Abstract
Aim
Nonalcoholic fatty liver disease (NAFLD), including nonalcoholic steatohepatitis (NASH), is known to be associated with type 2 diabetes mellitus (T2DM) in high rate. The improvement in hepatic function due to sodium-glucose co-transporter 2 (SGLT2) inhibitors has been reported in T2DM patients with and without NAFLD. However, only a few studies have attempted to evaluate the role of SGLT2 inhibitors in T2DM patients with biopsy-proven NASH, and no detailed prospective studies including the individual hepatic fibrosis stage have been reported. Therefore, we investigated the effect of canagliflozin on hepatic function in T2DM patients with biopsy-confirmed NASH.
Methods
T2DM patients with NASH (hepatic fibrosis stage 1–3 confirmed via liver biopsy, n=10) were enrolled and received canagliflozin (100 mg) once a day for 12 weeks. The primary end point was change in serum alanine aminotransferase (ALT) levels from baseline to week 12. Secondary end points were liver function/fibrosis markers, metabolic parameters, and safety.
Results
The change in ALT from baseline to week 12 was −23.9 U/L (95% CI −48.1 to 0.3, P=0.0526). Significant improvements in several hepatic function/fibrosis markers, such as aspartate aminotransferase, fibrosis-4 index, and FM-fibro index, and metabolic parameters including hemoglobin A1c and body weight were found. No serious or liver-related adverse events were reported. Regarding individual patients, different trends in ALT-lowering effects between stage 1 and stage 2/3 subjects were observed; the degree of ALT-lowering effect tended to be greater in the stage 1 group than in the stage 2/3 group.
Conclusion
Our results suggest that canagliflozin is effective and well-tolerated in patients with T2DM and NASH. Canagliflozin may be useful for the treatment of T2DM patients with NASH, especially those in early stages of NASH.
Introduction
Nonalcoholic fatty liver disease (NAFLD), including nonalcoholic steatohepatitis (NASH), is associated with type 2 diabetes mellitus (T2DM).Citation1–Citation3 In Japan, the prevalence of T2DM is ~50% in patients with NAFLD and increases with progression of the fibrosis stage; DM is a significant risk factor for advanced fibrosis.Citation4
Advanced NASH increases the risks of cirrhosis and hepatocellular carcinoma.Citation3 A reduction in serum alanine aminotransferase (ALT) level (≥30% reduction from the baselineCitation5 or ≥30% reduction from the baseline and decrease to ≤40 U/LCitation6) was associated with amelioration of liver fibrosis progression in NASH patients. Therefore, control of serum ALT via suitable interventions is important to prevent NASH progression. Although pioglitazone has been shown to improve serum ALT levels and histological features in NASH patients with insulin resistance, concerns such as body weight gain and congestive heart failure exist.Citation7 Therefore, new NASH treatment strategies are needed.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors suppress glucose reabsorption in the renal tubules and exert antihyperglycemic effects. In addition to glucose lowering, SGLT2 inhibitors improve multiple risk factors such as body weight and blood pressure.Citation8–Citation10 Because some SGLT2 inhibitors including canagliflozin and empagliflozin have shown cardiovascular and renal protective effects in T2DM patients with a history or high risk of cardiovascular disease,Citation11,Citation12 they are a potential therapeutic option for preventing diabetic complications. Improvement in hepatic function due to SGLT2 inhibitors has also been reported in T2DM patients with and without NAFLD.Citation13–Citation18 We also previously reported that treatment of canagliflozin (100 mg) for 12 weeks significantly improved ALT levels compared with placebo in T2DM patients with ALT >31 U/L.Citation19 However, studies on the role of SGLT2 inhibitors in T2DM patients with biopsy-proven NASH are limited, and no detailed prospective studies including individual hepatic fibrosis stage data have been reported.Citation16,Citation20,Citation21 Therefore, we performed the pilot study to investigate the efficacy and safety of canagliflozin in T2DM patients with biopsy-confirmed NASH.
