control media were prepared with 0.1 N NaOH and 10% BSA without lipid. PA solution was freshly made before each experiment.
Cell culture
The mouse embryonic hypothalamic cell line, mHypoE-44, was purchased from Cedarlane Cellutions Biosystems (Burlington, Ontario, Canada) and maintained in DMEM (Gibco, Waltham, MA, USA) with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin (Gibco) in a humidified incubator with 5% CO2 atmosphere at 37°C. Cells were grown to 70%–80% confluency and serum starved for 1 hour before leptin treatment.
Cell viability assays
Cell viability was assessed by Cell Counting Kit-8 (CCK-8) and a trypan blue staining assay. In brief, 2×105 mHypoE-44 cells were plated in each well of a 96-well plate with 100 µL medium. After different treatments were applied, cell viability was assessed by the CCK-8 (Dojindo, Japan) according to the manufacturer’s instructions. Meanwhile, cells were collected and stained with trypan blue (Thermo Fisher Scientific). The number and the percentage of trypan blue-positive cells were counted and calculated using Countess™ Automated Cell Counter from Thermo Fisher Scientific.
TUNEL assay
TUNEL assay was performed with an in situ cell death detection kit (Roche) according to the manufacturer’s instruction. Cells were TUNEL-stained and subjected to DNA staining with Hoechst 33342. TUNEL-positive cells were detected and counted using the confocal microscope. At least 200 cells in 5 random scope fields were analyzed to calculate the TUNEL-positive percentage.
Western blotting
Cells were collected, and the whole cell lysates were prepared by sonication in CelLytic MT buffer from Sigma-Aldrich Co. Samples consisting of 50 µg of protein were loaded on a denaturing 4%–20% precast SDS-PAGE gel (Bio-Rad Laboratories Inc., Hercules, CA, USA) and then transferred to immobilon-FL PVDF membranes (EMD Millipore, Billerica, MA, USA) by electrophoresis. The membranes were blocked in Odyssey Blocking Buffer (PBS) (LI-COR) at room temperature for 1 hour and then incubated with specific primary antibodies at 4°C overnight. Blots were incubated with species-specific secondary antibodies at room temperature for 1 hour the next day. The signals were detected by ECL reagent (Thermo Fisher Scientific) and analyzed by ImageJ software (LOCI, University of Wisconsin, USA).
Statistical analysis
All data shown were expressed as the mean ± SEM using unpaired Student’s t-tests or two-way ANOVA with Bonferroni posttests (GraphPad Software, Inc., La Jolla, CA, USA). The data represented at least three independent experiments, and P-values were calculated using GraphPad Prism software 7.0.
Results
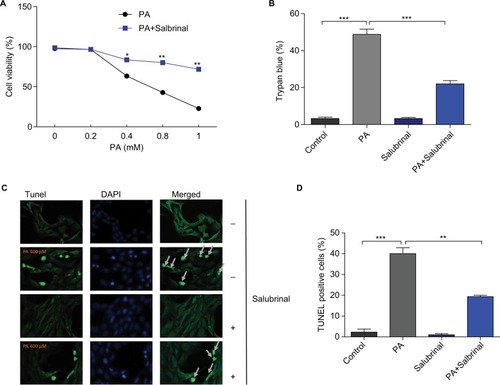
Salubrinal prevents hypothalamic cells from PA-induced cell death
Within the central nervous system, the hypothalamus is a critical site in sensing nutrient status, regulating food intake and energy expenditure, and maintaining energy homeostasis.Citation17–Citation19 We therefore carried out experiments using the mHypoE-44 cell line, a mouse embryonic hypothalamic cell line expressing AgRP and leptin receptor. To determine the effects of salubrinal on PA-induced hypothalamic cell death, mHypoE-44 cells were pretreated with salubrinal prior to PA treatment at the indicated concentrations. Cell viability was then assessed by CCK-8 (), trypan blue staining assay (), and TUNEL assay (). As expected, PA treatment led to a reduction of hypothalamic cell viability where this cytotoxic effect was significantly diminished by salubrinal pretreatment (). The results from the trypan blue staining assay also demonstrated that salubrinal significantly decreases PA-induced cell death, consistent with the previous data. Similar results were also observed in the TUNEL assay experiments. After the treatment of salubrinal, the number of TUNEL-positive cells was significantly reduced compared with the control group in response to PA. Taken together, these results suggest that salubrinal protects hypothalamic cells from PA-induced cell death.
