Abstract
Background:
Astragalus possesses therapeutic effects for type 2 diabetes (T2D), while its action mechanisms remain to be elucidated. In view of the pathogenic associations between gut microbiota and T2D, we explored the effect of astragalus on gut-microbiota composition of T2D mice.
Materials and methods:
Modulation effects of astragalus on gut microbiota of T2D-model mice were assessed by 16S rRNA gene sequencing.
Results:
Inhibited blood-glucose and body-weight levels of T2D mice by astragalus were accompanied by gut microbiota–composition alteration. Astragalus administration significantly increased gut-microbiota richness and diversity in T2D mice and significantly altered the abundance of several bacterial taxa, inducing increased abundance of Lactobacillus and Bifidobacterium. PICRUSt software revealed the relationship between astragalus and T2D.
Conclusion:
Due to previously reported decreased gut-microbiota richness and diversity and reduced abundance of key species of Lactobacillus and Bifidobacterium, more studies are encouraged to explore the contribution of gut-microbiota alteration by astragalus to its anti-T2D effect.
Introduction
Type 2 diabetes (T2D) is a chronic metabolic disease caused by the interaction of inherited and environmental factors. It was estimated that almost 425 million adults worldwide suffered from diabetes in 2017, and this number is projected to increase to 628 million by 2045.Citation1 The steadily increasing number of people living with T2D has created a global economic burden.Citation2 In addition, people with T2D are prone to developing severe complications, such as cardiovascular disease, diabetic nephropathy, diabetic neuropathy, and diabetic retinopathy.Citation3–Citation6 Therefore, there is an urgent need for prevention and early intervention of T2D.
Astragalus has been used in traditional Chinese medicines for thousands of years for its pharmacological effects. In the past decade, numerous studies on human and animal models have shown that astragalus has an antidiabetic effect.Citation7–Citation9 Owing to the poor bioavailability of the main active components of astragalus, such as saponins and flavonoids, more effort is needed to explore its underlying action mechanisms.Citation10–Citation12
In recent years, many animal and humans studies have suggested that gut microbiota may play an etiological role in T2D,Citation13–Citation16 and gut microbiota have been proposed to be potential therapeutic targets of this disease. As such, it is rational to hypothesize that astragalus may exert anti-T2D effects through altering the composition of gut microbiota. This stimulated us to explore the effect of oral administration of astragalus on gut microbiota of T2D-model mice by 16S rRNA gene sequencing, providing clues to understand the mechanism of action of this natural agent.
Materials and methods
Astragalus in brown-yellow fine-powder form containing 70% astragalan and 10% total saponins, was purchased from Huayue Chemical Products (Henan, China). Carboxymethylcellulose sodium (CMC-Na) was obtained from Sigma-Aldrich (St Louis, MO, USA). Accu-Chek was purchased from Roche Diagnostics (Mannheim, Germany). BKS.Cg-Dock7m +/+ Leprdb/Nju mice (5 weeks old) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China).
Mice were housed one per cage in a specific pathogen–free animal lab and maintained under standard conditions: a 12-hour light/dark cycle with room temperature at 22°C±2°C and 50%±5% humidity, and with ad libitum access to food and water. Ten mice were acclimatized to the laboratory environment for 1 week before the experiment. All mice were divided into two equal groups randomly: a control group and an astragalus-administered group. The astragalus-administered group received astragalus dissolved in 0.5% CMC-Na by gavage at a dosage of 1g/kg body weight per day, with mice fed a standard diet with 0.5% CMC-Na buffer as the control group. Mice were treated for 15 days once daily. Animal experiments were approved by the Animal Use Subcommittee of the Shandong University of Technology. Our use of experimental animals was in compliance with the Guide for the Care and Use of Laboratory Animals. Blood samples were withdrawn from an orbit vein after 12 hours’ fasting. Blood was centrifuged at 3,000 rpm for 10 minutes to obtain plasma. Fasting blood glucose (FBG) was measured with the Accu-Chek according to the manufacturer’s instructions.