Methods
Study design and patients
A single-arm, exploratory study was performed to investigate the efficacy and safety of canagliflozin in T2DM patients with NASH. The inclusion criteria were as follows: T2DM patients with hemoglobin A1c (HbA1c) ≥6.0% and <10.0%, ALT ≥31 U/L, age ≥20 and <75 years, hepatic fibrosis stage 1–3Citation22,Citation23 confirmed via liver biopsies during 1 year prior to the start of the study, and daily alcohol consumption of <30 g/day in males and <20 g/day in females. The exclusion criteria were as follows: T1DM, history of class IV heart failure per the New York Heart Association functional classification, malignancy, platelet count <105/µL, estimated glomerular filtration rate <45 mL/min/1.73 m2, and SGLT2 inhibitor treatment within 12 weeks prior to the start of the study. The severity of hepatic fibrosis stage was scored as: stage 1, zone 3 perisinusoidal fibrosis; stage 2, zone 3 perisinusoidal fibrosis with portal fibrosis; stage 3, zone 3 perisinusoidal fibrosis and portal fibrosis with bridging fibrosis; and stage 4, cirrhosis.Citation22,Citation23
Intervention and outcomes
Canagliflozin (100 mg) was orally administered to patients before breakfast for 12 weeks. Visits were scheduled every 4 weeks during the treatment. The primary end point was change in serum ALT levels from baseline. Secondary end points included changes from baseline to week 12 in aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GTP), alkaline phosphatase (ALP), hyaluronic acid, type IV collagen 7S, waist circumference, NAFIC score, fibrosis-4 index (FIB-4 index), FM-fibro index calculated from hyaluronic acid and type IV collagen 7S,Citation24 liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) measured via transient elastography (FibroScan; ECHOSENS, Paris, France), HbA1c, fasting plasma glucose, fasting serum insulin, serum C-peptide, platelet count, uric acid, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, body composition measured using a multifrequency impedance body composition analyzer (InBody 720; InBody Japan, Tokyo, Japan), and responses to the Dutch Eating Behavior Questionnaire (DEBQ) and to the 8-Item Short Form Health Survey (SF-8). Exploratory end points included changes in other parameters including serum glucagon and ferritin. The safety evaluation included assessments of adverse events (AEs) described using the Medical Dictionary for Regulatory Activities/Japanese version 20.1 and laboratory values.
Sample size calculation
We assumed a mean difference of −10 U/L in the change in ALT levels from baseline to the end of the treatment period with a SD of 15 U/L based on results from a previous studyCitation19 and found that 20 patients were required for a power (paired t-test) of 81% at a two-sided significance level of 5%.
Statistical analyses
Data were represented as mean values with SD or 95% CI values. All changes between baseline and week 12 were analyzed using two-tailed paired t-tests. As subsidiary evaluations, we also analyzed changes after 4 and 8 weeks. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using the statistical analysis system version 9.4 (SAS Institute Inc., Cary, NC, USA). Data management and statistical analyses were conducted by the EP-CRSU Co. Ltd. (Tokyo, Japan).
Ethical considerations
The study was conducted in compliance with the Declaration of Helsinki and the “Ethical guidelines for medical and health research involving human subjects”.Citation25 It was approved by the institutional review board at Kyoto Prefectural University of Medicine and is registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000023044). All patients provided written informed consent.
Results
Patients
Ten patients (seven males and three females) were enrolled in the study and included in efficacy and safety analysis. Patient characteristics are shown in . The mean ± SD of the baseline ALT, HbA1c, and body mass index were 99.1 ± 52.6 U/L, 7.09 ± 0.87%, and 29.09 ± 2.50 kg/m2, respectively; age and duration of T2DM were 54.9 ± 12.9 and 4.64 ± 4.38 years, respectively. Fibrosis stages included stage 1 (n=5), stage 2 (n=3), and stage 3 (n=2).
Table 1 Patient demographics and characteristics
Efficacy
A decreasing trend in ALT levels was observed from week 4 (), and the mean change in ALT levels from baseline to week 12 (primary end point) was −23.9 U/L (95% CI −48.1 to 0.3, P=0.0526) (), corresponding to the percent change of −19.94% (95% CI −40.40 to 0.53). AST, HbA1c, and body weight decreased from week 4 and were significantly reduced at week 12 () ().
Figure 1 Changes in (A) ALT, (B) AST, (C) HbA1c, and (D) body weight over 12 weeks.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, hemoglobin A1c.
