Abstract
Background
Metabolic syndrome (MS) is known to be associated with hypertension, insulin resistance, and dyslipidemia, and it raises the risk for cardiovascular diseases and diabetes mellitus. Telmisartan is used in clinic as an angiotensin II receptor blocker and it is also identified as activating peroxisome proliferator-activated receptors δ (PPARδ). Activation of PPARδ produced beneficial effects on fatty acid metabolism and glucose metabolism. This study aims to investigate the effects of telmisartan on the modulation of MS in rats fed a high-fat/high-sodium diet.
Methods
Rats were fed with a high-fat/high-sodium diet and received injections of streptozotocin at low dose to induce MS. Then, rats with MS were treated with telmisartan. The weight, glucose tolerance, and insulin sensitivity were measured. The lipid profiles were also obtained. The weights of retroperitoneal and epididymal fat pads were determined. The role of PPARδ in telmisartan treatment was identified in rats pretreated with the specific antagonist GSK0660.
Results
The results showed that telmisartan, but not losartan, significantly reduced plasma glucose and plasma insulin, and improved insulin resistance in rats with MS. Telmisartan also decreased blood pressure and lipids more significantly than losartan. Moreover, GSK0660 effectively reversed the effects of telmisartan in the MS rats. In the MS group, telmisartan activated PPARδ to enhance the levels of phosphorylated GLUT4 in muscle or the expression of phosphoenolpyruvate carboxykinase (PEPCK) in the liver, which was also abolished by GSK0660. Telmisartan is useful to ameliorate hypertension and insulin resistance in rats with MS. Telmisartan improves the insulin resistance through increased expression of GLUT4 and down-regulation of PEPCK via PPARδ-dependent mechanisms.
Conclusion
Telmisartan has been proven to ameliorate MS, particularly in the prediabetes state. Therefore, telmisartan is suitable to develop for the management of MS in clinics.
Introduction
Metabolic syndrome (MS) is a cluster of risk factors for metabolic abnormalities and cardiovascular disease. It includes abdominal obesity, dyslipidemia, hypertension, and hyperglycemia.Citation1 Prevalence of MS is rapidly increasing worldwide.Citation2 Approximately 31 % of the world’s adult population is estimated to have MS.Citation3 Moreover, MS is associated with a 2.5-fold increase in cardiovascular- and diabetes-related mortalities.Citation4
Diet is a potential factor that could be responsible for the rise in MS and the associated cardiovascular pathologies.Citation5 The diet pattern in Western countries is generally characterized by high intake of carbohydrates and saturated fat. The increase in calorific intake has been associated with many diet-induced complications, including MS, cardiovascular diseases, and nonalcoholic fatty liver disease. High dietary fat intake is associated with oxidative stress and an activation of the proinflammatory transcription factors.Citation6
High salt intake is also a significant environmental factor and is strongly associated with high blood pressure (BP). It has been previously indicated that essential hypertension is frequently related to insulin resistance and compensatory hyperinsulinemia.Citation7 MS patients also exhibit enhanced BP in response to sodium intake.Citation8 Insulin resistance could activate the renin-angiotensin system (RAS) by increasing the expression of angiotensinogen, angiotensin II (AT2), and angiotensin receptor (AR), which may contribute to the development of hypertension.Citation9 It has been recently discovered that adipocytes also produce aldosterone in response to AT2.Citation10
The peroxisome proliferator-activated receptor δ (PPARδ) is a transcription factor that belongs to the super-family of nuclear receptors.Citation11 Activation of PPARδ has beneficial effects on fatty acid and glucose metabolism.Citation12 Moreover, PPARδ could enhance fatty acid β-oxidation and attenuate MS.Citation13 PPARδ activation could prevent obesity and exert protective effects on hypertension and on the early manifestations of atherosclerosis in high-fat (HF) diet-fed mice.Citation14
Telmisartan, an AR blocker (ARB), has the highest affinity for AT2 receptors among the available ARBs.Citation15 Telmisartan has a profound role in the improvement of glucose homeostasis in skeletal muscle, which is associated with activation of PPARδ.Citation16 Several studies have revealed that telmisartan improves insulin sensitivity in patients with hypertension or the early stages of diabetes mellitus.Citation17,Citation18
Many animal models were used to study disorders of MSCitation19 in a manner to mimic the major signs of MS. In the induction of animal models, various approaches were applied in rodents including dietary manipulation, genetic modification, and drugs.Citation19 However, limitations of each model were observed. Dietary approaches included the use of a single type of diet or a combination of diets, such as high-fructose,Citation20 HF,Citation21 high-fructose/HF,Citation22 which usually affects the whole-body metabolism, but the effects is limitedCitation23 and the symptom did not include hypertension. The genetic models of MS included leptin-deficient (ob/ob) or leptin receptor-deficient (db/db) mice. Unlike humans with MS, ob/ob mice did not develop dyslipidaemia,Citation24 and both ob/ob and db/db mice did not show hypertension.Citation25,Citation26 Although HF fed, spontaneously hypertensive rats show some symptoms of MS, they have genetically induced, rather than diet-induced, hypertension.Citation27 Models of drug-induced MS include glucocorticoid- inducedCitation28 and antipsychotic-inducedCitation29 models, which seem more suitable for the research of specific diseases. In this study, we established a MS model based on environmental effects, which promoted blood glucose, blood pressure, and blood fat using the HF, high-sodium (HS) diet intake and a low-dose of streptozotocin (STZ) injection. The main aim of this study was to investigate the effects of telmisartan on insulin resistance, hyperlipidemia, and hypertension in rats with MS.