Fresh mice feces were collected into individual sterile Ependorf tubes and then frozen immediately at −80°C until DNA extraction. DNA extraction from each fecal sample was conducted by phenol trichloromethane methods. The extracted DNA concentration was determined by NanoDrop (Thermo Fisher Scientific). After DNA extraction from the feces samples, we used PCR amplification and pyrosequenced the V3 and V4 regions of the bacterial 16S ribosomal RNA gene. Amplicon-sequencing libraries were sequenced using the Illumina Miseq platform for paired-end reads of 300 bp. Several α-diversity indices were analyzed to evaluate the effect of astragalus on gut-microbiota richness and diversity of T2D mice. Dominant bacterial community differences between groups were detected employing linear discriminant analysis combined with effect size measurements (LEfSe). LEfSe was used to identify species most characteristic of different sample types. LEfSe results were visualized using taxonomy bar-chart and cladogram plots, as implemented on the LEfSe website (http://huttenhower.sph.harvard.edu/galaxy). Microbial functions were predicted with PICRUSt software.Citation17 Relevant predicted genes and their functions were aligned with the Kyoto Encyclopedia of Gene and Genomes (KEGG) database and differences among groups compared with STAMP software.
FBG and body-weight parameters were analyzed with SPSS 16.0. Data comparisons among different groups were analyzed by ANOVA. Graphic presentations were achieved with GraphPad Prism 6 (GraphPad Software, San Diego, IL, USA).
Results
Effects on FBG and body-weight levels
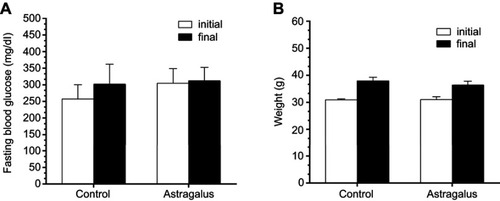
The effect of astragalus administration on FBG and body-weight levels was evaluated through comparison with the control group. The results showed that astragalus administration for 15 days reduced FBG and body weight, as shown in .
Overall structural alteration of gut microbiota
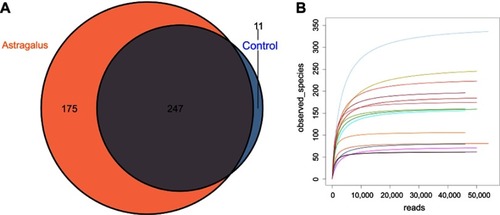
All fecal samples were examined using Illumina high-throughput sequencing. A data set consisting of 462,040 total sequence-read counts was generated, and the average number of sequences obtained was 46,204 for each sample. Altogether, 433 operational taxonomic units were exhibited at a 97% similarity level. According to the Venn diagram in , there were 247 shared operational taxonomic units between the two groups, with eleven unique to the control group and 175 to the astragalus-administered group. Rarefaction curves plateaued with the current sequencing, indicating that most gut microbial organisms in each sample were captured with the current sequencing depth ().
Figure 2 Evaluation of Illumina MiSeq data showing that astragalus altered the overall composition of gut microbiota in type 2 diabetes mice.
To explore the effect of astragalus on the richness and diversity of gut microbiota, we analyzed the α-diversity metrics (including Chao1, PD_whole_tree, Shannon, and Simpson) of the control and astragalus-administered groups. It was found that astragalus administration significantly increase gut-microbiota diversity and diversity of T2D mice (see ).