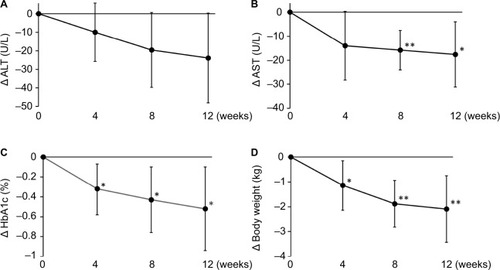
Table 2 Effects of canagliflozin on hepatic function/fibrosis markers and metabolic parameters (n=10)
The other secondary end points, γ-GTP as a hepatic function-related marker, and platelets, type IV collagen 7S, the FIB-4 index and the FM-fibro index as hepatic fibrosis markers, were significantly changed at week 12. Other hepatic fibrosis markers including the NAFIC score and LSM/CAP evaluated using transient elastography did not change ().
Changes in body composition were assessed using a multifrequency impedance body composition analyzer; body fat mass was significantly decreased, and no significant changes in muscle mass or body water content were seen (). The percentage changes in each component are shown in .
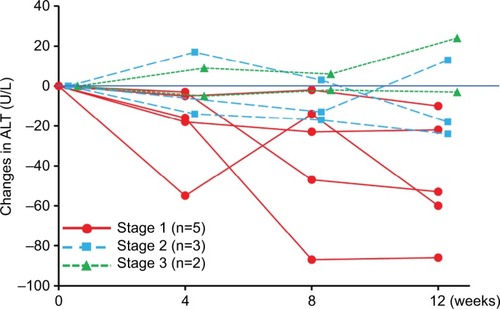
Changes in ALT in each subject are shown in ; different trends in ALT-lowering effects between stage 1 and stage 2/3 subjects were observed. Post hoc analysis showed that in the stage 1 and stage 2/3 populations, the baseline ALT values were 109.6 ± 59.7 and 88.6 ± 49.0 U/L, respectively; changes in the ALT values from baseline to week 12 were −46.2 U/L (95% CI −84.0 to −8.4) and −1.6 U/L (95% CI −26.8 to 23.6), respectively.
Figure 2 Time course of changes in ALT levels from baseline in individual subjects.
Abbreviation: ALT, alanine aminotransferase.
Other secondary end points and exploratory end points are shown in . Among them, serum ferritin was significantly decreased at week 12. The SF-8 and DEBQ, indicators of quality of life and dietary habits, respectively, were unchanged due to the treatment (data not shown).
Safety
AEs occurred in five patients; however, no serious or liver-related AEs were reported (). The changes in laboratory values are shown in .
Discussion
This study examined the efficacy and safety of canagliflozin in T2DM patients with NASH; the treatment showed improvement in several hepatic function/fibrosis markers and metabolic parameters and was well-tolerated. Canagliflozin decreased ALT levels from baseline (−23.9 U/L); however, it was not statistically significant (P=0.0526). This may have been due to a lack of statistical power. Canagliflozin significantly improved the hepatic fibrosis markers such as the FIB-4 index and the FM-fibro index, suggesting the possibility of improving hepatic fibrosis. Conversely, NAFIC score, LSM, and CAP did not change; improvement in these parameters may not be detected during short-term treatments. Improvement in several hepatic function/fibrosis markers (ALT, AST, FIB-4 index) was consistent with previous reports involving SGLT2 inhibitors,Citation20,Citation21 except the FM-fibro index, a new fibrosis marker,Citation24 with the present study being the first reporting its use in drug evaluation. Although accuracy of FM-fibro index for diagnosing NASH fibrosis had been evaluated in a pilotCitation26 or multicenter studies,Citation27 the drug evaluation data is also important for characterization of FM-fibro index as a biomarker of NASH. In terms of hepatic fibrosis stage, decrease in ALT tended to be greater in the stage 1 group (−46.2 U/L) than in the stage 2/3 group (−1.6 U/L), suggesting greater improvement in hepatic function in the stage 1 group; this may indicate the significance of canagliflozin treatment in early-stage NASH patients.