Methods
Animals
Male Sprague Dawley rats weighing 180–220 g were obtained from the National Animal Center (Taipei, Taiwan) and maintained in the animal center of Chi Mei Medical Center (Tainan, Taiwan). The animals were housed two rats per cage on a 12/12-hour light/dark cycle at a constant temperature (24 °C±1 °C) and humidity (60%±10%). This project was approved by the Institutional Animal Care and Use Committee of Chi Mei Medical Center (No. 105110330). The Guide for the Care was referred to during this study.
Rat model with MS
The rats were randomly fed either standard rat chow (13.43% kcal as fat; TestDiet®; Richmond, IN, USA) or HF/HS diet for 8 weeks. Custom HF diets (60% kcal as fat; LabDiet®; St Louis, MO, USA) were applied to prepare a HF/HS (4% NaCl) diet.Citation30 All rats freely received normal tap water.
After an 8-week feeding of a HF/HS diet, rats were starved for 12 hours then injected intraperitoneally with STZ at low dose (30 mg/kg)Citation31 and were continued to be fed the same diet during the experiments. After 7 days of STZ injections, the rats had hyperglycemia (>200 mg/dL), hyperlipidemia (total cholesterol [TC] >110 mg/dL and triglyceride [TG] >150 mg/dL), an increase in body weight (8% of initial weight) or mean arterial BP >130 mmHg, and a marked decrease in high-density lipoprotein (HDL) cholesterol (<35 mg/dL) that were used to confirm the development of MS.Citation32 Rats with MS were allowed the HF/HS diet until the end of the study.
Treatment protocols
Once MS occurred, models and controls were treated by oral gavage with telmisartan (8 mg/kg/day; Boehringer Ingelheim, Ingelheim am Rhein, Germany)Citation10 or losartan (8 mg/kg/day; Zydus Pharmaceuticals, Pennington, NJ, USA) for 4 weeks. Moreover, PPARδ antagonist GSK0660 (10 mg/kg; Sigma-Aldrich Co., St Louis, MO, USA) was intraperitoneally injected 30 minutes before telmisartan administration.
Food and water intake were measured daily. Body weight was monitored weekly. BP was determined at week 9 (before drug treatment) and week 13 (the end of 4-week periods of the drug treatment) using the tail-cuff method with a sphygmomanometer (Muromachi Kikai Co., Ltd., Tokyo, Japan).Citation33
At week 14, insulin tolerance tests (ITTs) were performed in the rats fasting overnight. According to a previous report,Citation34 rats were intraperitoneally injected with 0.75 IU/kg of regular insulin. Blood was collected from the tail vein of rats under anesthesia before injection and after 15, 30, 60, 90, and 120 minutes.
At the end of the study, livers and soleus muscles were collected from the sacrificed rats. The weight of retroperitoneal and epididymal fat pads were also measured. All samples were immediately frozen in liquid nitrogen and kept at –80°C for further assays.
Biochemical measurements
Blood samples were collected from the tail vein of rats that were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and all efforts were made to minimize the animals’ suffering. Blood glucose concentration was measuredCitation35 using commercial kits (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Serum insulin concentrations were also measured using commercialized enzyme-linked immunosorbent assay kits (Mercodia AB, Uppsala, Sweden). The following formula was used to calculate the homeostasis model assessment for insulin resistance (HOMA-IR): (fasting insulin [μU/mL] × fasting glucose [mg/dL])/405. Additionally, the area under the curve was evaluated for the glucose concentrations determined at 0, 30, 60, 90, and 120 minutes. The lipid profile, including concentrations of TC, TG, and HDL, was estimated using laboratory kit reagents (Randox Laboratories, Crumlin, UK). The low-density lipoprotein (LDL) levels were then calculated using Frieldwann’s equation.