Table 1 Gut-microbiota diversity and richness indices of control and astragalus-administered groups
Bacterial composition analysis
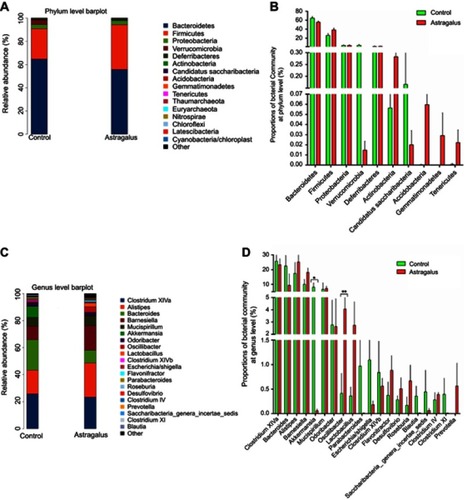
Bacterial composition in the astragalus-administered group and control group was then compared. As illustrated in ), Bacteroidetes, Firmicutes and Proteobacteria were the three dominant phyla in all samples. The 20 most abundant genera in the two groups are shown in . A total of three genera exhibited significant differences in abundance between the astragalus-administered group and the control group (). Further analysis found that the relative abundance of Oscillibacter significantly increased from 0.13% to 1.05% (P<0.01) after administration of astragalus.
Figure 3 Comparisons of bacterial community abundance between the control and astragalus- administered groups.
Taxonomic analysis
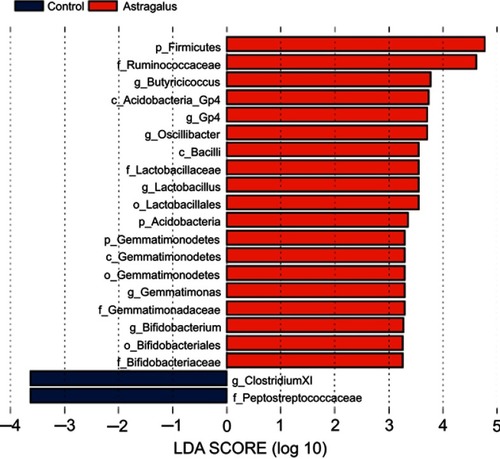
LEfSe was used to explore significant changes and relative richness in the bacterial community in the control and astragalus groups (). LEfSe results were visualized using taxonomy bar-chart and cladogram plots. Three phylum (Firmicutes, Acidobacteria, and Gemmatimonadetes) were enriched in the astragalus-adminisered group and none in the control group. One genus and six genera were enriched in the control and astragalus-administered group, respectively. Astragalus administration significantly inhibited the growth of Clostridium cluster XI, and increased the growth of Lactobacillus and Bifidobacterium in T2D mice.
Figure 4 Linear discriminant analysis (LDA) combined with effect-size measurements at all levels of control and astragalus-administered groups.
Metabolic function analysis
PICRUSt analysis was used to predict the metabolic functions of gut microbiota influenced by astragalus in T2D mice. The results revealed that 13 and 31 KEGG pathways were changed in the astragalus group at levels 2 () and 3 (), respectively, among which six were increased and seven decreased in comparison with the control group at level 2.
Figure 5 Predicted functions for the altered metagenome of gut microbiota in each group shown with Kyoto Encyclopedia of Gene and Genomes pathways.
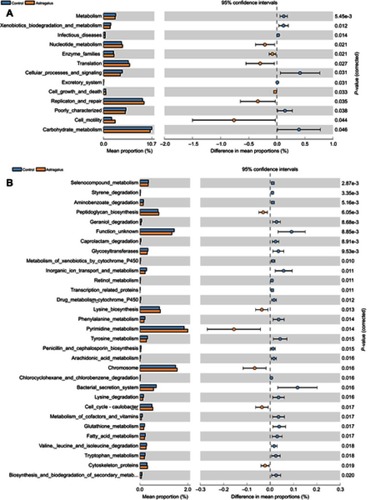
In particular, we found several interesting changes wherein 31 KEGG pathways at level 3 had changed. Firstly, the biosynthesis processes of bacteria, such as nucleotide metabolism, enzyme families, translation, cell growth, death, replication, repair, and motility (level 2) and nucleotide metabolism, including that of pyrimidine and cytoskeleton proteins (level 3), had increased in the astragalus group in comparison with the control group. In addition, the metagenome of the control group had been enriched in pathways related to xenobiotic biodegradation and metabolism, including styrene degradation, aminobenzoate degradation, caprolactam degradation, metabolism of xenobiotics by cytochrome P450, drug metabolism with cytochrome P450, and chlorocyclohexane and chlorobenzene degradation; lipid metabolism, including arachidonic-acid metabolism and fatty-acid metabolism; and animo-acid metabolism, including phenylalanine metabolism, tyrosine metabolism, lysine degradation, valine, leucine, and isoleucine degradation, and tryptophan metabolism.
