Abstract
Background and purpose: Ginsenoside Rg1 (GS Rg1), as an important active substance of Panax ginseng, has been proven to have elaborate cardioprotective effects. The purpose of this study was to detect that GS Rg1 attenuates cardiac oxidative stress and inflammation in streptozotocin (STZ)-induced diabetic rats (DM).
Methods: Cardiac function was assessed by heart rate and blood pressure. Markers relevant to myocardial oxidative stress and antioxidant capacity, and inflammatory reaction factors were detected. The mRNA and protein expression were detected by RT-qPCR and Western blot, respectively.
Results: GS Rg1 treatment significantly reduced the symptoms of cardiac hypertrophy and hypertension, and also decreased oxidative stress, inflammation response, NF-κB expression and NLRP3 inflammasome expression. GS Rg1 enhanced mitochondrial biogenesis by increasing PGC-1α, complex III and complex Ⅳ expression. GS Rg1 treatment significantly increased the expression of AMPK, Nrf2 and HO-1 in cardiac tissues.
Conclusion: GS Rg1 exhibited protective effect against STZ-induced cardiac dysfunction, which is potentially associated with AMPK/Nrf2/HO-1 signal pathway.
Introduction
At present, diabetes has become a global problem that threatens human health.Citation1 As the incidence of diabetes increases,the complications of diabetes have caused considerable concern. Diabetic cardiomyopathy is considered to be a disease of myocardial structural and functional abnormality independent of hypertension, coronary heart disease and related heart disease.Citation2 Among them, the occurrence of cardiovascular complications can easily lead to high morbidity and mortality in diabetic patients. At the same time, the risk of developing coronary heart disease and congestive heart failure increases in diabetic patients.Citation3 The risk of developing congestive heart failure events in diabetic patients is much higher than in non-diabetics.Citation4 According to clinical features, the main feature of diabetic cardiomyopathy is myocardial dilatation or hypertrophy, while left ventricular systolic or diastolic function is impaired. However, the underlying mechanisms for the pathogenesis of diabetic cardiomyopathy are not completely understood.
Oxidative stress, ammoniation, myocardial fibrosis, apoptotic cell death and other molecular mechanisms can consider to be used as potential factors in the pathogenesis of diabetic cardiomyopathy.Citation5 For oxidative stress, activation of 5ʹ adenosine phosphate-activated protein kinase (AMPK) plays a key role in improving mitochondrial activity and regulating cell metabolism.Citation6 Recently, studies have indicated that AMPK can suppress oxidative stress by stimulating upregulation of nuclear factor erythrocyte 2 related factor 2 (Nrf2)-dependent blood oxygenase (HO-1), and significant crosstalk is observed in mammalian inflammatory systems.Citation7 Therefore, we hypothesized that dysfunction of AMPK/Nrf2/HO-1 signaling pathway might play an important role in streptozotocin (STZ)-induced cardiac oxidative stress and inflammation.
GS Rg1 is an active ingredient from the Panax notoginseng, known for its cardioprotective effects.Citation8 Currently, many studies have reported the protective effect of GS Rg1, such as inhibiting cardiovascular cell apoptosis and reducing oxidative stress.Citation9 Moreover, most of the diseases studied are mainly hypertrophic cardiomyopathy, acute myocardial infarction, myocardial ischemia/reperfusion.Citation10–Citation12 However, few studies have demonstrated the myocardial protective effect of GS Rg1 by decreasing oxidative stress and inflammatory response in diabetic rats. Therefore, in the current study, we observed the effects of GS Rg1 on oxidative stress and inflammatory response in STZ-induced DM rats.
Materials and methods
Animal experimental
This study was approved by the Ethics Committee of Qingdao University. Male Wistar rats (200±20) g were purchased from laboratory animal center of Qindao University and randomly divided into diabetic group and control group. The rats of diet group were administered intraperitoneally with STZ (40 mg/kg body weight). The rats of control rats were administered with 0.1 M citrate buffer. One week after the administration of STZ, blood glucose concentration >300 mg/dL were considered diabetic.