Figure 1 Salubrinal protects hypothalamic cells against PA-induced cell death.
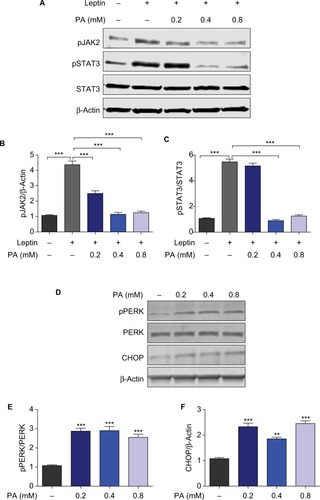
PA induces hypothalamic leptin resistance and ER stress
To study the effects of PA in regulating leptin signaling and ER stress in the hypothalamic cells, mHypoE-44 cells were treated with PA at indicated doses for 24 hours prior to leptin treatment. After 45 minutes, cells were collected and prepared for Western blot analysis. Leptin treatment increased levels of phospho-JAK2 and phospho-STAT3 where this increase was significantly diminished by PA challenge in a dose-dependent manner (). These results indicate that PA treatment is able to induce hypothalamic leptin resistance. ER stress is well known as one major cause of leptin resistance. To further assess the role of PA in ER stress, we treated mHy-poE-44 cells with PA only and subjected them to Western blot analysis for ER stress markers, phospho-PERK and CHOP (). Significant induction of these two markers was found in response to PA treatment, which implicates that PA promotes hypothalamic leptin resistance and ER stress.
Figure 2 PA provokes hypothalamic leptin resistance and ER stress.
Abbreviations: ER, endoplasmic reticulum; JAK2, Janus-activated kinase 2; STAT3, signal transducer and activator of transcription 3.
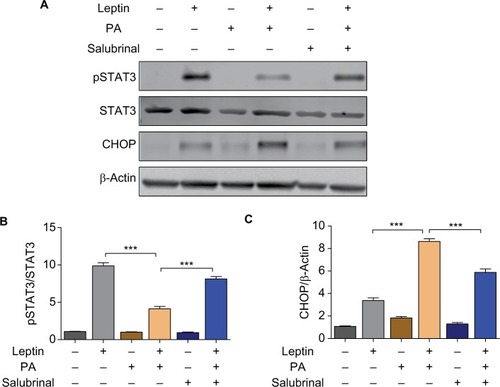
Salubrinal promotes hypothalamic leptin sensitivity via inhibiting PA-induced ER stress response
To verify the effect of salubrinal on PA-Induced leptin resistance and ER stress, mHypoE cells were co-treated with salubrinal, PA, and leptin. Cells were then subjected to Western blot analysis and probed for the expressions of phosoho-STAT3 and CHOP. Results demonstrated that the elevated expression levels of CHOP induced by PA treatment were significantly attenuated by co-treatment with salubrinal (). In addition, PA-induced impairment of leptin signaling was also rescued by salubrinal (). These results suggest that salubrinal promotes leptin sensitivity and protects hypothalamic cells against PA-induced ER stress.
Figure 3 Salubrinal promotes hypothalamic leptin sensitivity by inhibiting PA-induced ER stress response.
Abbreviations: ER, endoplasmic reticulum; JAK2, Janus-activated kinase 2; pSTAT3, phospho-STAT3; STAT3, signal transducer and activator of transcription 3.
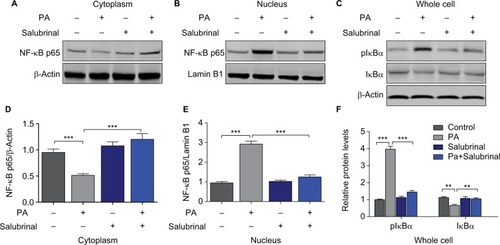
Salubrinal inhibits the activation of hypothalamic nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway induced by PA
NF-κB pathway is implicated in many biological progresses in the nervous system, including inflammation, stress response, and cell survival.Citation20,Citation21 To test whether PA treatment can activate NF-κB pathway and salubrinal is able to inhibit the activation, we challenged hypothalamic cells with salubrinal and PA. Western blot analysis probing for the NF-κB pathway demonstrated that salubrinal notably blocked the transportation of NF-κB p65 from cytoplasm to nucleus induced by PA treatment (). Furthermore, the degradation of IκB induced by PA was also diminished by salubrinal (). In sum, these results indicate that salubrinal attenuated the activation of the NF-κB pathway induced by PA treatment in hypothalamic cells.
