 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Resistin (RES) concentration increases in end-stage renal disease patients. However, there have been no studies defining the role of physical activity in RES concentrations in hemodialyzed (HD) patients. This study was aimed to determine metabolic and inflammatory effects, including RES, of 4-week supervised rehabilitation program in HD patients, with or without metabolic syndrome (MS).
Methods
The study was completed by 28 patients aged 56.9±13.3 years who were HD for 50.6±73.4 months, and 30 controls aged 61.5±8.3 years with normal renal function. Both the groups were divided into two subgroups with respect to MS. Individualized supervised rehabilitation program based on physiotherapy, including exercises, was provided to each subject for 4 weeks. Baseline and post-intervention complete blood count, glycated hemoglobin (HbA1c) and levels of serum RES, leptin, adiponectin, cystatin C, erythropoietin, high sensitivity C-reactive protein (hs-CRP), tumor necrosis factor alpha (TNF-α), interleukin-6, transforming growth factor- β1, plasminogen activator inhibitor-1 homocysteine, insulin, albumin, parathyroid hormone (PTH), and phosphorus were measured.
Results
Compared to controls, HD patients showed higher baseline leucocytes count and higher serum concentrations of RES, leptin, cystatin C, hs-CRP, TNF-α, homocysteine, phosphorus, PTH while hemoglobin, glucose, and albumin concentrations. A positive correlation between serum albumin and RES concentrations was observed in HD patients. Post-intervention RES increase was observed in HD patients without MS (post-intervention 34.22±8.89 vs baseline 30.16±11.04 ng/mL; P=0.046) while no change was observed in patients with MS and in the control group.
Conclusion
MS modifies a RES response to the rehabilitation program in HD patients.
Introduction
Discovered in 2001, resistin (RES) is a cysteine-rich protein of molecular weight 12.5 kDa and structurally similar to cytokines.Citation1,Citation2 RES is an adipokine, in mice it is mainly expressed by adipocytes. Its major sources in humans are monocytes and macrophages.Citation2–Citation4 In uremic patients, serum RES concentrations are elevated as compared to individuals with normal renal function.Citation5 It has not been concluded whether higher RES concentration is secondary to reduced renal clearance or reflects chronic inflammation.Citation6–Citation8 Hemodialysis does not eliminate RES as expected for its molecular size.Citation2 RES is an independent biomarker associated with all-cause and cardiovascular mortality in senile patients, hypertensive individuals, and at early stages of chronic kidney disease.Citation9,Citation10 The correlation between RES concentration and metabolic indicators in individuals with normal renal function remains unexplained.Citation11 Despite its name, the effect of this adipokine on the development of insulin resistance in humans has never been explicitly demonstrated.Citation12,Citation13 There have also been some contradictory reports on the relation of RES with obesity, insulin resistance, and type 2 diabetes.Citation4,Citation14–Citation16 Like uremia, obesity is a chronic inflammatory condition. Although low shares of human RES originate from fat cells, its concentration correlates with the body mass and responds to weight changes.Citation17,Citation18
In hemodialyzed (HD) patients, the serum RES concentration is increased, but it does not correlate with insulin resistance,Citation19 and hemodialysis improves insulin sensitivity and decreased insulin clearance.Citation20–Citation22 In healthy subjects, the association between RES and inflammatory markers has been confirmed.Citation23 Similarly, in HD patients, a relationship between RES and concentrations of high sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and the number of leucocytes has been observed.Citation24,Citation25
Regular physical exercise reduces mortality and morbidity of many diseases and demonstrates a cardio-protective effect.Citation26 However, what seems most important is the anti-inflammatory effect of regular physical exercise caused, among others, by the secretion of anti-inflammatory cytokines by the muscles.Citation26–Citation28 Exercise training intervention in individuals with normal renal function reduces RES, leptin, IL-6, and homocysteine (Hcy) concentrations especially in the overweight or obese population.Citation23
Despite numerous reports indicating the anti-inflammatory effect of physical exercise in chronic conditions,Citation26,Citation29,Citation30 including early stage of chronic kidney disease (CKD),Citation27 the relationship between regular exercise and inflammation indices in HD patients remains unclear.Citation31
The effect of enhanced physical activity on reduced RES concentrations in healthy individuals has been well documented.Citation32 However, no studies have been conducted to define the role of physical activity in RES concentrations in HD patients.
Metabolic syndrome (MS) is a recognized and proven risk factor for cardiovascular disease.Citation33 Uremia itself is a very strong cardiovascular risk factor, and one wonders whether the presence of MS adds to increasing the risk. It is possible that the MS interferes with physiologically observed responses to physical activity program. The phenomenon of reverse epidemiology occurs in uremic patients.Citation34,Citation35 Higher body mass index (BMI) correlates with lower all-cause and cardiovascular mortality.Citation33 We hypothesized that the response of HD patients to exercise training intervention may vary depending on the presence or absence of the MS.
The aim of the study was to investigate a metabolic and inflammatory response, with a special focus on RES, to the 4-week supervised rehabilitation program including individualized aerobic exercises in end-stage renal disease patients treated with hemodialysis, with or without MS.
Subjects and methods
This prospective interventional study comprised 28 consecutive patients of a dialysis center (study group HD), aged 56.9±13.3 years (; in the range from 25 to 75 years), 57% women, who were treated with hemodialysis for 50.6±73.4 months (in the range from 5 to 272 months) and fulfilled the criteria for participation and completed the program. These subjects were recruited at the dialysis center in Katowice, Poland. A control group (C) comprised 30 consecutive patients of the rehabilitation day ward, aged 61.5±8.3 years (
; in the range from 37 to 76 years), 50% women, who fulfilled the criteria for participation and completed the program. These subjects were recruited at the Department of Rehabilitation at University Hospital (No. 7 SUM, Upper Silesian Medical Center, Katowice, Poland; ).