Western blotting analysis
The Western blotting analysis was was performed according to the previous method.Citation52 Total protein lysates were extracted in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris [pH 7.5], and 5 mM ethylenediaminetetraacetic acid), containing a protease and phosphatase inhibitor cocktail (Sigma-Aldrich Co.). The protein concentration was determined with the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The following primary antibodies were used at 4°C overnight: anti-PPARδ (1:1000) and anti-GLUT4 (1:1000) (Abcam, Cambridge, UK); and anti-phosphoenolpyruvate carboxykinase (anti-PEPCK) (1:1000) (EMD Millipore, Billerica, MA, USA) was used as an internal control. The next day, the blots were incubated in the secondary antibodies at room temperature for 1 hour. Protein bands were visualized using the enhanced chemiluminescence kit (PerkinElmer Inc., Boston, MA, USA). The optical densities of the bands were determined using software (Gel-Pro® Analyzer Version 4.0 software; Media Cybernetics Inc., Rockville, MD, USA).
Statistical analyses
All results are provided as mean ± SEM of each group. All statistical analyses were carried out by SPSS, Version 21 (IBM Corporation, Armonk, NY, USA). Differences between the two groups were determined by two-way repeated measures ANOVA. For comparisons between two independent groups, a Student’s t-test was used. Significance was accepted at p<0.05.
Results
Telmisartan ameliorated hyperglycemia and insulin resistance in MS rats
Rats fed the HF/HS diet for 8 weeks followed by an injection of STZ (30 mg/kg) developed MS because they became hyperphagic, obese, hyperlipidemia, insulin resistant, and hypertensive. The MS group displayed moderate glucose intolerance characterized by a 2.0-fold increase in fasting serum glucose and 2.2-fold increase in HOMA-IR index. In ITT experiments, the MS group showed higher glucose levels in serum that were improved by telmisartan treatment compared with the normal control group.
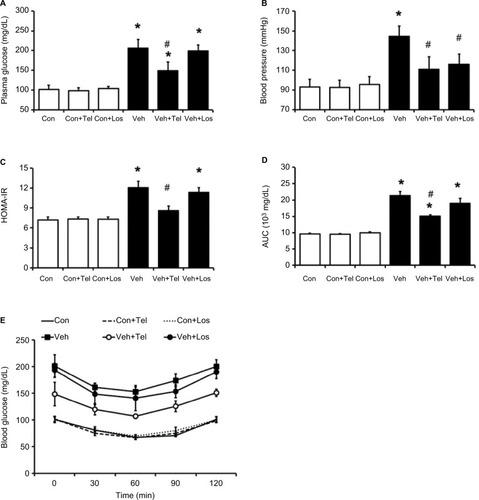
Blood glucose was significantly reduced in MS rats treated with telmisartan compared with the vehicle-treated MS group (−28%, P<0.05) (). Similarly, the HOMA-IR was significantly attenuated in the telmisartan-treated group (–29%, P<0.05). However, losartan did not affect the glucose levels in MS rats ().
Figure 1 Effects of telmisartan or losartan on MS rats.
Abbreviations: AUC, area under the curve; Con, control; HOMA-IR, homeostatic model assessment for insulin resistance; ITT, insulin tolerance test; Los, losartan; MS, metabolic syndrome; SD, Sprague Dawley; Veh, vehicle; Tel, telmisartan.
Effects of telmisartan on body weight, food intake and white adipose mass in MS rats
The MS group showed a 14% increase in body weight compared with the control group. Also, the MS group significantly increased retroperitoneal fat mass and epididymal fat mass in a different way to the control group (). In addition, the average daily food intake and water intake were elevated in MS groups more markedly than in the control group ().
Table 1 Effects of telmisartan (Tel) or losartan (Los) on the metabolic parameters in normal rat and MS rats
Table 2 Effects of telmisartan or losartan on food intake and water intake in normal rats and MS rats
Telmisartan significantly reduced the rise in body weight of MS rats compared with the vehicle-treated MS rats. Retro-peritoneal fat mass and epididymal fat mass were also noted to be reduced by telmisartan in the MS group at the end of experiments. However, a similar change was not observed in MS rats administered with losartan. Additionally, telmisartan or losartan did not influence body weight or fat mass in rats that received normal chow diet ().
Telmisartan improved lipid metabolism in MS rats
Lipids in normal control or MS rats treated with telmisartan or losartan shown in . Significant increases in TC, TG, HDL, and LDL cholesterol concentrations were observed in MS rats.