The STZ-induced DM rats subsequently apportioned randomly and equally to a DM control group (administered saline, ip), GS Rg1 group (20 mg/kg/day, ip) and AMPK inhibitor compound C group (CC, 20 mg/kg/day, ip). The assigned treatments were administered for 8 weeks. Based on previous studies, we use GS Rg1(20 mg/kg) as pharmacological approach for the administration of diabetic-induced cardiomyopathy.Citation13,Citation14 The rats in the non-diabetic control group were normal, and these rats served as non-diabetic controls in the following experiment. Body weight of rats was measured weekly.
Heart rate and blood pressure measurement
Heart rate and blood pressure were measured using a noninvasive tail-cuff method (Narco BioSystem, Houston, TX, USA) in all rats. The rats were placed at 37°C for 10 mins at room temperature and then placed in an acrylic restrainer. The mean arterial pressure (MAP) was read and recorded. Finally, the average value of six individuals is counted as final value.
Preparation of myocardial tissue and blood samples
After 8 weeks of treatment, all rats were sacrificed under an overdose of 10% chloral hydrate (700 mg/kg body weight). When blood sample was obtained from the heart of the rat, 10% chloral hydrate (350 mg/kg body weight) was used to anesthetize rats. Then, 3 mL of blood samples was collected for subsequently determining the levels of blood glucose, lactate dehydrogenase (LDH), creatine kinase (CK)-MB and aspartate aminotransferase (AST). Subsequently, rat heart tissues were rapidly dissected and frozen at −80°C until RT-qPCR and Western blot. The myocardial hypertrophy index (ratio of heart weight/body weight) was determined.
Detection of markers relevant to myocardial oxidative stress and antioxidant capacity
The supernatants of myocardial tissues from each group were evaluated for levels of thiobarbituric acid reactive substances (TBARS), hydrogen peroxide, peroxynitrite, superoxide dismutase (SOD), catalase, GPx and glutathione peroxidase (GSH) using commercial kits (Cayman Chemicals, USA). ROS in cardiac homogenates were estimated fluorometrically.Citation15
Determination of inflammatory reaction factors
Inflammatory reaction factors (IL-1β, IL-6 and TNF-α) were detected using ELISA kits (Thermo Fisher Scientific, USA) in heart homogenate and plasma, respectively.
cDNA synthesis and quantitative real-time PCR
Total RNA (1 µg) was extracted from the cardiac tissue using TRIzol reagent (Takara, Tokyo, Japan). Using 1 µg total RNA, first-strand cDNA was synthesized by the AMV enzyme in 20 µL reaction mixture (Takara, Tokoyo, Japan). Using 2 µL reverse transcriptase products, quantitative PCR was performed in a final volume of 20 µL using gene-specific primers. The primers sequence is listed in . Amplification was carried out as follows: 95°C, 30 s, 1 cycle; 95°C, 5 s and 60°C 1 min for 40 cycles; subsequently, the melting curve was determined. Gene transcripts were quantified with SYBR Premix Ex Taq kit (Takara, Tokyo, Japan). The levels of gene expression were evaluated by quantitative real-time PCR with CFX96 system (Thermo Fisher Scientific, USA). The target gene expression level relative to the endogenous control gene (GAPDH) was calculated by 2−ΔΔCt method.Citation16
Table 1 List of primer sequences
Analysis of relevant protein levels by Western blot
The cardiac tissues were harvested, washed three times in sterile saline and then homogenized in RIPA buffer (Beyotime Institute of Biotechnology, Suzhou, People’s Republic of China) containing a protease inhibitor cocktail (Thermo Scientific, Rockford, IL, USA). Following centrifugation at 16,000 × g at 4°C for 30 mins, the supernatant was collected. Protein was quantified using a BCA Protein Assay kit (Beyotime Institute of Biotechnology), then separated by 10% SDS-PAGE. Following transfer to a PVDF membrane (Beyotime Institute of Biotechnology), the primary antibodies were then incubated at 4°C overnight. Subsequently, they were incubated with the appropriate horseradish peroxidase-conjugated anti-rabbit (ZB-2301) secondary antibodies (ZSGB-BIO) for 1 hr at room temperature and visualized under a chemiluminescence system (Bio-Rad, USA). The bands were quantified using MultiGauge version 3.2 software. Relative expression levels of proteins were calculated by integrated grey values of the bands normalized with reference protein. The primary antibodies: AMPK (1:800; ab3759, Abcam, Boston, MA, USA), p-AMPK (Thr 172) (1:500; ab23875, Abcam), Nrf2 (1:800; ab137550, Abcam), HO-1 (1:1000; ab13243, Abcam), PGC-1α (1:800; ab54481, Abcam), NF-κB (1:800; ab131546, Abcam).