Figure 1 Recruitment of patients to the study.
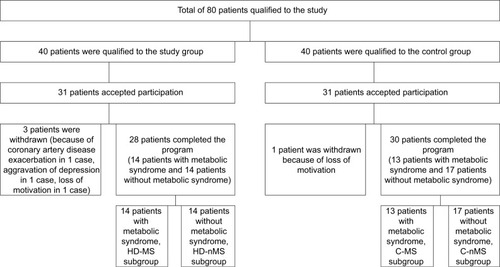
Written informed consent was obtained from each subject after the aim of the study, protocol, and risks were presented and explained.
Inclusion criteria involved: age >18 years, end-stage renal failure with the hemodialysis treatment duration >3 months (for the study group) or normal kidney function (assessed by eGFR, general urinalysis, 24-hours albumin excretion, and renal ultrasound results) – for the control group, capacity to perform low-level intensity exercise, and written informed consent for participation in the study.
Exclusion criteria included: a systemic rheumatic disease, cancer, conditions preventing/inability to perform low-level intensity exercise (New York Heart Association class IV heart failure, advanced mobility disorders), history of clinically overt infection during past 3 months, immunosuppressive therapy, and pregnancy ().
All subjects were further divided according to the MS. Diagnosis of MS was based on the presence of at least three out of five distinct, clinically relevant cardio-metabolic risk factors for the European population, that is, waist circumference, WC: female ≥80 cm, male ≥94 cm; fasting triglyceride concentration, TG ≥150 mg/dL or treatment of hypertriglyceridemia; fasting high-density lipoprotein cholesterol: female <50 mg/dL, male <40 mg/dL or use of concentration-increasing drugs; systolic blood pressure, sSBP ≥130 mmHg or diastolic blood pressure, DBP ≥85 mmHg or use of antihypertensive drugs; fasting serum glucose concentration ≥100 mg/dL or use of medication for hyperglycemia, according to the International Diabetes Federation, National Heart, Lung, and Blood Institute, and American Heart Association.Citation36
Fourteen patients of the study group met criteria of MS (HD-MS subgroup) while 14 others did not (HD-non-MS [nMS] subgroup). Also, 13 individuals of the control group met these criteria (C-MS subgroup) while 17 subjects did not (C-nMS subgroup).
The study was approved by the Bioethical Committee of the Medical University of Silesia in Katowice (resolution no. KNW/0022/KB1/44/II/06/14/17) and was carried out according to the Declaration of Helsinki regarding human studies.
Measurements
The patients were evaluated for their body weight, height, BMI and waist circumference, Charlson comorbidity index (CCI) and cardiopulmonary exercise testing (CPET). CPET is an exercise test with analysis of breathing gases. Ventilation and gas exchange were measured breath-by-breath with an automated metabolic measurement system (ZAN 680). The CPET was performed on a treadmill ergometer (TM425). Before each test, the device was calibrated with standard calibration gases. The heart rate, SBP, and DBP at rest, the end of each stage, peak exercise, and during recovery were assessed.
Fasting blood samples (at least 12 hours after the last meal) were taken between 6:00 and 7:00 am on the post-dialysis day before initiation of the program and on the day next to the completion of the of the 4-week physical activity program. Post-dialysis day refers to another day without hemodialysis.
Laboratory tests included blood hemoglobin concentration, leucocytes and lymphocytes count, and HbA1c percentage, as well as hs-CRP, albumin, glucose, insulin, creatinine, PTH, sodium, potassium, and phosphorous serum concentrations. Homeostasis Model Assessment (HOMA), according to the formula: fasting state glycaemia (mmol/L) × fasting insulin (µIU/mL)/22.5, was used to quantify insulin resistance. We derived eGFR from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation.Citation37
Kt/V (natural logarithm of the ratio of urea concentrations before and after dialysis) was used to quantify the adequacy of dialysis.
Serum samples were collected at −80°C for cytokine and hormonal tests. Serum concentrations of RES, adiponectin, cystatin C, erythropoietin, TNF-α, IL-6, transforming growth factor beta 1 (TGF-β1), and plasminogen activator inhibitor-1 (PAI-1) were assessed with the use of ELISA tests, leptin – radio-immuno-assay test and Hcy – with the use of the lumino-immuno-assay test.
Rehabilitation program
Initial rehabilitation assessment including anamnesis, physical and anthropometric examination, and functional capacity assessment was performed in each subject. Treadmill stress test according to the modified Bruce protocol with continuous gas analysis was used to assess ventilatory anaerobic threshold (VAT). The anaerobic threshold (VAT) was estimated using the V-slope method.Citation38 Cardiac rehabilitation exercise programs were used as the base of rehabilitation program. Individualized supervised rehabilitation program based on physiotherapy was provided to each subject for 4 weeks, 6 days a week, 2 hours a day. A regular physical activity program was also carried out on dialysis days. Hemodialysis sessions were conducted in the afternoon hours.
This program included 20–40 minute (25±10 minutes) of aerobic endurance training (AET) – walking, treadmill walking, forward and back walking, and cycling on stationary ergometers. Intensity of exercise in patients after CPET was about 10% below VAT. Every time, the intensity of exercise was closed to VAT. In patients without CPET (15.5%), the intensity of AET was closed between 40% and 60% of heart rate reserve. During all sessions of AET the rating of perceived exertion based on Borg 6–20 scale was below 13.Citation39 This exercise was complemented with isometric exercises, free active exercises, balance and coordination exercises, active breathing exercises, exercises in part or full unloading, manual therapy, medical massage, relaxation exercises, transcutaneous electrical nerve stimulation, laser therapy, ultrasound and light therapy, and magnetic field therapy. All participants accomplished at least 90% of the prescribed training sessions.