Figure 4 Salubrinal inhibits the activation of hypothalamic NF-κB pathway induced by PA treatment.
Abbreviation: NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells,
Discussion
With an increase in consumption of the Western-style diet, characterized as an HFD, obesity has become a global problem related to the rise of a wide range of diseases. Much effort has been made to explore novel chemicals that may help protect against HFD-induced metabolic syndromes. In the present study, we aim to testify the protective role of salubrinal against PA-induced hypothalamic cell death, leptin resistance, and ER stress. Furthermore, we want to determine the mechanism underlying its protective role.
The hypothalamus is the main site in the central nervous system in controlling energy homeostasis. In the present study, we performed our experiments using the hypothalamic cell line, mHypoE-44. This cell line has a neuronal morphology and is derived and immortalized from mouse embryonic day 17 hypothalamic primary cultures by retroviral transfer of SV40 T-Ag. In addition, mHypoE-44 cells are known to express neuropeptide Y, AgRP, and leptin receptor. As a major component in the HFD, PA is reported to cause hypothalamic inflammation and insulin resistance.Citation22,Citation23 In addition, PA also leads to insulin signaling impairment. However, the correlation between PA and hypothalamic cell death is still elusive. ER stress, which is well known as a major cause of leptin resistance, can also regulate neuron death, neuron inflammation, and neurogenesis.Citation24–Citation26 In our study, we found that exposure to PA results in hypothalamic cell death, leptin resistance, and ER stress. Co-treatment with salubrinal largely reverses these effects of PA and protects hypothalamic cells from apoptosis. These results delineate the specific role of PA in hypothalamic leptin signaling and establish a putative agent, salubrinal, to reverse the adverse effects of over-nutrition.
Finally, we aimed to determine the underlying mechanism by which salubrinal acts in the hypothalamic cell death. The pro-inflammatory NF-κB pathway plays various roles in the nervous system, including regulation of neuroprotection, neuron proliferation, and inflammation.Citation27 It has been reported that HFD induces chronic hypothalamic inflam mation and increases the secretion of pro-inflammatory cytokines in the hypothalamus.Citation28 Moreover, leptin resistance is also associated with neuroinflammation.Citation29 This is consistent with our findings in the present study that PA treatment activates the NF-κB pathway. When activated, NF-κB enters into the nucleus and induces the transcription of pro-inflammatory genes. Meanwhile, IκB is phosphorylated then degraded. Currently, we have verified that salubrinal inhibits the activation of the NF-κB pathway and attenuates the degradation of IκB induced by PA treatment. These results suggest that the NF-κB pathway is critical for the protective role of salubrinal against PA-induced hypothalamic cell death and ER stress.
In sum, we have revealed the protective role of salubrinal in PA-induced hypothalamic cell death via inhibiting ER stress and promoting leptin sensitivity. Our results demonstrate that salubrinal can be a potential therapeutic chemical used in preventing HFD-induced obesity.
Acknowledgments
This research was supported by Funds for Major Science and Technology Projects from Changzhou Health and Family Planning Commission (No. ZD201512).
Disclosure
The authors report no conflicts of interest in this work.