Statistical analysis
All experimental data were analyzed using SPSS 20.0 software (Chicago, IL, USA). Student’s t-test was utilized as the comparison between the two groups. P<0.05 indicated that the data were significantly different.
Results
Effects of GS Rg1 treatment on MAP, heart rate, body weight and hypertrophy index in STZ-induced diabetic rats
As shown in , , compared with control rats, the MAP, heart rate and hypertrophy index of DM rats were significantly increased (p<0.05). shows no change in the body weight of diabetic rats (p>0.05).
Figure 1 Effects of GS Rg1 on the indicators in the control and diabetic rats. (A) The MAP levels. (B) The heart rate. (C) The body weight. (D) The hypertrophy index. (E) The blood glucose levels. (F) The LDH levels. (G) The CK-MB levels. (H) The AST levels. P<0.05. (*) vs C; (#) vs C+GS Rg1 20; (&) vs D.

After 8 weeks of GS Rg1 injections in DM rats, we found that the MAP, heart rate and body weight of GS Rg1 treatment were significantly decreased (p<0.05; –). Furthermore, GS Rg1 administration also remarkably decreased hypertrophy index of heart in DM rats (p<0.05; ).
Effects of GS Rg1 on glycaemic control and myocardiac injury markers in STZ-induced diabetic rats
As depicted in , the STZ administration significantly increased blood glucose concentrations (p<0.05), which was efficiently decreased by GS Rg1 (p<0.05; ). Previous research has shown that serum enzyme activities of LDH, CK-MB and AST usually can be used as a marker to detect myocardial infarction.Citation16 Therefore, we tested the expression of the above indicators. The results are shown in . Compared with control rats, LDH (p<0.05; ), CK-MB (p<0.05; ) and AST (p<0.05; ) expression levels were significantly increased in the plasma in DM rats, which all were efficiently lessened by GS Rg1 treatment.
Effects of GS Rg1 on myocardial oxidative stress in STZ-induced diabetic rats
Studies have shown that oxidative stress is associated with diabetic cardiomyopathy.Citation17 Therefore, we tested the relevant indicators expression of cardiac oxidative stress. As shown in , the expression levels of TBARS (p<0.05; ), ROS (p<0.05; ), hydrogen peroxide (p<0.05; ) and peroxynitrite (p<0.05; ) significantly increased in DM group rats compared with control group, suggesting that STZ administration significantly increased oxidative stress in the hearts of diabetic rats. On the contrary, GS Rg1 treatment remarkably reduced these oxidative stress markers in the myocardium of STZ-induced diabetic rats (p<0.05; ).
Figure 2 Effects of GS Rg1 on the cardiac oxidative stress and antioxidant defense system in control and diabetic rats. (A) Cardiac TBARS levels. (B) Cardiac ROS levels. (C) Cardiac hydrogen peroxide levels. (D) Cardiac peroxynitrite levels. (E) Myocardial SOD levels. (F) Myocardial catalase levels. (G) Myocardial GSH levels. (H) Myocardial GPx levels. P<0.05. (*) vs C; (#) vs C+GS Rg1 20; (&) vs D.