Statistics
Statistical analysis was carried out by using Statistica 13.1 (Stat Soft, Inc., Tulsa, OK, USA). Results are presented as mean and standard deviation or as medians. The Student’s paired t-test or the Wilcoxon test was used to compare the variables before and after rehabilitation program. The Student’s independent t-test was used to compare HD group and C group. Mann–Whitney U test was applied for non-parametric variables. Relationships between variables were determined with the Spearman rank correlation test. The level of significance was set at P<0.05.
Ethics statement
The results of scientific studies are anonymized and will be stored as such in an electronic form in a structured Statistica format on the premises of the facility conducting the study. The data will be stored for a period of 15 years. If requested, it can be made available in an anonymized form to the publisher within the scope of the presented work. Personal data related to this research are kept on the premises of the medical facility where the studies have been conducted. The data are subject to provisions on data protection in accordance with the law (Patients’ Rights and Patients’ Rights Ombudsman Article 26 section 4).
Results
Comparison of HD group and C group
The most prevalent causes of end-stage renal failure were: glomerulonephritis (25.1%), diabetic renal disease (21.4%), and hypertensive nephropathy (21.4%). The mean CCI in the HD and C groups were 4.5±3.6 and 1.03±0.61, respectively (P<0.001). The vascular access was through an arterio-venous (AV) fistula in 24 patients (85.6%) or through AV graft or dialysis catheter. Bicarbonate dialysis sessions lasted 4–4.5 hours and was performed 3–5 times a week using of high-flux dialysis machines. The dialysis fluid flow rate was 500 mL/min and blood flow 250–350 mL/min. Dialysis adequacy index (Kt/V) was 1.41±0.23. HD and C groups were demographically and anthropometrically comparable, showing no significant differences in age, sex, and BMI. HD group compared to C group showed significantly higher serum concentrations of creatinine, potassium RES, leptin, cystatin C, hs-CRP, TNF-α, Hcy, WBC, phosphorus, and PTH. Individuals with normal renal function had higher hemoglobin, glucose, and albumin concentration ().
Table 1 Baseline characteristics of the patients participating in the study (n=58)
Comparison of HD-MS subgroup and HD-nMS subgroup
HD-MS patients showed significantly higher serum leptin, PAI-1, and phosphorus compared to HD-nMS subgroup (40.23±31.99 vs 19.60±31.78 ng/mL; P=0.01 and 52.24±24.14 vs 34.63±18.01 ng/mL; P=0.04 and 5.55±1.00 vs 4.92±1.45 mg/dL; P=0.049, respectively). HD-MS subgroup to HD-nMS subgroup showed significantly higher HbA1c (6.19±1.55% vs 4.97%±0.80%; P=0.01). Other study parameters did not differ significantly between the subgroups with and without the MS ().
Table 2 Baseline comparison the biochemical markers in the study group (HD) and control group (C) with the metabolic syndrome (MS) and without the metabolic syndrome (nMS)
Comparison of C-MS subgroup and C-nMS subgroup
C-MS participants had higher leptin and TNF-α concentrations also number of leucocytes than the C-nMS subgroup (14.78±13.17 vs 8.10±9.22 ng/mL; P=0.04, 9.22±2.55 vs 6.70±2.17 pg/mL; P=0.007, 6.93±2.51 vs 5.31±1.23×10³/µL; P=0.03, respectively). C-MS subgroup compared to C-nMS subgroup showed significantly higher HbA1c (6.39±1.34% vs 5.37±0.75%; P=0.02). Other study parameters did not differ significantly between the subgroups with and without the MS ().
Comparison of HD group and C group with and without the MS
HD-MS subgroup, compared to C-MS subgroup, showed significantly higher serum concentrations of RES (36.26±14.47 vs 6.81±2.19 ng/mL; P<0.001), cystatin C (6,720±2,273 vs 2,032±1,034 ng/mL; P<0.001), hs-CRP (9.26±9.62 vs 1.50±1.39 mg/L; P<0.001), TNF-α (22.08±11.46 vs 9.22±2.55 pg/mL; P<0.001), Hcy (22.00±10.03 vs 13.64±2.55 µmol/L; P=0.001), phosphorus (5.55±1.00 vs 4.06±0.61 mg/dL; P<0.001), PTH (261.5±210.6 vs 52.14±21.50 pg/mL; P=0.001). Similar differences were demonstrated between HD-nMS patients and C-nMS subgroup, that is, elevated RES (30.16±11.04 vs 5.98±3.45 ng/ mL; P<0.001), cystatin C (6031±1,653 vs 1,604±581 ng/mL; P<0.001), hs-CRP (5.49±5.68 vs 0.87±0.82 mg/L; P<0.001), TNF-α (18.39±12.07 vs 6.70±2.17 pg/mL; P<0.001), Hcy (20.98±5.46 vs 12.55±3.95; P<0.001), phosphorus (4.92±1.45 vs 3.87±0.68 mg/dL; P<0.001), PTH (158.7±152.2 vs 57.92±28.40 pg/mL; P=0.01). Serum leptin concentration was significantly higher in HD-MS patients compared to C-MS subgroup (40.23±31.99 vs 14.78±13.17 ng/mL; P=0.01) while no significant differences were found between HD-nMS vs C-nMS. Total white cell and lymphocyte counts were significantly higher in HD-nMS patients compared to C-nMS subgroup (7.21±1.71 vs 5.31±1.23×10³/µL; P=0.002 and 1.80±0.48 vs 1.32±0.37×10³/µL; P=0.02, respectively). Irrespective of the presence or absence of the MS, serum albumin concentrations were lower in HD group compared to group C (41.40±5.12 vs 48.02±2.53 g/L; P<0.001 and 39.61±6.68 vs 47.55±3.70 g/L; P<0.001, respectively). Other study parameters did not differ significantly between dialyzed patients and individuals with normal renal function ().