Telmisartan attenuated the plasma TC, TG and LDL levels significantly and increased the HDL levels. However, losartan produced a little improvement in the lipids in MS rats compared with telmisartan-treated MS rats.
Effects of telmisartan and losartan on BP
Mean arterial BP was significantly increased in MS rats and mild hypertension was observed compared with the control group (144.3±10.7 vs 92.9±7.9 mmHg, P<0.05). A significant reduction in BP was observed in MS rats treated with telmisartan or losartan (telmisartan: 110±12.7 mmHg and losartan: 115.8±10.6 mmHg) compared with those not treated (144.3±10.7 mmHg, P<0.05) ().
PPARδ antagonist inhibited the effects of telmisartan in MS rats
To investigate the role of PPARδ in the effects of telmisartan in MS rats, the selective PPARδ antagonist GSK0660 was pretreated with telmisartan.
GSK0660 significantly reduced the beneficial effects of telmisartan in blood glucose (from 153.0±17.1 mg/dL to 185.6±14.2 mg/dL, P<0.05) (). Additionally, TG and TC levels in the telmisartan-treated MS group were reversed markedly (). Moreover, pretreatment with GSK0660 reversed the reduction of HOMA-IR index and the improvement of ITT, which were induced by telmisartan in MS rats (P<0.05) (). In addition, the BP-lowering effect of telmisartan was also inhibited by GSK0660; GSK0660 reversed BP from 137±1 mmHg to 152±1 mmHg (P<0.05) showinĝ30% recovery in the telmisartan-treated MS group (). Administration of GSK0660 also reversed the glucose and lipid profiles modified by telmisartan in MS rats but not in the control group. In addition, GSK0660 also reversed the body weight and adipose mass in MS rats attenuated by telmisartan ().
Figure 2 PPARδ antagonist GSK0660 inhibited the effects induced by chronic telmisartan treatment in MS rats.
Abbreviations: AUC, area under the curve; Con, control; HOMA-IR, homeostatic model assessment; ITT, insulin tolerance test; MS, metabolic syndrome; PPARδ, peroxisome proliferator-activated receptor delta; SD, Sprague Dawley; Tel, telmisartan; Veh, vehicle.
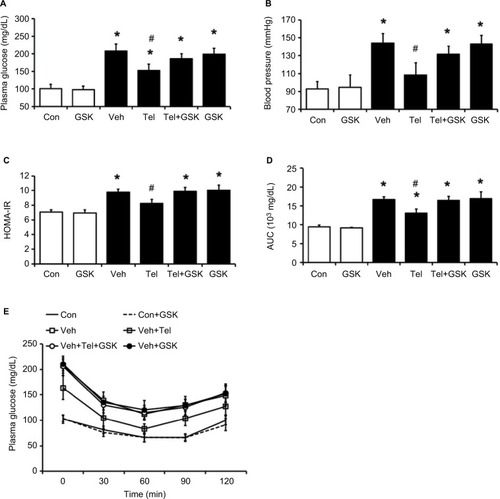
Table 3 GSK0660 inhibited the effects of telmisartan on various metabolic parameters in MS rats
Telmisartan ameliorates MS through PPARδ-dependent mechanisms in liver and skeletal muscle
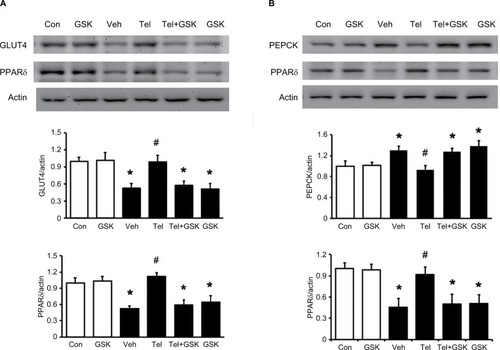
The protein levels of GLUT4 in skeletal muscle and PEPCK in the liver of MS rats were determined. As shown in , the decreased GLUT4 and PPARδ expression in the soleus muscle of MS rats were reversed by telmisartan. Additionally, the increased hepatic PEPCK level in MS rats was also markedly reduced by telmisartan (). But similar changes were not observed in losartan-treated MS rats. Moreover, the effects of telmisartan were attenuated by GSK0660 in MS rats (). Therefore, the results suggested that the effects of telmisartan in liver and skeletal muscle were PPARδ dependent.
Figure 3 Effects of telmisartan or losartan on PPARδ and related signal expression in skeletal muscle and liver.