Discussion
In recent years, many traditional Chinese medicines have been tried to treat T2D,Citation18–Citation20 among which astragalus is considered a promising antidiabetic natural agent, but its mechanism of action needs to be explored. Inspired by the recent findings of gut-microbiota regulation in interpreting the pharmacology of anti-T2D agents,Citation21 the present work studied alterations ingut microbiota of T2D mice through astragalus administration. It was found that astragalus prevented increases of FBG levels and body weight. Characterization of gut microbiota showed that astragalus administration significantly increased microbial diversity and richness and altered the relative abundance of several key bacterial species.
It has been reported that gut-microbiota diversity and richness decrease in diabetic mice compared to controls.Citation22 The “normalization” effect of astragalus observed in the current study may make an important contribution to its pharmacological effect. In addition, both human and animal studies have indicated that abundance of Lactobacillus and Bifidobacterium decreases in diabetic rats and T2D patients.Citation23,Citation24 Djurasevic et al found that virgin coconut oil affected some secondary parameters in diabetic rats and significantly increased the abundance of probiotic bacteria, such as Lactobacillus, Allobaculum, and Bifidobacterium spp.Citation25 Previous studies also found increased relative abundance of Bifidobacterium in T2D patients after treatment with metformin.Citation26,Citation27 Although no metabolic pathway associated with differential bacteria was found in functional prediction analysis, the biosynthesis processes of bacteria was increased in the astragalus group compared to the control group. This suggested that the addition of astragalus promoted cellular processes, but the current findings need to be further verified.
To summarize, the present findings indicated that inhibition of FBG and body-weight levels in T2D mice was associated with alterations in gut-microbiota composition. Increased gut-microbiota diversity and richness and regulation of key bacterial species abundance may be involved in the antidiabetic effect of astragalus. Further studies are needed to evaluate the contribution of gut-microbiota alteration to the anti-T2D activity of astragalus, which is important to understand the pharmacology of this agent better.
Abbreviation list
T2D, type 2 diabetes; CMC-Na, carboxymethylcellulose sodium.
Acknowledgments
This work was supported by the Shandong Provincial Natural Science Foundation (grant ZR2018MH010), Shandong Provincial Key Research and Development Program (grant 2018GSF121001), and the Talent Program of Zibo.
Disclosure
The authors report no conflicts of interest in this work.
References
- International Diabetes Federation. IDF Diabetes Atlas . 8th ed. Brussels: IDF; 2017.