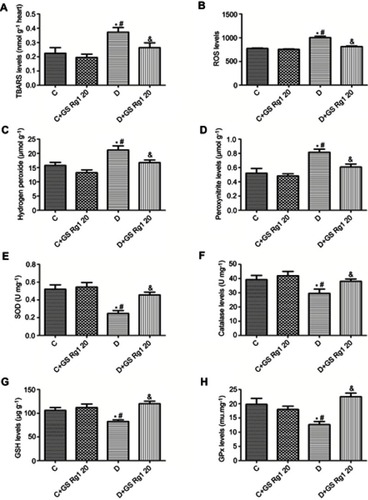
Effects of GS Rg1 on antioxidant defense system in STZ-induced diabetic rats myocardium
Based on the results of the above oxidative stress indicators, we speculated whether GS Rg1 would affect the antioxidant defense system of STZ-induced diabetic rats. The results are shown in . Compared with the rats of control group, total SOD (p<0.05; ), catalase (p<0.05; ), GSH (p<0.05; ) and GPx (p<0.05; ) expression levels were significantly decreased in the antioxidant defense system in STZ-induced diabetic rats, indicating STZ treatment significantly attenuated the role of the antioxidant defense system in the myocardium. However, GS Rg1 administrated significantly reversed these indicators of antioxidant defense system in STZ-induced diabetic rats (p<0.05; –).
Effects of GS Rg1 on inflammation in STZ-induced diabetic rats
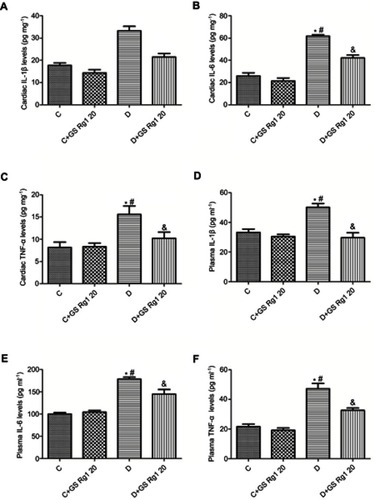
DM patients often have chronic inflammation. Therefore, to validate the anti-inflammatory effect of GS Rg1 in STZ-induced diabetic rats, the levels of inflammatory factors IL-1β, IL-6 and TNF-α were examined. As shown in , compared with control group rats, the expression levels of IL-1 (p<0.05; ), IL-6 (p<0.05; ) and TNF-α (p<0.05; ) were significantly elevated in the myocardial of STZ-induced diabetic rats. However, GS Rg1 treatment significantly attenuated the expression levels of IL-1β, IL-6 and TNF-α in the myocardium of STZ-induced diabetic rats (). In addition, we also tested these inflammatory cytokines in the plasma. We found that a similar kind of results was observed in plasma inflammatory cytokines from GS Rg1 administered STZ-induced diabetic rats and control rats (p<0.05; –).
Figure 3 Effects of GS Rg1 supplementation on cardiac and plasma inflammatory cytokines of control and diabetic rats. (A) Cardiac IL-1β levels. (B) Cardiac IL-6 levels. (C) Cardiac TNF-α levels. (D) Plasma IL-1β levels. (E) Plasma IL-6 levels. (F) Plasma TNF-α levels. The significance was set at P<0.05. (*) vs C; (#) vs C+GS Rg1 20; (&) vs D.
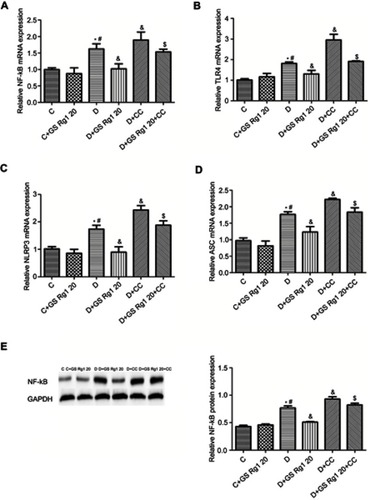
Effects of GS Rg1 on myocardial Nf-κB expression and inflammasome in STZ-induced diabetic rats
As shown in , vcompared with control group rats, the mRNA and protein expression of NF-κB in the myocardial were significantly increased in STZ-induced diabetic rats (p<0.05). On the contrary, GS Rg1 treatment significantly reduced mRNA (p<0.05; ) and protein (p<0.05; ) expression of cardiac NF-κB in DM rats whereas CC treatment enhanced the same. Meanwhile, co-administration with CC markedly reversed the inhibitory effect of GS Rg1 on NF-κB (p<0.05; , ). Next, we evaluated the expression of inflammasome components (NLRP3 and ASC) and its upstream activator (TLR4). The results were shown that the mRNA expression levels of TLR4 (p<0.05; ), NLRP3 (p<0.05; ) and ASC (p<0.05; ) significantly increased in DM rats. GS Rg1 administrate effectively decreased mRNA expression of above gene, whereas CC markedly enhanced them (p<0.05; –). However, co-administration with CC prevented the inhibitor effect of GS Rg1 on mRNA expression of inflammasome components.