Abbreviations: Con, control; PEPCK, phosphoenolpyruvate carboxykinase; PPARδ, peroxisome proliferator-activated receptor delta; Los, losartan; MS, metabolic syndrome; SD, Sprague Dawley; Tel, telmisartan; Veh, vehicle.
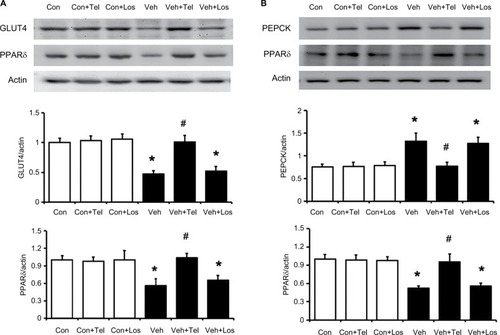
Figure 4 Effects of the PPARδ antagonist GSK0660 on expressions of PPARδ and related signals in skeletal muscle and liver isolated from the telmisartan-treated MS rats.
Abbreviations: Con, control; GSK, GSK0660; PEPCK, phosphoenolpyruvate carboxykinase; PPARδ, peroxisome proliferator-activated receptor delta; MS, metabolic syndrome; SD, Sprague Dawley; Tel, telmisartan; Veh, vehicle.
Discussion
A rat model was successfully developed for MS using the modified diet and pancreatic toxin described in this study. Male SD rats received a HF/HS diet for 8 weeks, followed by a low-dose STZ (30 mg/kg) injection. The MS rats showed insulin resistance, impaired glucose tolerance, obesity, dyslipidemia, and hypertension. Moreover, this MS model mimics the main changes that occur in humans.
In this study, we found that telmisartan improved glucose and/or lipid profiles in MS rats through PPARδ activation. Telmisartan significantly decreased the plasma insulin and markedly reduced plasma glucose. Telmisartan also alleviated the impaired insulin resistance and ameliorated the responses of ITT and HOMA-IR in MS rats. Addition ally, telmisartan significantly attenuated the increased adipose mass and reduced increased plasma TC and TG concentrations that may be due to modification of adipocyte biology and metabolism.Citation36 This resulted in an increase of energy expenditure and decrease of dietary-induced obesity and/or accumulation of visceral fat.Citation37,Citation38 It is consistent with a previous report that telmisartan activates PPARδ expression and reduces weight gain and HF-induced obesity.Citation39 In this study, telmisartan produced a beneficial effect on hyperglycemia, insulin resistance, and lipid metabolism more effectively than losartan in the MS model, which is consistent with a previous report.Citation40 In clinics, telmisartan showed effects on metabolic parameters to a greater degree than losartan in hypertensive patients with MS.Citation41 A large-scale clinical study reported that hypertensive patients with type 2 diabetes had reduced plasma glucose and serum TG concentrations after 6 months’ treatment with telmisartan compared with baseline values.Citation42 Telmisartan may induce beneficial effects on MS by direct blockade of the AT1 receptor. Some studies have established that AT1 receptor stimulation by AT2 contributes to insulin resistance and its associated deleterious metabolic profile.Citation43,Citation44 Therefore, the AT1 receptor blockade ameliorates the disorders and partially explains the beneficial effects of telmisartan on insulin resistance. A recent study indicated that the prevention of weight gain by telmisartan is partly attributed to an Ang-(1-7)-dependent mechanism.Citation45 However, it also indicated that lowering of BP in fructose-fed rats by the use of other antihypertensive drugs, such as calcium channel blockers,Citation46,Citation47 failed to show a metabolic impact, which suggests that telmisartan improved insulin resistance via BP-independent mechanisms. Moreover, our study showed that the beneficial effects of telmisartan on MS were markedly reversed by GSK0660 at the dose sufficient to block PPARδ. Therefore, telmisartan may activate PPARδ to improve glucose and lipid metabolism and prevent the increase of insulin resistance induced by diet.Citation48
Increase in insulin sensitivity is mainly induced by the enhancement of insulin signals. PPARδ is the most abundant isoform among the three PPARs in skeletal muscle.Citation49 Alternatively, Ang II (via AT receptor) is the predominant component of the RAS, which appears to be antagonistic to insulin action and contributes to insulin resistance.Citation50 Ang II impairs the insulin-induced activation of IRS1 and Akt in addition to GLUT4 membrane translocation in skeletal muscle cells.Citation51 Although it is an ARB, telmisartan could activate PPARδ to increase the oxidative capacity and result in the usage of glucose or breakdown of fat.Citation52 Insulin induces GLUT4 translocation to the cellular membrane to facilitate glucose uptake in skeletal muscle. Insulin resistance leads to defective PI3K/Akt signaling, reduced GLUT4 expression, and impaired insulin-stimulated glucose uptake.Citation49 The present study demonstrated that telmisartan activates PPARδ in the skeletal muscle of MS rats, which is consistent previous research.Citation16 Moreover; telmisartan attenuated the increased expression of hepatic PEPCK in a dose-related manner. It has been documented that PPARδ functions as a nuclear sensor of dietary fats, capable of modulating immune response through regulation of metabolic programs in the liver.Citation53 Therefore, telmisartan could activate PPARδ to alter peripheral insulin sensitivity and improve pancreatic β-cell function.