In addition to reduction in ALT, improvement in HbA1cCitation28 and body weight reductionCitation29 are also important for preventing NASH progression. Adipose tissue inflammation is also associated with NASH progressionCitation30; canagliflozin reduced fat mass, which may lead to inhibition of inflammatory cytokine release from adipocytes.Citation31 The effects of SGLT2 inhibitors on these factors may contribute to prevention of NASH progression.
NAFLD/NASH with diabetes increases the risk of developing cardiovascular disease and serious hepatic diseases, particularly NASH, cirrhosis, and hepatocellular carcinoma.Citation32,Citation33 Moreover, malignant neoplasia is the leading cause of death and liver cancer is the second leading cause of cancer death in Japanese DM patients.Citation34 Therefore, interventions using SGLT2 inhibitors in T2DM patients with early-stage NASH may be important to prevent cardiovascular disease and liver cancer.
The limitations of this study were the small sample size, the short-term treatment period, and the lack of a control group.
Conclusion
Canagliflozin is effective and well-tolerated in patients with T2DM and NASH; canagliflozin may be especially useful for those in early stages of NASH. A long-term, large-scale, randomized control study is necessary to confirm the benefit of SGLT2 inhibitor treatment in patients with T2DM and NASH.
Data sharing statement
The datasets generated and/or analyzed during the current study are not publicly available because of lack of agreement for disclosing individual raw data in public but are available from the corresponding author on reasonable request.
Author contributions
Y Seko,TN and YI contributed to the study design, data collection and analysis. Y Sumida, TO, MK, HI, and TH contributed to the study design. All authors contributed to data interpretation, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was funded by Mitsubishi Tanabe Pharma Corporation. The authors would like to thank Ms A Yano (EP-CRSU Co., Ltd.) for coordinating the study and Ms Y Yamamoto (EP-CRSU Co., Ltd.) for conducting the statistical analyses.
Disclosure
Y Sumida has received speakers’ bureau fees from Astellas Pharma Inc., AstraZeneca K.K., Eli Lilly Japan K.K., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd. and has received a research grant from Bristol-Myers Squibb Co. TO has received consulting fee from Bristol-Myers Squibb Co., and Gilead Sciences, Inc. MK, HI, and TH are employees of Mitsubishi Tanabe Pharma Corporation. YI has received speakers’ bureau fees from Mitsubishi Tanabe Pharma Corporation and AU has received research support from Bristol-Myers Squibb Co. Y Seko, TN, KY, MM, and KY report no conflicts of interest in this work.
Supplementary materials
Table S1 Percent change of body composition after canagliflozin treatment at week 12 (n=10)
Table S2 Effects of canagliflozin on other efficacy end points (n=10)
Table S3 Adverse events
Table S4 Laboratory variables (n=10)
References
- Vernon G Baranova A Younossi ZM Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults Aliment Pharmacol Ther 2011 34 3 274 285 21623852
- Reid AE Nonalcoholic steatohepatitis Gastroenterology 2001 121 3 710 723 11522755
- Younossi ZM Koenig AB Abdelatif D Fazel Y Henry L Wymer M Global epidemiology of nonalcoholic fatty liver disease – meta-analytic assessment of prevalence, incidence, and outcomes Hepatology 2016 64 1 73 84 26707365
- Nakahara T Hyogo H Yoneda M Japan Study Group of Nonalcoholic Fatty Liver Disease Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients J Gastroenterol 2014 49 11 1477 1484 24277052
- Seko Y Sumida Y Tanaka S Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients Hepatol Res 2015 45 10 E53 E61 25429984
- Hoofnagle JH Van Natta ML Kleiner DE Non-alcoholic Steatohepatitis Clinical Research Network (NASH CRN) Vitamin E and changes in serum alanine aminotransferase levels in patients with nonalcoholic steatohepatitis Aliment Pharmacol Ther 2013 38 2 134 143 23718573
- Watanabe S Hashimoto E Ikejima K Japanese Society of Gastroenterology; Japan Society of Hepatology Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis J Gastroenterol 2015 50 4 364 377 25708290
- Kashiwagi A Maegawa H Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus J Diabetes Investig 2017 8 4 416 427