Figure 4 Effects of GS Rg1 treatment on inflammatory response in cardiac tissues of control and diabetic rats. (A) NF-κB mRNA expression. (B) TLR4 mRNA expression. (C) NLRP3 mRNA expression. (D) ASC mRNA expression. (E) NF-B protein expression. Values are represented as mean±SD. P<0.05. (*) vs C; (#) vs C+GS Rg1 20; (&) vs D; ($) vs D+GS Rg1 20.
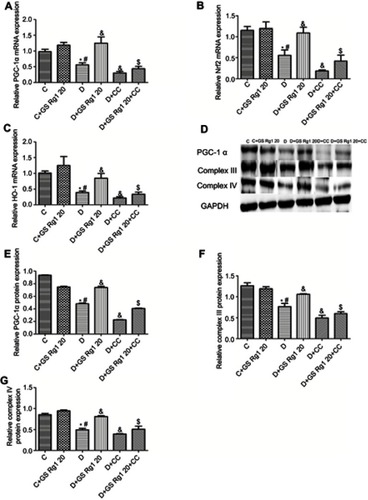
Effects of GS Rg1 on mitochondrial biogenesis in STZ-induced diabetic rats
Studies have shown that oxidative stress is known to cause mitochondrial dysfunction.Citation18 As shown in , compared with control group rats, the mRNA levels of PGC-1α (p<0.05; ) and protein levels of myocardial PGC-1α (p<0.05; ), complex III (p<0.05; ) and complex IV (p<0.05; ) in DM rats significantly decreased. However, GS Rg1 treatment significantly enhanced the same gene expression in DM rats, whereas CC markedly decreased the same gene levels (). Meanwhile, co-administration with CC significantly reversed the effect of GS Rg1.
Figure 5 Effects of GS Rg1 administration on the expression of the mitochondrial biogenesis proteins, Nrf2, HO-1 in the hearts of control and diabetic rats. (A) mRNA expression of PGC-1α. (B) mRNA expression of Nrf2. (C) mRNA expression of HO-1. (E) protein expression of PGC-1α. (F) protein expression of complex III. (G) protein expression of complex V. P<0.05. (*) vs C; (#) vs C+GS Rg1 20; (&) vs D; ($) vs D+GS Rg1 20.
Effects of GS Rg1 on Nrf2 and HO-1mRNA expression levels in cardiac tissues of STZ-induced diabetic rats
As shown in , compared with control group rats, the mRNA expression of Nrf2 (p<0.05; ) and HO-1 (p<0.05; ) significantly decreased in the cardiac tissue of DM rats. GS Rg1 administration significantly enhanced the Nrf2 and HO-1 mRNA expression in heart tissues of DM rats, whereas CC treatment significantly reduced Nrf2 and HO-1 mRNA expression (p<0.05; –). However, co-administration with CC reversed the effect of GS Rg1.
Figure 6 Effects of GS Rg1 administration on the protein expression levels of AMPK/Nrf2/HO-1 signaling components in the hearts of control and diabetic rats. Myocardial expression of (A) AMPK, p-AMPK; (B) Nrf2 and (C) HO-1. Values are represented as mean±SD. P<0.05. (*) vs C; (#) vs C+GS Rg1 20; (&) vs D; ($) vs D+GS Rg1 20.