Elevated BP is associated with metabolic disorders.Citation54 In this study, HS intake was an important factor associated with the exacerbation of hypertension.Citation55 Excessive salt intake may stimulate ROS production to increase the oxidative stress in various organs including muscle, liver and fat tissues in rats.Citation56,Citation57 HS diet also causes a decrease in the activity of circulating RAS to lower Ang II levels, which may induce the compensatory upregulation of AT receptors.Citation58 Telmisartan, as a long-acting ARB, showed the antihypertensive effect more effectively than losartan, which is consistent with a previous report.Citation59 A 3-year study confirmed the advantage of telmisartan in controlling BP and reducing the risk of MS.Citation60 Telmisartan may cause an AT receptor blockade to result in a fall of peripheral resistanceCitation59 or a PPAR-dependent increase in eNOS expression and activity.Citation61 PPARδ has been suggested as a potential therapeutic target in the treatment of hypertensive subjects with insulin resistance. We also confirmed that systemic blockade of PPARδ seems to be associated with the elevation of BP in MS rats. Chronic PPARδ agonist administration in the hypertensive rats induced a marked decrease in BP.Citation58 In addition, the PPARδ agonist also induced the upregulation of hepatic lipid oxidation processes to suppress Ang II-induced dysfunctional adipogenesis and lipid accumulation.Citation61
Conclusion
In summary, we have provided experimental evidence that telmisartan is effective in ameliorating hypertension, hyper-insulinemia, and hypertriglyceridemia through activation of PPARδ in rats with MS. Therefore, the preclinical data support that treatment with telmisartan is suitable for managing patients with MS after clinical trials in the future.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interests in this work.
References
- Srikanthan K Feyh A Visweshwar H Shapiro JI Sodhi K Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population Int J Med Sci 2016 13 1 25 38 26816492
- Misra A Singhal N Khurana L Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils J Am Coll Nutr 2010 29 3 Suppl 289S 301S 20823489
- Engin A The definition and prevalence of obesity and metabolic syndrome Adv Exp Med Biol 2017 960 1 17 28585193
- Lakka HM Laaksonen DE Lakka TA The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men JAMA 2002 288 21 2709 2716 12460094
- Hassannejad R Kazemi I Sadeghi M Longitudinal association of metabolic syndrome and dietary patterns: a 13-year prospective population-based cohort study Nutr Metab Cardiovasc Dis 2018 28 4 352 360 29458993
- Shankar E Vykhovanets EV Vykhovanets OV High-fat diet activates pro-inflammatory response in the prostate through association of Stat-3 and NF-kappaB Prostate 2012 72 3 233 243 21604287
- Ferrannini E Natali A Capaldo B Lehtovirta M Jacob S Yki-Jarvinen H Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. European Group for the Study of Insulin Resistance (EGIR) Hypertension 1997 30(5 1144 1149 9369268
- Chen J Gu D Huang J Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study Lancet 2009 373 9666 829 835 19223069
- Zhou MS Schulman IH Zeng Q Link between the renin-angiotensin system and insulin resistance: implications for cardiovascular disease Vasc Med 2012 17 5 330 341 22814999
- Muller-Fielitz H Hubel N Mildner M Vogt FM Barkhausen J Raasch W Chronic blockade of angiotensin AT(1) receptors improves cardinal symptoms of metabolic syndrome in diet-induced obesity in rats Br J Pharmacol 2014 171 3 746 760 24490862
- Tyagi S Gupta P Saini AS Kaushal C Sharma S The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases J Adv Pharm Technol Res 2011 2 4 236 240 22247890
- Kramer DK Al-Khalili L Guigas B Leng Y Garcia-Roves PM Krook A Role of AMP kinase and PPARdelta in the regulation of lipid and glucose metabolism in human skeletal muscle J Biol Chem 2007 282 27 19313 19320 17500064
- Tanaka T Yamamoto J Iwasaki S Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome Proc Natl Acad Sci U S A 2003 100 26 15924 15929 14676330