Effect of rehabilitation program on the study parameters
As compared to pre-exercise levels, elevation of serum RES was observed in HD-nMS patients at the day next to the program completion (30.16±11.04 vs 34.22±8.89 ng/mL; P=0.046) while no change was observed in HD-MS patients. Serum RES remained unchanged in the C-nMS subgroup while non-significant reduction (6.81±2.19 vs 6.26±1.91 ng/ mL; P=0.07) in C-MS individuals was observed. Non-significant reduction of cystatin C concentrations (6,720±2,273 vs 5,780±2,054 ng/mL; P=0.098) and elevation of insulin concentrations (12.55±5.95 vs 18.10±8.88 µIU/mL; P=0.049) in HD-MS patients was observed ().
Table 3 Comparison of the biochemical markers before and after the rehabilitation program
Post-intervention changes in the concentrations of other cytokines and hormones in the HD and C groups did not reach the level of statistical significance.
RES correlations
Correlation between RES and albumin concentrations
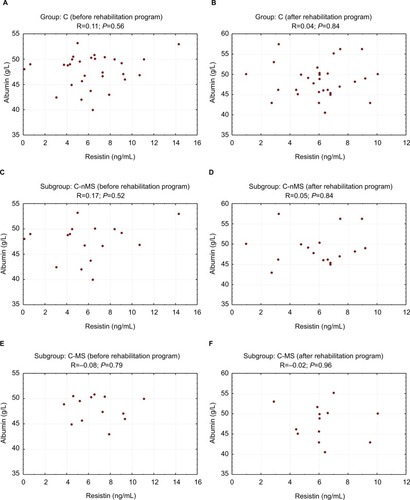
HD group showed correlation between serum albumin and RES concentrations. Pre-exercise correlation was statistically significant in HD-MS patients (R=0.58; P=0.03) and non-significant in HD-nMS patients (R=0.49; P=0.07). Following the 4-week program of regular physical exercise, the correlation in HD-MS patients was no longer statistically significant (R=0.26; P=0.37) while the correlation between RES and albumin became significant in HD-nMS patients (R=0.69; P=0.007). Group C demonstrated no correlation between serum albumin and RES concentrations, regardless of the presence or absence of the MS ( and ).
Figure 2 Correlation between resistin and albumin concentrations before and after the rehabilitation program (study group).
Abbreviations: HD, hemodialyzed; MS, metabolic syndrome; nMS, non-metabolic syndrome.
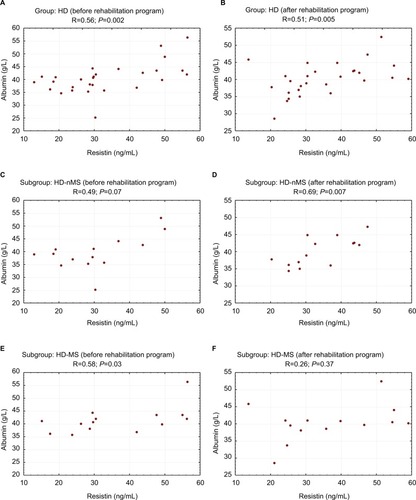
Figure 3 Correlation between resistin and albumin concentrations before and after the rehabilitation program (control group).
Abbreviations: C, control group; MS, metabolic syndrome; nMS, non-metabolic syndrome.
Correlation between RES and insulin concentrations and HOMA-IR
Before the program, HD-nMS patients showed a significant negative correlation between RES and insulin concentrations (R=−0.66; P=0.01) as well as between RES and HOMA-IR (R=−0.68; P=0.007). After the completion of the rehabilitation program, no significant correlations were noted between RES and insulin or HOMA-IR in HD-nMS patients ().
Table 4 Correlation between serum resistin and biochemical markers before and after the rehabilitation program
No significant pre- or post-rehabilitation program correlations were found in HD-MS patients and group C.
Other correlations
In HD-MS, the pre-rehabilitation program positive correlation between RES and leptin concentrations was non-significant (R=0.49; P=0.07) while the correlation between RES and IL-6 was significant (R=0.62; P=0.02). The same group also showed significant positive correlations between RES and the leukocyte (R=0.70; P=0.007) and lymphocyte (R=0.77; P=0.002) counts as well as between RES and phosphorous concentration (R=0.68; P=0.007) ().
Following the rehabilitation program, HD-MS patients still showed a positive and significant correlation between RES and IL-6 concentrations (R=0.61; P=0.02) and the leukocyte count (R=0.61; P=0.02) while correlation between RES and the lymphocyte count and phosphorous was no longer significant.
No significant correlations were observed between these parameters in HD-nMS.
Negative correlation between RES and PTH concentrations was observed in HD-MS patients before the exercise program (R=−0.65; P=0.01). No such correlation was revealed following the completion of the program.
HD-nMS patients showed negative significant correlation between RES and PAI-1 concentrations after physical exercise program (R=−0.55; P=0.04). No correlation between serum RES and these parameters was observed before and after physical exercise in HD-MS subgroup.
C-nMS participants showed a positive pre-rehabilitation program correlation between serum RES and the leukocyte count (R=0.54; P=0.02) as well as between RES and IL-6 (R=0.56; P=0.04). In the C-nMS subgroup, a positive correlation was observed between RES and TNF-α concentrations (R=0.51; P=0.04) post-rehabilitation program. Other correlations were not significant.