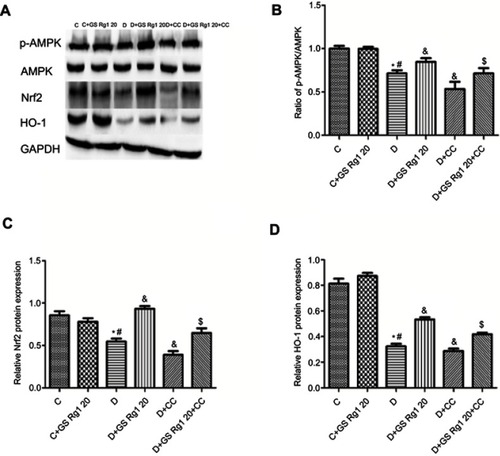
Effects of GS Rg1 on AMPK/Nrf2/HO-1 signaling pathway in STZ-induced diabetic rats
As shown in , we found that p-AMPK/AMPK ratio significantly decreased in DM rats myocardium, which was reversed after GS Rg1 administration (p<0.05; ), whereas CC treatment significantly diminished p-AMPK/AMPK ratio. However, co-administration with CC significantly blocked GS Rg1-mediated phosphorylation of AMPK (p<0.05; ). Similarly, GS Rg1 administration significantly increased the Nrf2 and HO-1 expression levels in DM rats (p<0.05; –). However, co-administration with CC markedly reversed the effect of GS Rg1 (p<0.05; –).
Discussion
In this study, we established a diabetes model with STZ 40 mg/kg injection. Then, we examined the relevant indicators of myocardium and determined that STZ administration can cause myocardial impairment.
Excessive production of ROS may exceed the activity of antioxidant enzymes, leading to the occurrence of oxidative stress. Growing evidence shows that excessive levels of superoxide radicals induced by hyperglycemia in diabetes can contribute to oxidative stress and changes in cardiac structure, which ultimately leads to diabetic myocardial damage.Citation17 Under normal physiological conditions, although ROS are continuously produced, the antioxidant defense system is sufficient to prevent ROS-related damage. Diabetic cardiomyopathy is an imbalance between ROS production and clearance, leading to oxidative stress being amplified by cascades and possibly leading to damage and apoptosis in normal tissues.Citation19 Hyperglycemia can increase the sensitivity of STZ-induced diabetic rats myocardial tissue to oxidative stress, mainly because the functional activities of SOD, catalase, GSH and GPX are decreased, and the antioxidant defense response is weakened. In this study, we used GS Rg1 administration to STZ-induced diabetic rats, found that GS Rg1 significantly reduced cardiac TBARS, ROS, hydrogen peroxide, peroxynitrite expression, and recovered cardiac antioxidant enzymes SOD, catalase, GPx and GSH levels. Therefore, the free radical scavenging properties and antioxidant effects of GS Rg1 contribute to the alleviation of STZ-induced myocardial oxidative stress.
It has been reported that hyperglycemia-induced myocardial tissue oxidative stress is associated with inflammation by overproduction of pro-inflammatory cytokines.Citation20 NF-κB is a pleiotropic transcription factor that controls the expression of multiple target genes and is primarily involved in inflammation. When NF-κB is blocked, oxidative stress and inflammatory responses in myocardial tissue are simultaneously reduced, and cardiac dysfunction in type 2 diabetic mice is alleviated.Citation21 In the heart, activation of NF-κB signaling is associated with different pathophysiological environments, including myocardial infarction, heart failure, cardiac hypertrophy and diabetic cardiomyopathy.Citation22 Our study found that the expression of NF-κB mRNA and protein was increased in STZ-induced diabetic rats, and GS Rg1 treatment decreased its expression, suggesting that GS Rg1 may inhibit NF-κB- mediated cardiac inflammation. At the same time, studies have shown that NF-κB transactivation and Toll-like receptor 4 (TLR4)-mediated ROS production are mechanisms of inflammatory body initiation and regulation.Citation23 The NLRP3 pathway affects insulin sensitivity and increases myocardial cytokine levels as well as macrophage infiltration.Citation24 Moreover, NLRP3 inflammatory bodies regulate downstream inflammatory events of lipotoxicity and glucose toxicity during the development of T2 DM.Citation25 In the present study, we found that the expression of TLR4, NLRP3 and ASC in the heart of STZ-induced diabetic rats was significantly upregulated, while GS Rg1 reduced the inflammation of the NLRP3 inflammatory body. At the same time, surprisingly, after the combination of CC, we found that CC inhibited the inhibitory effect of GS Rg1 on myocardial inflammation in DM rats mediated by NF-κB and NLRP3 inflammatory neutrophils in an AMPK-dependent manner.