- Toral M Gomez-Guzman M Jimenez R Chronic peroxisome proliferator-activated receptorbeta/delta agonist GW0742 prevents hypertension, vascular inflammatory and oxidative status, and endothelial dysfunction in diet-induced obesity J Hypertens 2015 33 9 1831 1844 26147382
- Kakuta H Sudoh K Sasamata M Yamagishi S Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers Int J Clin Pharmacol Res 2005 25 1 41 46 15864875
- Li L Luo Z Yu H Telmisartan improves insulin resistance of skeletal muscle through peroxisome proliferator-activated receptor-delta activation Diabetes 2013 62 3 762 774 23238297
- Shinohara T Takahashi N Abe I Telmisartan effectively improves insulin sensitivity in hypertensive patients with insulin resistance Obes Res Clin Pract 2011 5(4 e267 360
- Sanchez RA Masnatta LD Pesiney C Fischer P Ramirez AJ Telmisartan improves insulin resistance in high renin nonmodulating salt-sensitive hypertensives J Hypertens 2008 26 12 2393 2398 19008718
- Wong SK Chin KY Suhaimi FH Fairus A Ima-Nirwana S Animal models of metabolic syndrome: a review Nutr Metab (Lond) 2016 13 65 27708685
- Thirunavukkarasu V Anitha Nandhini AT Anuradha CV Lipoic acid attenuates hypertension and improves insulin sensitivity, kallikrein activity and nitrite levels in high fructose−fed rats J Comp Physiol B 2004 174 8 587 592 15565449
- Ghibaudi L Cook J Farley C van Heek M Hwa JJ Fat intake affects adiposity, comorbidity factors, and energy metabolism of spraguedawley rats Obes Res 2002 10 9 956 963 12226145
- Zhuhua Z Zhiquan W Zhen Y A novel mice model of metabolic syndrome: the high-fat-high-fructose diet-fed ICR mice Exp Anim 2015 64 4 435 442 26134356
- Panchal SK Brown L Rodent models for metabolic syndrome research J Biomed Biotechnol 2011 2011 35 1982
- Van den Bergh A Vanderper A Vangheluwe P Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness Cardiovasc Res 2008 77 2 371 379 18006491
- Mark AL Shaffer RA Correia ML Morgan DA Sigmund CD Haynes WG Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice J Hypertens 1999 17 12 Pt 2 1949 1953 10703894
- Dong YF Liu L Kataoka K Aliskiren prevents cardiovascular complications and pancreatic injury in a mouse model of obesity and type 2 diabetes Diabetologia 2010 53 1 180 191 19894030
- Shin SJ Lim JH Chung S Peroxisome proliferator-activated receptor-alpha activator fenofibrate prevents high-fat diet-induced renal lipotoxicity in spontaneously hypertensive rats Hypertens Res 2009 32 10 835 845 19644507
- Geer EB Islam J Buettner C Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism Endocrinol Metab Clin North Am 2014 43 1 75 102 24582093
- Chen J Huang XF Shao R Chen C Deng C Molecular mechanisms of antipsychotic drug-induced diabetes Front Neurosci 2017 11 643 29209160
- Dobrian AD Schriver SD Lynch T Prewitt RL Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity Am J Physiol Renal Physiol 2003 285(4 F619 628 12799306
- Kaur G Meena C Amelioration of obesity, glucose intolerance, and oxidative stress in high-fat diet and low-dose streptozotocin-induced diabetic rats by combination consisting of “curcumin with piperine and quercetin” ISRN Pharmacol 2012 2012 957283 22474599
- Suman RK Ray Mohanty I Borde MK Maheshwari U Deshmukh YA Development of an experimental model of diabetes co-existing with metabolic syndrome in rats Adv Pharmacol Sci 2016 2016 9463476 26880906
- Li YX Cheng KC Asakawa A Role of musclin in the pathogenesis of hypertension in rat PLoS One 2013 8 8 e72004 23940802
- Bowe JE Franklin ZJ Hauge-Evans AC King AJ Persaud SJ Jones PM Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models J Endocrinol 2014 222 3 G13 25 25056117
- Kuo SC Chung HH Huang CH Cheng JT Decrease of hyperglycemia by syringaldehyde in diabetic rats Horm Metab Res 2014 46 1 8 13 23918689
- Araki K Masaki T Katsuragi I Tanaka K Kakuma T Yoshimatsu H Telmisartan prevents obesity and increases the expression of uncoupling protein 1 in diet-induced obese mice Hypertension 2006 48 1 51 57 16717145
- Fujisaka S Usui I Kanatani Y Telmisartan improves insulin resistance and modulates adipose tissue macrophage polarization in high-fat-fed mice Endocrinology 2011 152 5 1789 1799 21427223