- Inagaki N Harashima SI Iijima H Canagliflozin for the treatment of type 2 diabetes: a comparison between Japanese and non-Japanese patients Expert Opin Pharmacother 2018 19 8 895 908 29799286
- Davies MJ Merton KW Vijapurkar U Balis DA Desai M Canagliflozin improves risk factors of metabolic syndrome in patients with type 2 diabetes mellitus and metabolic syndrome Diabetes Metab Syndr Obes 2017 10 47 55 28184166
- Neal B Perkovic V Mahaffey KW CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes N Engl J Med 2017 377 7 644 657 28605608
- Zinman B Wanner C Lachin JM EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes N Engl J Med 2015 373 22 2117 2128 26378978
- Leiter LA Forst T Polidori D Balis DA Xie J Sha S Effect of canagliflozin on liver function tests in patients with type 2 diabetes Diabetes Metab 2016 42 1 25 32 26575250
- Ito D Shimizu S Inoue K Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial Diabetes Care 2017 40 10 1364 1372 28751548
- Shibuya T Fushimi N Kawai M Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective randomized controlled pilot study Diabetes Obes Metab 2018 20 2 438 442 28719078
- Seko Y Sumida Y Tanaka S Effect of sodium glucose cotrans-porter 2 inhibitor on liver function tests in Japanese patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus Hepatol Res 2017 47 10 1072 1078 27925353
- Kuchay MS Krishan S Mishra SK Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial) Diabetes Care 2018 41 8 1801 1808 29895557
- Sumida Y Murotani K Saito M Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective, single-arm trial (LEAD trial) Hepatol Res Epub 2018 7 27
- Seko Y Sumida Y Sasaki K Effects of canagliflozin, an SGLT2 inhibitor, on hepatic function in Japanese patients with type 2 diabetes mellitus: pooled and subgroup analyses of clinical trials J Gastroenterol 2018 53 1 140 151 28669071
- Tobita H Sato S Miyake T Ishihara S Kinoshita Y Effects of dapa-gliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open-label, uncontrolled Study Curr Ther Res Clin Exp 2017 87 13 19 28912902
- Akuta N Watanabe C Kawamura Y Effects of a sodium-glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: preliminary prospective study based on serial liver biopsies Hepatol Commun 2017 1 1 46 52 29404432
- Brunt EM Kleiner DE Wilson LA Belt P Neuschwander-Tetri BA NASH Clinical Research Network (CRN) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings Hepatology 2011 53 3 810 820 21319198
- Brunt EM Janney CG Di Bisceglie AM Neuschwander-Tetri BA Bacon BR Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions Am J Gastroenterol 1999 94 9 2467 2474 10484010
- Yoshimura K Okanoue T Ebise H Identification of novel noninvasive markers for diagnosing nonalcoholic steatohepatitis and related fibrosis by data mining Hepatology 2016 63 2 462 473 26390046
- Japanese Ministry of Health, Labor and Welfare Ethical guidelines for medical and health research involving human subjects Available from: http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkan-boukouseikagakuka/0000080278.pdf Accessed August 29, 2018
- Okanoue T Ebise H Kai T A simple scoring system using type IV collagen 7S and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis J Gastroenterol 2018 53 1 129 139 28589339
- Itoh Y Seko Y Shima T Accuracy of non-invasive scoring systems for diagnosing non-alcoholic steatohepatitis-related fibrosis: multicenter validation study Hepatol Res Epub 2018 7 04
- Hamaguchi E Takamura T Sakurai M Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis Diabetes Care 2010 33 2 284 286 19880582
- Vilar-Gomez E Yasells-Garcia A Martinez-Perez Y Development and validation of a noninvasive prediction model for nonalcoholic steatohepatitis resolution after lifestyle intervention Hepatology 2016 63 6 1875 1887 26849287
- Tilg H Moschen AR Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis Hepatology 2010 52 5 1836 1846 21038418
- Garvey WT Van Gaal L Leiter LA Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes Metabolism 2018 85 32 37 29452178
- Cusi K Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions Diabetologia 2016 59 6 1112 1120 27101131
- Kawamura Y Arase Y Ikeda K Large-scale long-term follow-up study of Japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma Am J Gastroenterol 2012 107 2 253 261 22008893
- Nakamura J Kamiya H Haneda M Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: Report of the Committee on Causes of Death in Diabetes Mellitus J Diabetes Investig 2017 8 3 397 410