- Bommer C , Sagalova V , Heesemann E , et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care . 2018;41:963–970. doi:10.2337/dc17-1962 29475843
- Beckman JA , Creager MA , Libby P . Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA . 2002;287:2570–2581.12020339
- Chen L , Magliano DJ , Zimmet PZ . The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol . 2011;8:228–236. doi:10.1038/nrendo.2011.183 22064493
- Kraemer FB , Ginsberg HN , Gerald M , Reaven M . Demonstration of the central role of insulin resistance in type 2 diabetes and cardiovascular disease. Diabetes Care . 2014;37:1178–1181. doi:10.2337/dc13-2668 24757223
- Hoogwerf BJ . Complications of diabetes mellitus. Int J Diabetes Dev Ctries . 2005;25:63–69. doi:10.4103/0973-3930.22774
- Lau KM , Lai KK , Liu CL . Synergistic interaction between astragali radix and rehmanniae radix in a chinese herbal formula to promote diabetic wound healing. J Ethnopharmacol . 2012;141:250–256. doi:10.1016/j.jep.2012.02.025 22366433
- Zhang K , Pugliese M , Pugliese A , Passantino A . Biological active ingredients of traditional Chinese herb astragalus membranaceus on treatment of diabetes: a systematic review. Mini Rev Med Chem . 2015;15:315–329. doi:10.2174/1389557515666150227113431 25723453
- Wang Y , Lin C , Ren Q , Liu Y , Yang X . Astragaloside effect on TGF-β1, SMAD2/3, and α-SMA expression in the kidney tissues of diabetic KKAy mice. Int J Clin Exp Pathol . 2015;8:6828.26261569
- Yu K , Chen F , Li C . Absorption, disposition, and pharmacokinetics of saponins from Chinese medicinal herbs: what do we know and what do we need to know more? Curr Drug Metab . 2012;13:577–598. doi:10.2174/1389200211209050577 22292787
- Manach C , Williamson G , Morand C , Scalbert A , Rémésy C . Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr . 2005;81:230S–242S.15640486
- Shen L , Ji HF . Intestinal microbiota and metabolic diseases: pharmacological Implications. Trends Pharmacol Sci . 2016;37:169–171. doi:10.1016/j.tips.2015.11.010 26706621
- Barlow GM , Yu A , Mathur R . Role of the gut microbiome in obesity and diabetes mellitus. Nutr Clin Pract . 2015;30:787–797. doi:10.1177/0884533615609896 26452391
- Sohail MU , Althani A , Anwar H , Rizzi R , Marei HE . Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J Diabetes Res . 2017;2017:9631435. doi:10.1155/2017/9631435 29082264
- Brunkwall L , Orho-Melander M . The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia . 2017;60:943–951. doi:10.1007/s00125-017-4278-3 28434033
- Karlsson FH , Tremaroli V , Nookaew I , et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature . 2013;498:99–103. doi:10.1038/nature12184 23719380
- Kanehisa M , Goto S , Sato Y , Furumichi M , Tanabe M . KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res . 2012;40:109–114.
- Li W , Zheng H , Bukuru J , De Kimpe N . Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol . 2004;92:1–21. doi:10.1016/j.jep.2003.12.031 15099842
- Hsu PC , Tsai YT , Lai JN , Wu CT , Lin SK , Huang CY . Integrating traditional Chinese medicine healthcare into diabetes care by reducing the risk of developing kidney failure among type 2 diabetic patients: a population-based case control study. J Ethnopharmacol . 2014;156:358–364. doi:10.1016/j.jep.2014.08.029 25178949
- Poon TY , Ong KL , Cheung BM . Review of the effects of the traditional Chinese medicine rehmannia six formula on diabetes mellitus and its complications. J Diabetes . 2011;3:184–200. doi:10.1111/j.1753-0407.2011.00130.x 21631896
- Wu H , Esteve E , Tremaroli V , et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med . 2017;23:850–858. doi:10.1038/nm.4265 28530702
- Zhang Q , Yu H , Xiao X , Hu L , Xin F , Yu X . Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ . 2018;6:e4446. doi:10.7717/peerj.4446 29507837
- Yan X , Feng B , Li P , Tang Z , Wang L . Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an animal study. J Diabetes Res . 2016;2016:2093171. doi:10.1155/2016/2093171 27631013
- Barengolts E , Green SJ , Eisenberg Y , et al. Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS One . 2018;13:e0194171. doi:10.1371/journal.pone.0194171 29596446
- Djurasevic S , Bojic S , Nikolic B , et al. Beneficial effect of virgin coconut oil on alloxan-induced diabetes and microbiota composition in rats. Plant Foods Hum Nutr . 2018;73:295–301. doi:10.1007/s11130-018-0689-7 30168039
- Forslund K , Hildebrand F , Nielsen T , et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature . 2015;528:262–266. doi:10.1038/nature15766 26633628
- de la Cuesta-Zuluaga J , Mueller NT , Corrales-Agudelo V , et al. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care . 2017;40:54–62. doi:10.2337/dc16-1324 27999002