- Choi GJ Kim HM Kang H Kim J Effects of telmisartan on fat distribution: a meta-analysis of randomized controlled trials Curr Med Res Opin 2016 32 7 1303 1309 27010868
- He H Yang D Ma L Telmisartan prevents weight gain and obesity through activation of peroxisome proliferator-activated receptor-delta-dependent pathways Hypertension 2010 55 4 869 879 20176998
- Khan AH Imig JD Telmisartan provides better renal protection than valsartan in a rat model of metabolic syndrome Am J Hypertens 2011 24 7 816 821 21415842
- Vitale C Mercuro G Castiglioni C Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome Cardiovasc Diabetol 2005 4 6 15892894
- Michel MC Bohner H Koster J Schafers R Heemann U Safety of telmisartan in patients with arterial hypertension: an open-label observational study Drug Saf 2004 27 5 335 344 15061687
- Cooper ME Tikellis C Thomas MC Preventing diabetes in patients with hypertension: one more reason to block the renin-angiotensin system J Hypertens Suppl 2006 24 1 S57 63 16601575
- Jandeleit-Dahm KA Tikellis C Reid CM Johnston CI Cooper ME Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes J Hypertens 2005 23 3 463 473 15716683
- Schuchard J Winkler M Stolting I Lack of weight gain after angiotensin AT1 receptor blockade in diet-induced obesity is partly mediated by an angiotensin-(17)/Mas-dependent pathway Br J Pharmacol 2015 172 15 3764 3778 25906670
- Oron-Herman M Sela BA Rosenthal T Risk reduction therapy for syndrome X: comparison of several treatments Am J Hypertens 2005 18 3 372 378 15797656
- Zorad S Dou JT Benicky J Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma Eur J Pharmacol 2006 552 1–3 112 122 17064684
- Chen ZC Lee KS Chen LJ Wang LY Niu HS Cheng JT Cardiac peroxisome proliferator-activated receptor delta (PPARdelta) as a new target for increased contractility without altering heart rate PLoS One 2013 8 5 e64229 23724037
- Angione AR Jiang C Pan D Wang YX Kuang S PPARdelta regulates satellite cell proliferation and skeletal muscle regeneration Skelet Muscle 2011 1 1 33 22040534
- Perlstein TS Henry RR Mather KJ Effect of angiotensin receptor blockade on insulin sensitivity and endothelial function in abdominally obese hypertensive patients with impaired fasting glucose Clin Sci (Lond) 2012 122 4 193 202 21861845
- Wei Y Sowers JR Nistala R Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells J Biol Chem 2006 281 46 35137 35146 16982630
- Wang YX Zhang CL Yu RT Regulation of muscle fiber type and running endurance by PPARdelta PLoS Biol 2004 2 10 e294 15328533
- Liu S Hatano B Zhao M Role of peroxisome proliferator-activated receptor {delta}/{beta} in hepatic metabolic regulation J Biol Chem 2011 286 2 1237 1247 21059653
- Hermida RC Chayan L Ayala DE Association of metabolic syndrome and blood pressure nondipping profile in untreated hypertension Am J Hypertens 2009 22 3 307 313 19131935
- Weinberger MH Miller JZ Luft FC Grim CE Fineberg NS Definitions and characteristics of sodium sensitivity and blood pressure resistance Hypertension 1986 8 6 Pt 2 II127 134 3522418
- Uetake Y Ikeda H Irie R High-salt in addition to high-fat diet may enhance inflammation and fibrosis in liver steatosis induced by oxidative stress and dyslipidemia in mice Lipids Health Dis 2015 14 6 25888871
- Lenda DM Boegehold MA Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation Am J Physiol Heart Circ Physiol 2002 282 2 H395 402 11788385
- Nickenig G Strehlow K Roeling J Zolk O Knorr A Bohm M Salt induces vascular AT1 receptor overexpression in vitro and in vivo Hypertension 1998 31 6 1272 1277 9622141
- Sueta D Koibuchi N Hasegawa Y Telmisartan exerts sustained blood pressure control and reduces blood pressure variability in metabolic syndrome by inhibiting sympathetic activity Am J Hypertens 2014 27 12 1464 1471 24871627
- Peng J Zhao Y Zhang H Prevention of metabolic disorders with telmisartan and indapamide in a Chinese population with high-normal blood pressure Hypertens Res 2015 38 2 123 131 25273554
- Yuen CY Wong WT Tian XY Telmisartan inhibits vasoconstriction via PPARgamma-dependent expression and activation of endothelial nitric oxide synthase Cardiovasc Res 2011 90 1 122 129 21156825