Discussion
This study is the first one to evaluate the influence of a supervised rehabilitation program on a range of serum hormones and cytokines including RES in HD patients with respect to the diagnosis of MS. Our results confirm previous observationsCitation2,Citation6,Citation7 that uremic patients have elevated concentrations of this adipokine. The MS had no significant impact on RES concentrations in individuals with normal renal function and those with renal failure. However, we found an unexpected RES increase in dialyzed patients without the MS following 4-week rehabilitation program, contrary to dialyzed patients with MS and to the control group. Apart from the fact that it needs confirmation in further studies and that it could be a result of a pre-analytical or laboratory error, a possible explanation of this observation would be expected. It has been demonstrated that contracting muscles increase the production of IL-6 and, in consequence, its concentration in the serum.Citation40,Citation41 On the other hand, it was observed that IL-6 can stimulate the secretion of RES.Citation42 In our study, we observed a non-significant IL-6 concentrations increase after physical exercise in HD-MS patients and HD-nMS patients and we also observed significant correlations between IL-6 and RES (only in patients with the MS). In patients without the MS, there is only a tendency for such correlation after physical exercise program. Therefore, it cannot be excluded that the observed RES increase immediately after completion of the rehabilitation program was associated with the influence of IL-6 on RES production in patients with diminished clearance of these cytokines which are poorly removed during hemodialysis. Unfortunately, we were unable to determine a delayed effect of the rehabilitation program on the RES concentration. RES elevation following the rehabilitation program in dialyzed patients may not be considered explicit unfavorable due to the phenomenon of reverse epidemiology in the dialyzed patients. A hypothesis suggesting a favorable change might be supported by a positive correlation between RES and albumin concentrations and negative correlation between concentrations of RES and plasminogen activator (PAI-1). Albumin concentration is an important index of nutritional status, a negative inflammatory marker, and one of the major negative predictors of mortality in dialyzed patients. Hence, it might suggest that changes in RES concentrations parallel to albumin concentration change reflect beneficial metabolic effect. However, this assumption is contrary to observations of Kaynar et alCitation43 who demonstrated positive correlation between the degree of protein-energy malnutrition and RES and adiponectin concentrations. On the other hand, a hypothesis suggesting beneficial effect of serum RES elevation in HD-nMS patients seems to be supported by a negative correlation between concentrations of this cytokine and PAI-1 which is a well-defined cardiovascular risk factor.Citation44 The correlations specified above allow no explicit definition of a cause–effect relationship between the study parameters as they merely indicate the existence of some relationship. A suggestion that RES elevation might have beneficial effects has been rejected by other authorsCitation32 who demonstrated a decrease in serum RES in individuals with normal renal function after exercise training intervention. The present study failed to confirm such relationship which might have been caused by a small study sample. The physiological and pathophysiological roles of RES remain unclear. Numerous authors have emphasized its association with the inflammatory condition.Citation45–Citation47 However, only few studies demonstrated a direct relationship between RES concentrations and clinical manifestations of inflammation. Some reports indicate that both high and low RES concentrations were predictors of frequent hospitalization. There are some reports indicating that low RES concentrations are associated with the need for more frequent hospitalization of HD patients. Thus, this relationship may not be linear but U-shaped.Citation48 Spoto et al observed that the effect of RES on cardiovascular risk was modified by adiponectin concentration.Citation49 Studies of the role of RES have also shown that old age and serum concentration of this adipokine exceeding 127.4 ng/mL were independent risk factors for death in HD patients.Citation50 Also some studies suggest a beneficial effect of RES in individuals with normal renal function. It was observed that serum RES concentrations were significantly lower in neonates with intrauterine infection compared to healthy neonates (17.8 vs 27 ng/mL). The differences did not depend on sex, type of delivery, or general condition of a neonate after birth.Citation51 Other studies showed a negative correlation between RES concentration and BNP and NT-proBNP concentrations.Citation52 Most probably, there are multiple mechanisms underlying RES synthesis and effects.
Our findings allow no explicit conclusions and should be considered with caution. We are unable to determine the clinical significance of the study. Further research with a larger research sample is needed. This is the first study on the impact of exercise training intervention on RES concentration in HD patients.
None of the previous publications reported significant reduction of proinflammatory cytokines, for example, CRP, IL-6, TNF-α, after physical activity programsCitation27,Citation31,Citation53 in HD patients. We also did not observe such a relationship although cystatin C reduction in HD-MS patients approached statistical significance. We believe this is the first one comprising an inpatient rehabilitation program, where exercise was taken daily under specialist supervision. Physical effort was meticulously controlled and all the participants completed 90%–100% of the individually tailored physical exercise program. So far, investigations of physical effort of HD patients concerned exercise taken twice or three times a week for 15–60 minutes during first 2 hours of hemodialysis session,Citation54–Citation57 ambulatory rehabilitation programs,Citation56 or exercises done by the patient at home.Citation57–Citation60 Observation period usually wasted 1 to 24 monthsCitation54,Citation57,Citation61,Citation62 or longer.Citation63,Citation64 Previous studies of the effect of physical effort in HD patients focused on effort tolerance, ability to walk, muscular strength, quality of life, quality of sleep, and the restless feet syndrome.Citation65–Citation67 Only few contributions addressed the question of the effect of physical activity on cardiovascular riskCitation47,Citation68,Citation69 and dialysis efficiencyCitation70 in HD patients. Compared to individuals with normal renal function, our study showed RES elevation as well as higher concentrations of cystatin C, hs-CRP, TNF-α, homocystein, and PTH. These results are supported by other authors, suggesting that elevated RES, cystatin C, hs-CRP, and TNF-α concentrations in HD patients are most probably the consequence of inflammatory condition and reduced elimination of cytokines due to renal failure.Citation71 The MS was associated with significantly higher leptin concentrations. In HD patients with the MS, higher leptin concentrations were associated with impaired renal function. In HD patients without the MS, renal function did not seem to have a marked effect on leptin concentrations.