References
- Friedman JM Obesity: causes and control of excess body fat Nature 2009 459 7245 340 342 19458707
- Wang YC McPherson K Marsh T Gortmaker SL Brown M Health and economic burden of the projected obesity trends in the USA and the UK Lancet 2011 378 9793 815 825 21872750
- Hodson L Skeaff CM Fielding BA Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake Prog Lipid Res 2008 47 5 348 380 18435934
- Hudgins LC Hellerstein M Seidman C Neese R Diakun J Hirsch J Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet J Clin Invest 1996 97 9 2081 2091 8621798
- Karmi A Iozzo P Viljanen A Increased brain fatty acid uptake in metabolic syndrome Diabetes 2010 59 9 2171 2177 20566663
- Ubhayasekera SJ Staaf J Forslund A Bergsten P Bergquist J Free fatty acid determination in plasma by GC-MS after conversion to Weinreb amides Anal Bioanal Chem 2013 405 6 1929 1935 23307129
- Penke M Schuster S Gorski T Gebhardt R Kiess W Garten A Oleate ameliorates palmitate-induced reduction of NAMPT activity and NAD levels in primary human hepatocytes and hepatocarcinoma cells Lipids Health Dis 2017 16 1 191 28974242
- Zhang Y Proenca R Maffei M Barone M Leopold L Friedman JM Positional cloning of the mouse obese gene and its human homologue Nature 1994 372 6505 425 432 7984236
- Hübschle T Thom E Watson A Roth J Klaus S Meyerhof W Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation J Neurosci 2001 21 7 2413 2424 11264315
- Yang G Lim CY Li C FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1 J Biol Chem 2009 284 6 3719 3727 19049975
- Pagliassotti MJ Kim PY Estrada AL Stewart CM Gentile CL Endoplasmic reticulum stress in obesity and obesity-related disorders: an expanded view Metabolism 2016 65 9 1238 1246 27506731
- Ozcan L Ergin AS Lu A Endoplasmic reticulum stress plays a central role in development of leptin resistance Cell Metab 2009 9 1 35 51 19117545
- Purkayastha S Zhang H Zhang G Ahmed Z Wang Y Cai D Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress Proc Natl Acad Sci U S A 2011 108 7 2939 2944 21282643
- Kim JS Heo RW Kim H Salubrinal, ER stress inhibitor, attenuates kainic acid-induced hippocampal cell death J Neural Transm (Vienna) 2014 121 10 1233 1243 24728926
- Wu L Luo N Zhao HR Salubrinal protects against rotenone-induced SH-SY5Y cell death via ATF4-parkin pathway Brain Res 2014 1549 52 62 24418467
- Cakir I Cyr NE Perello M Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing J Biol Chem 2013 288 24 17675 17688 23640886
- Schwartz MW Woods SC Porte D Seeley RJ Baskin DG Central nervous system control of food intake Nature 2000 404 6778 661 671 10766253
- He W Lam TK Obici S Rossetti L Molecular disruption of hypothalamic nutrient sensing induces obesity Nat Neurosci 2006 9 2 227 233 16415870
- Lam TK Schwartz GJ Rossetti L Hypothalamic sensing of fatty acids Nat Neurosci 2005 8 5 579 584 15856066
- Baldwin AS Series introduction: the transcription factor NF-kappaB and human disease J Clin Invest 2001 107 1 3 6 11134170
- Hoesel B Schmid JA The complexity of NF-κB signaling in inflammation and cancer Mol Cancer 2013 12 86 23915189
- Posey KA Clegg DJ Printz RL Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet Am J Physiol Endocrinol Metab 2009 296 5 E1003 E1012 19116375
- Delarue J Magnan C Free fatty acids and insulin resistance Curr Opin Clin Nutr Metab Care 2007 10 2 142 148 17285001
- Brown MK Naidoo N The endoplasmic reticulum stress response in aging and age-related diseases Front Physiol 2012 3 263 22934019
- Anuncibay-Soto B Pérez-Rodriguez D Santos-Galdiano M Salubrinal and robenacoxib treatment after global cerebral ischemia. Exploring the interactions between ER stress and inflammation Bio-chem Pharmacol 2018 151 26 37
- Sprenkle NT Sims SG Sánchez CL Meares GP Endoplasmic reticulum stress and inflammation in the central nervous system Mol Neurode-gener 2017 12 1 42
- Wang R Chen S Liu Y All-trans-retinoic acid reduces BACE1 expression under inflammatory conditions via modulation of nuclear factor κB (NFκB) signaling J Biol Chem 2015 290 37 22532 22542 26240147
- Morselli E Fuente-Martin E Finan B Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα Cell Rep 2014 9 2 633 645 25373903
- de Git KC Adan RA Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation Obes Rev 2015 16 3 207 224 25589226