Previous studies have demonstrated that mitochondrial dysfunction promotes the production of oxidative free radicals, leading to cardiac oxidative stress in diabetic rats.Citation26 Overexpression of PGC-1α in the heart strongly enhances mitochondrial DNA content,Citation27 whereas knockdown of PGC-1α leads to decreased expression of the citrate cycle and phosphatidylation genes.Citation28 In our study, chronic GS Rg1 treatment enhanced the expression of PGC-1α, complex III and V subunits. At the same time, co-administration with CC, we found that CC significantly inhibited the promotion of GS Rg1 on myocardial mitochondrial biosynthesis in DM rats, indicating that GS Rg1 can be activated by AMPK pathway to improve mitochondrial biogenesis.
Next, we investigated the AMPK/Nrf2/HO-1 signal, a well-recognized metabolic stress sensor that reduces cardiac oxidative stress in the context of STZ-induced type 2 diabetes by GS Rg1 administration. Oxidative stress can cause AMPK phosphorylation, thereby stimulating the expression of Nrf2 and its downstream antioxidant enzymes, including HO-1.Citation29 In addition, activation of Nrf2 protects cardiomyocytes against doxorubicin-induced cardiomyopathy by inducing mitochondrial biosynthesis.Citation30 Surprisingly, Nrf2 also blocks the inflammatory response by inhibiting the transcription of pro-inflammatory cytokines.Citation31 Therefore, we believe that the Nrf2/HO-1 axis acts as a marker for antioxidant enzymes, and that GS Rg1 is found to be effective in increasing the expression of Nrf2, which activates AMPK in DM rats myocardium. To further reveal that the AMPK signaling pathway is involved in GS Rg1-induced cardioprotection effects, AMPK inhibitors are given to diabetic rats with GS Rg1. CC combination therapy not only prevented GS Rg1 from inducing AMPK phosphorylation but also reduced the expression of Nrf2 and HO-1 in diabetic myocardium. AMPK stimulation regulates cellular energy content by increasing ATP generation through increasing mitochondrial pool via upregulation of mitochondrial biogenesis, which is requisite to restrain diabetes-triggered oxidative stress.Citation32 Meanwhile, p-AMPK may be involved in other pathway regulation. These results indicate that GS Rg1 promotes AMPK/Nrf2/HO-1 pathway to improve STZ-mediated myocardial oxidative stress and inflammation.
In conclusion, we have demonstrated that GS Rg1 administration has a beneficial effect on cardiac oxidative stress and inflammation in STZ-induced diabetic rats. The underlying mechanisms may be involved in AMPK/Nrf2/HO-1 pathway.
Ethical approval
All animal experiments were performed in accordance with the regulations of the Ethics Committee of the Affiliated Hospital of Qingdao University and were consistent with the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Disclosure
The authors declare that they have no conflicts of interest.
References
- van Dieren S , Beulens JW , van der Schouw YT , Grobbee DE , Nealb B . The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil . 2010;17(Suppl 1):S3–S8. 20489418
- Boudina S , Abel ED . Diabetic cardiomyopathy revisited. Circulation . 2007;115(52):3213–3223.17592090
- Asrih M , Steffens S . Emerging role of epigenetics and miRNA in diabetic cardio- myopathy. Cardiovasc Pathol . 2013;22(2):117–125.22951386
- Qazi MU , Malik S . Diabetes and cardiovascular disease: original insights from the Framingham heart study. Glob Heart . 2013;8(1):43–48.23544179
- Miki T , Yuda S , Kouzu H , Miura T . Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev . 2013;18(2):149–166.22453289
- Zong H , Ren JM , Young LH , et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA . 2002;99(25):15983–15987.12444247
- Mo C , Wang L , Zhang J , et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS- stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal . 2014;20(4):574–588.23875776
- Lü JM , Yao Q , Chen C . Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol . 2009 7(3):293–302.19601854
- Lee CH , Kim JH . A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res . 2014;38(3):161–166.25378989