Another unexpected observation was an increase of insulin concentration (12.55±5.95 vs 18.10±8.88 µIU/mL; P=0.049) after the rehabilitation program in HD patients with MS. Initially insulin concentrations were higher in control group compared to dialyzed patients. Therefore, exercise training intervention could increase synthesis and release of insulin. Also, one might speculate that higher insulin levels was due to decreased insulin clearance in end stage renal disease.Citation72 On the other hand, it is knownCitation73 that excessive physical exercise may lead to counter-regulatory hyperglycemia. It is possible that despite being careful, the exercises applied in HD patients with the MS were too strenuous for them. In the present study, higher RES concentrations were not associated with insulin resistance. A negative correlation between RES and insulin concentrations as well as HOMA-IR index in the subgroup of HD patients without the MS prior to the rehabilitation program was observed. However, results of other studies do not allow unequivocal definition of the relationship between RES and insulin resistance. No correlation between RES concentrations and insulin resistance was observed in non-obese patients on peritoneal dialysis.Citation74 On the other hand, in an extensive Finnish cross-sectional health examination survey, RES turned out to be an independent predictor of the MS and correlated with insulin resistance.Citation75 The MS in our HD patients and individuals with normal renal function was associated with higher leptin concentrations. Other authors confirmed this observation.Citation45 PAI-1 concentration was higher in HD patients with the MS compared to HD patients without the MS, while individuals with normal renal function and the MS showed a significant increase in TNF-α concentrations compared to individuals with normal renal function without the MS.
Strength and limitations
There are several limitations of this study. First, it comprised a relatively small number of participants what was a result of a complex method. Second, the observation period was relatively short. However, the authors’ focus was to evaluate the effect of daily physical exercise performed by inpatient under close supervision. The third limitation stems from the fact that our study only comprised patients of a single dialysis center, which however facilitated the elimination of confounding factors associated with different standards and procedures.
Strength of our study was the daily scheme of individually tailored exercises, in inpatient conditions ensuring permanent supervision by an interdisciplinary team that resulted in very high compliance. So far, such a scheme has never been undertaken in HD patients. Another advantage was that all measurements and laboratory tests were performed using the same methods and by the same research team.
The added value of this study was an implementation of the regular physical activity habit in our patients.
Conclusion
A metabolic and inflammatory response for the 4-week supervised rehabilitation program is changed in end-stage renal disease HD patients as compared to controls with normal renal function. MS modifies a RES response to the rehabilitation program in HD patients. An increase of RES concentration in HD patients without the MS immediately after completion of the rehabilitation program needs further exploration.
Acknowledgments
We wish to acknowledge and thank all physiotherapists, physicians, nurses, and participants for their assistance and contribution to this study. This project was supported by National Science Center, Poland (Project NN 404273740) and by the Medical University of Silesia in Katowice grant to statutory work (contract KNW-1-121/N/7/Z).
Disclosure
The funding body played no role in the formulation of the design, methods, subject recruitment, data collection, analysis, or preparation of this paper. The results presented in this paper have not been published previously in whole or part. The authors report no conflicts of interest in this work.
References
- Kim KH Lee K Moon YS Sul HS A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation J Biol Chem 2001 276 14 11252 11256 11278254
- Karbowska A Boratyńska M Resistin KM A pathogenic factor or a biomarker of metabolic disorders and inflammation? Postepy Hig Med Dosw 2009 63 485 491
- Pang SS Le YY Yy L Role of resistin in inflammation and inflammation-related diseases Cell Mol Immunol 2006 3 1 29 34 16549046
- Menzaghi C Bacci S Salvemini L Serum resistin, cardiovascular disease and all-cause mortality in patients with type 2 diabetes PLoS One 2014 8 6 e64729 23755138
- Dan S Aditya P Banerjee P Bal C Roy H Banerjee I Effect of chronic kidney disease on serum resistin level Niger J Clin Pract 2014 17 6 735 738 25385911
- Malyszko J Malyszko JS Kozminski P Pawlak K Mysliwiec M Elevated resistin is related to inflammation and residual renal function in haemodialysed patients Nephrology 2007 12 3 246 253 17498119
- Kawamura R Doi Y Osawa H Circulating resistin is increased with decreasing renal function in a general Japanese population: the Hisayama Study Nephrol Dial Transplant 2010 25 10 3236 3240 20339098
- Moreno LO Salvemini L Mendonca C Serum resistin and glomerular filtration rate in patients with type 2 diabetes PLoS One 2015 10 3 e0119529 25811174
- Marouga A Dalamaga M Kastania AN Circulating resistin is a significant predictor of mortality independently from cardiovascular comorbidities in elderly, non-diabetic subjects with chronic kidney disease Biomarkers 2016 21 1 73 79 26667298
- Fontana A Spadaro S Copetti M Association between resistin levels and all-cause and cardiovascular mortality: a new study and a systematic review and meta-analysis PLoS One 2015 10 3 e0120419 25793385
- Bajnok L Seres I Varga Z Relationship of serum resistin level to traits of metabolic syndrome and serum paraoxonase 1 activity in a population with a broad range of body mass index Exp Clin Endocrinol Diabetes 2008 116 10 592 599 18465683
- Bo S Gambino R Pagani A Relationships between human serum resistin, inflammatory markers and insulin resistance Int J Obes 2005 29 11 1315 1320
- Janowska J Zahorska-Markiewicz B Olszanecka-Glinianowicz M Relationship between serum resistin concentration and proinflammatory cytokines in obese women with impaired and normal glucose tolerance Metabolism 2006 55 11 1495 1499 17046552
- Lim S Koo BK Cho SW Association of adiponectin and resistin with cardiovascular events in Korean patients with type 2 diabetes: the Korean atherosclerosis study (KAS): a 42-month prospective study Atherosclerosis 2008 196 1 398 404 17178123
- Niu XH Li L Li JY Song Q Jin MM Liu JX Serum resistin positively correlates with serum lipids, but not with insulin resistance, in first-degree relatives of type-2 diabetes patients: an observational study in China Medicine 2017 96 16 e6622 28422857
- Singh AK Tiwari S Gupta A Association of resistin with insulin resistance and factors of metabolic syndrome in north Indians Indian J Clin Biochem 2015 30 3 255 262 26089609
- Schwartz DR Lazar MA Human Resistin: Found in Translation From Mouse to Man. Trends in endocrinology and metabolism Trends Endocrinol Metab 2011 22 7 259 265 21497511
- Montazerifar F Bolouri A Paghalea RS Mahani MK Karajibani M Obesity, serum resistin and leptin levels linked to coronary artery disease Arq Bras Cardiol 2016 107 4 348 353 27627223
- Axelsson J Bergsten A Qureshi AR Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance Kidney Int 2006 69 3 596 604 16395259
- Kobayashi S Maejima S Ikeda T Nagase M Impact of dialysis therapy on insulin resistance in end-stage renal disease: comparison of haemodialysis and continuous ambulatory peritoneal dialysis Nephrol Dial Transplant 2000 15 1 65 70
- Sudha MJ Salam HS Viveka S Udupa AL Assessment of changes in insulin requirement in patients of type 2 diabetes mellitus on maintenance hemodialysis J Nat Sci Biol Med 2017 8 1 64 68 28250677
- Nashar K Egan BM Relationship between chronic kidney disease and metabolic syndrome: current perspectives Diabetes Metab Syndr Obes 2014 7 7 421 435 25258547
- Gondim OS de Camargo VT Gutierrez FA Benefits of regular exercise on inflammatory and cardiovascular risk markers in normal weight, overweight and obese adults PLoS One 2015 10 10 e0140596 26474157
- Cohen G Ilic D Raupachova J Hörl WH Resistin inhibits essential functions of polymorphonuclear leukocytes J Immunol 2008 181 6 3761 3768 18768828
- O’Sullivan TF Smith AC Watson EL Satellite cell function, intramuscular inflammation and exercise in chronic kidney disease Clin Kidney J 2018 72
- Gleeson M Bishop NC Stensel DJ Lindley MR Mastana SS Nimmo MA The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease Nat Rev Immunol 2011 11 9 607 615 21818123
- Viana JL Kosmadakis GC Watson EL Evidence for anti-inflammatory effects of exercise in CKD J Am Soc Nephrol 2014 25 9 2121 2130 24700875
- Dungey M Hull KL Smith AC Burton JO Bishop NC Inflammatory factors and exercise in chronic kidney disease Int J Endocrinol 2013 2013 2 1 12
- Pedersen BK Saltin B Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases Scand J Med Sci Sports 2015 25 Suppl 3 1 72
- Beavers KM Brinkley TE Nicklas BJ Effect of exercise training on chronic inflammation Clin Chim Acta 2010 411 11–12 785 793 20188719
- Dungey M Young HML Churchward DR Burton JO Smith AC Bishop NC Regular exercise during haemodialysis promotes an anti-inflammatory leucocyte profile Clin Kidney J 2017 10 6 813 821 29225811
- Marcelino-Rodríguez I Almeida Gonzalez D Alemán-Sánchez JJ Inverse association of resistin with physical activity in the general population PLoS One 2017 12 8 e0182493 28771611
- Małgorzewicz S Aleksandrowicz-Wrona E Owczarzak A Debska-Slizień A Rutkowski B Łysiak-Szydłowska W Adipokines and nutritional status for patients on maintenance hemodialysis J Ren Nutr 2010 20 5 303 308 20071195
- Kalantar-Zadeh K Abbott KC Salahudeen AK Kilpatrick RD Horwich TB Survival advantages of obesity in dialysis patients Am J Clin Nutr 2005 81 3 543 554 15755821
- Kalantar-Zadeh K Kilpatrick RD Mcallister CJ Greenland S Kopple JD Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions Hypertension 2005 45 4 811 817 15699452
- Alberti KG Eckel RH Grundy SM Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity Circulation 2009 120 16 1640 1645 19805654
- Levey AS Stevens LA Schmid CH A new equation to estimate glomerular filtration rate Ann Intern Med 2009 150 9 604 612 19414839
- Albouaini K Egred M Alahmar A Wright DJ Cardiopulmonary exercise testing and its application Heart 2007 93 10 1285 1292 17890705
- Borg GA Psychophysical bases of perceived exertion Med Sci Sports Exerc 1982 14 5 377 381 7154893
- Febbraio MA Hiscock N Sacchetti M Fischer CP Pedersen BK Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction Diabetes 2004 53 7 1643 1648 15220185
- Ihalainen J Walker S Paulsen G Acute leukocyte, cytokine and adipocytokine responses to maximal and hypertrophic resistance exercise bouts Eur J Appl Physiol 2014 114 12 2607 2616 25145982
- Bokarewa M Nagaev I Dahlberg L Smith U Tarkowski A Resistin, an adipokine with potent proinflammatory properties J Immunol 2005 174 9 5789 5795 15843582
- Kaynar K Kural BV Ulusoy S Is there any interaction of resistin and adiponectin levels with protein-energy wasting among patients with chronic kidney disease Hemodial Int 2014 18 1 153 162 23919731
- Arikan H Koc M Tuglular S Ozener C Akoglu E Elevated plasma levels of PAI-1 predict cardiovascular events and cardiovascular mortality in prevalent peritoneal dialysis patients Ren Fail 2009 31 6 438 445 19839820
- Saluk J Bansal V Hoppensteadt D Syed D Abro S Fareed J Prevalence of metabolic syndrome in patients with end stage renal disease and relevance of biomarkers Int Angiol 2016 35 1 47 56 25476033