- Zhang YJ , Zhang XL , Li MH , et al. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J Cardiovasc Pharmacol . 2013;62(1):50–57.23846802
- Wang XD , Gu TX , Shi EY , Lu CM , Wang C . Effect and mechanism of panaxoside Rg1 on neovascularization in myocardial infarction rats. Chin J Integr Med . 2010;16(2):162–166.20473743
- Li X , Chen JX , Sun JJ . [Protective effects of Panax notoginseng saponins on experimental myocardial injury induced by ischemia and reperfusion in rat]. Zhongguo Yao Li Xue Bao . 1990;11(1):26–29.2403009
- Yu H , Zhen J , Yang Y , Gu J , Wu S , Liu Q . Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress-induced apoptosis in a streptozotocin-induced diabetes rat model. J Cell Mol Med . 2016;20(4):623–631.26869403
- Yu HT , Zhen J , Pang B , Gu JN , Wu SS . Ginsenoside Rg1 ameliorates oxidative stress and myocardial apoptosis in streptozotocin-induced diabetic rats. J Zhejiang Univ-Sci B . 2015 16(5):344–354.25990051
- Maity P , Bindu S , Dey S , et al. Indomethacin, a non-steroidal anti-inflammatory drug, develops gastropathy by inducing reactive oxygen species-mediated mitochondrial pathology and associated apoptosis in gastric mucosa: a novel role of mitochondrial aconitase oxidation. J Biol Chem . 2009;284(5):3058–3068.19049974
- Badole SL , Chaudhari SM , Jangam GB , Kandhare AD , Bodhankar SL . Cardioprotective activity of pongamia pinnata in streptozotocin-nicotinamide induced diabetic rats. Biomed Res Int . 2015;2015:403291.25954749
- Golbidi S , Botta A , Gottfred S , Nusrat A , Laher I , Ghosh S . Glutathione administration reduces mitochondrial damage and shifts cell death from necrosis to apoptosis in ageing diabetic mice hearts during exercise. Br J Pharmacol . 2014;171(23):5345–5360.25039894
- Okonko DO , Shah AM . Heart failure: mitochondrial dysfunction and oxidative stress in CHF. Nat Rev Cardiol . 2015;12(1):6–8.25421167
- Singal PK , Bello-Klein A , Farahmand F , Sandhawalia W . Oxidative stress and functional deficit in diabetic cardiomyopathy. Adv Exp Med Biol . 2001;498:213–220.11900371
- Hussain T , Tan B , Yin Y , Blachier F , Tossou MC , Rahu N . Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev . 2016;2016:7432797.27738491
- Mariappan N , Elks CM , Sriramula S , et al. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res . 2010;85(3):473–483.19729361
- Maier HJ , Schips TG , Wietelmann A , et al. Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci . 2012;109(29):11794–11799.22753500
- Boaru SG , Borkham-Kamphorst E , Van de Leur E , Lehnen E , Liedtke C , Weishirchen R . NLRP3 inflammasome expression is driven by NF-kappaB in cultured hepatocytes. Biochem Biophys Res Commun . 2015;458(3):700–706.25686493
- Kawaguchi M , Takahashi M , Hata T , et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation . 2011;123(6):594–604.21282498
- Vandanmagsar B , Youm YH , Ravussin A , et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med . 2011;17(2):179–188.21217695
- Boudina S , Sena S , Theobald H , et al. Mitochondrial energetics in the heart in obesity- related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes . 2007;56(10):2457–2466.17623815
- Russell LK , Mansfield CM , Lehman JJ , et al. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1 alpha promotes mitochondrial biogenesis and reversible cardio- myopathy in a developmental stage-dependent manner. Circ Res . 2004;94(4):525–533.14726475
- Arany Z , He H , Lin J , et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab . 2005;1(4):259–271.16054070
- Zimmermann K , Baldinger J , Mayerhofer B , et al. Activated AMPK boosts the Nrf2/HO-1 signaling axis-a role for the unfolded protein response. Free Radic Biol Med . 2015;88:417–426.25843659
- Piantadosi CA , Carraway MS , Babiker A , et al. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory Factor-1. Circ Res . 2018;103(11):1232–1240.
- Kobayashi EH , Suzuki T , Funayama R , et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun . 2016;7:11624.27211851
- Kukidome D , Nishikawa T , Sonoda K , et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes . 2006;55(1):120–127.16380484