- Sweigert PJ Bansal VK Hoppensteadt DA Saluk JL Syed DA Fareed J Inflammatory and metabolic syndrome biomarker analysis of vascular outcomes in end-stage renal disease Int J Angiol 2017 26 1 043 048
- Lehrke M Reilly MP Millington SC Iqbal N Rader DJ Lazar MA An inflammatory cascade leading to hyperresistinemia in humans PLoS Med 2004 1 2 e45 15578112
- Chung W Jung ES Shin D Low resistin level is associated with poor hospitalization-free survival in hemodialysis patients J Korean Med Sci 2012 27 4 377 381 22468100
- Spoto B Mattace-Raso F Sijbrands E Resistin and all-cause and cardiovascular mortality: effect modification by adiponectin in end-stage kidney disease patients Nephrol Dial Transplant 2013 28 Suppl 4 iv181 iv187 23975745
- Chi PJ Liou HH Hsu BG Tasi JP Relationship between resistin and mortality in maintenance hemodialysis patients Clin Nephrol 2016 86 9 125 131 27389928
- Stojewska M Wnęko-Masłoń B Nawrat A Serum resistin concentration in full-term newborns with intrautherine infections. Stężenie rezystyny w surowicy noworodków donoszonych, z zakażeniami wewnątrzmacicznymi Polish Journal of Paediatrics 2016 91 325 331
- Liu W Jiang L Chen J Association of adipokines with blood pressure, arterial elasticity and cardiac markers in dialysis patients: cross-sectional analysis of baseline data from a cohort study Nutr Metab 2017 14 34
- Gołębiowski T Kusztal M Weyde W A program of physical rehabilitation during hemodialysis sessions improves the fitness of dialysis patients Kidney Blood Press Res 2012 35 4 290 296 22377500
- Giannaki CD Hadjigeorgiou GM Karatzaferi C A single-blind randomized controlled trial to evaluate the effect of 6 months of progressive aerobic exercise training in patients with uraemic restless legs syndrome Nephrol Dial Transplant 2013 28 11 2834 2840 23929523
- Liao MT Liu WC Lin FH Intradialytic aerobic cycling exercise alleviates inflammation and improves endothelial progenitor cell count and bone density in hemodialysis patients Medicine 2016 95 27 e4134 27399127
- Young HML March DS Graham-Brown MPM Effects of intra-dialytic cycling exercise on exercise capacity, quality of life, physical function and cardiovascular measures in adult haemodialysis patients: a systematic review and meta-analysis Nephrol Dial Transplant 2018 33 8 1436 1445 29608708
- Bohm C Stewart K Onyskie-Marcus J Esliger D Kriellaars D Rigatto C Effects of intradialytic cycling compared with pedometry on physical function in chronic outpatient hemodialysis: a prospective randomized trial Nephrol Dial Transplant 2014 29 10 1947 1955 25061127
- Manfredini F Mallamaci F D’Arrigo G Exercise in patients on dialysis: a multicenter, randomized clinical trial J Am Soc Nephrol 2017 28 4 1259 1268 27909047
- Tao X Chow SK Wong FK A nurse-led case management program on home exercise training for hemodialysis patients: a randomized controlled trial Int J Nurs Stud 2015 52 6 1029 1041 25840898
- Matsuzawa R Hoshi K Yoneki K Exercise training in elderly people undergoing hemodialysis: a systematic review and meta-analysis Kidney Int Rep 2017 2 6 1096 1110 29270518
- Konstantinidou E Koukouvou G Kouidi E Deligiannis A Tourkantonis A Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs J Rehabil Med 2002 34 1 40 45 11900261
- Chen JL Godfrey S Ng TT Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial Nephrol Dial Transplant 2010 25 6 1936 1943 20100734
- Anding K Bär T Trojniak-Hennig J A structured exercise programme during haemodialysis for patients with chronic kidney disease: clinical benefit and long-term adherence BMJ Open 2015 5 8 e008709
- Kouidi E Grekas D Deligiannis A Tourkantonis A Outcomes of long-term exercise training in dialysis patients: comparison of two training programs Clin Nephrol 2004 61 Suppl 1 31 38
- Esteve Simo V Junqué Jiménez A Moreno Guzmán F Benefits of a low intensity exercise programme during haemodialysis sessions in elderly patients Nefrologia 2015 35 4 385 394 26306966
- Heiwe S Jacobson SH Exercise training in adults with CKD: a systematic review and meta-analysis Am J Kidney Dis 2014 64 3 383 393 24913219
- Bennett PN Fraser S Barnard R Effects of an intradialytic resistance training programme on physical function: a prospective stepped-wedge randomized controlled trial Nephrol Dial Transplant 2016 31 8 1302 1309 26715763
- Miller BW Cress CL Johnson ME Nichols DH Schnitzler MA Exercise during hemodialysis decreases the use of antihypertensive medications Am J Kidney Dis 2002 39 4 828 833 11920350
- Frih B Jaafar H Mkacher W Ben Salah Z Hammami M Frih A The effect of interdialytic combined resistance and aerobic exercise training on health related outcomes in chronic hemodialysis patients: the Tunisian randomized controlled study Front Physiol 2017 8 288 28620308
- Kirkman DL Roberts LD Kelm M Wagner J Jibani MM Macdonald JH Interaction between intradialytic exercise and hemodialysis adequacy Am J Nephrol 2013 38 6 475 482 24296748
- Akagun T Caliskan Y Yazici H Elevated resistin levels are associated with inflammation in hemodialysis patients with failed renal allografts Int J Artif Organs 2014 37 5 358 363 24811303
- Guthoff M Wagner R Vosseler D Impact of end-stage renal disease on glucose metabolism-a matched cohort analysis Nephrol Dial Transplant 2017 32 4 670 676 28407130
- Kjaer M Hollenbeck CB Frey-Hewitt B Galbo H Haskell W Reaven GM Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes J Appl Physiol 1990 68 5 2067 2074 2193907
- Yoo DE Lee MJ Oh HJ Low circulating adiponectin levels are associated with insulin resistance in non-obese peritoneal dialysis patients Endocr J 2012 59 8 685 695 22673293
- Malo E Ukkola O Jokela M Resistin is an indicator of the metabolic syndrome according to five different definitions in the Finnish Health 2000 survey Metab Syndr Relat Disord 2011 9 3 203 210 21332410
