Abstract
Introduction
Type 2 diabetes (T2D) is a widely distributed disease that affects large population worldwide. This study aimed to verify the role of Ginkgo biloba (GB) extract and magnetized water (MW) on the survival rate and functional capabilities of pancreatic β-cells in type 2 diabetic rats.
Materials and methods
T2D was induced by feeding the rats on a high-fat diet (20% fat, 45% carbohydrate, 22% protein) for eight weeks followed by intra-peritoneal injection of a single low dose of streptozotocin (25mg/Kg). Forty rats were randomly assigned to four groups (n=10 rats) as follows: non treated control and three diabetic groups. One diabetic group served as a positive control (diabetic), while the other two groups were orally administered with water extract of GB leaves (0.11 g/kg/day) and MW (600 gauss) for four weeks, respectively.
Results
The β-cell mass and insulin expression in these cells increased markedly after both treatments, particularly in GB treated group. In addition, the immune-expression of the two antioxidant enzymes; glutathione and superoxide dismutase 2 (SOD2) in the pancreatic tissue demonstrated a down-regulation in GB and MW treated groups as compared with the diabetic group.
Conclusion
A four-week treatment of GB and MW protected pancreatic β-cell cells and improved their insulin expression and antioxidant status in type 2 diabetic rats.
Introduction
Diabetes mellitus (DM) is classified into two main subtypes: 1 and 2. Type 1 DM results from the destruction of the pancreatic β-cells and lack of insulin secretion; it is accompanied by high blood glucose concentrations and ketoacidosis.Citation1,Citation2 However, Type 2 DM (T2D) is more common and is frequently linked to obesity.Citation3,Citation4
It has been previously shown that T2D could affect the pancreatic endocrine (islets of Langerhans) and exocrine systems (pancreatic acini). T2D in many cases is accompanied by a decrease in body weight and many digestive disturbances, which may rely on the enzymatic functional defect of the pancreatic exocrine system.Citation5–Citation7
Even more, T2D is accompanied by high blood insulin levels and hyperlipidemia.Citation8 Maintaining optimal blood glucose levels can delay further DM progression. Whereas, a steady rise in plasma glucose levels occurs regardless of the degree of control or type of treatment. Therefore, β-cell function declines linearly with time, and it was reported that after 10 years more than 50% of patients require insulin therapy.Citation9 The underlying changes in β-cell function have been well described,Citation10,Citation11 and β-cell mass decreases steadily during the course of T2D.Citation12,Citation13 It is strongly accepted that T2D is a worldwide chronic progressive syndrome. Therefore, a high likelihood demand for insulin therapy is important to maintain at optimal glycemic status.Citation14
Free radicals play a major role in the pathogenesis of T2D and most likely its further complications. Nevertheless, the formation of reactive oxygen species (ROS) is a direct consequence of hyperglycemia.Citation15 Increased ROS and decreased antioxidant systems induce a critical oxidative stress in diabetic patients. Substances having ROS scavenging ability can have potential effectiveness in diabetic animals with high oxidative stress level.Citation16 Modulation of the levels of common antioxidants including vitamins A, C, and E, glutathione, and the enzymes superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase can be applied to counteract this oxidative stress condition.Citation17–Citation19
It has been suggested that dietary antioxidants may play a role in reducing the risk of T2D as well as its complications.Citation20 The extracts derived from Ginkgo biloba (GB) have been frequently used in traditional medicine and has been shown to exhibit antioxidant potency.Citation21 GB extract leads to significant alterations in antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) and total antioxidant status.Citation22
The magnetized water (MW), however, has been also reported to reduce blood glucose, improve antioxidant status, and lipid profiles in streptozotocin-induced diabetes in rats.Citation21,Citation23 This protective effect of MW is induced by elevating the concentration of glutathione peroxidase (GSH-Px) in serum after one or two months of exposure.Citation24
The role of natural antioxidants ie GB and MW in protecting β-cells is so far not mentioned in the available literature. This novel study was designed to verify the protective role of administration of GB and MW on pancreatic β-cells.
Materials and methods
Animals and experimental design
This experiment was performed on 40 adult males Wister rats weighing 200±20 g. The experiment was performed in the animal house, Jazan University, KSA, and was approved by the ethical committee of Jazan University. We followed our previously published protocol for designing the current experiments.Citation21 Shortly, animals were housed in separate cages under normal day and night cycles. The animals were divided into two main groups: a control group (n=10) and a diabetic group (n=30). The control group was fed standard laboratory ration and allowed free access to water. The diabetic group was further subdivided into three groups (10 rats each). Group I was kept as non-treated control, diabetic group. Group II was orally administered with water extraction of GB leaves (0.11 mg/kg/day/four weeks) purchased from Novo Mesto Company, Slovenia, Diabetic+ GB. Group III was orally administered magnetic treated (magnetized) water for four weeks, Diabetic+ MW.
Ethical statement
All experiments were carried out in accordance with Jazan University, KSA laws and University guidelines for the care of experimental animals. The data used to support the findings of this study are included within the article.
Induction of T2D
T2D was induced by feeding the rats on a high-fat diet (20% fat, 45% carbohydrate, 22% protein) for eight weeks At the beginning of the ninth week, animals fasted for 12 hrs then injected intraperitoneally by a single dose (25 mg/kg) of streptozotocin (STZ) purchased from Sigma Chemical Co, St. Louis, MO, USA. After injection, rats were given 10% glucose for the next 24hours to avoid fatal hypoglycemia that may result from the massive pancreatic insulin release following STZ injection.Citation25 After three days, the development of diabetes was confirmed by measuring glucose levels in blood samples obtained from the tail vein. Rats with blood glucose level over 200 mg/dl were considered diabetic.
Preparation of MW
The MW was prepared by passing drinking water through our hand-made electro-magnet unit.Citation21 A transistor-controlled DC current is flowing in two coils connected in series. A potentiometer was used to control magnetic field strength. Water was pumped through a flexible tube by a water pump installed inside the unit. The distance between the magnetic coils was about 15 mm. The produced magnetic strength was 600 G (measured by WT10A Teslameter), it was uniform and perpendicular to the water flow. Water flow was at a relatively low speed (0.34 L/min) to avoid overflow. The 600G is an average strength that has been tested to cause no pathological lesions in experimental rats.Citation26
Semithin sectioning
Small tissue samples (2 mm thick) of the pancreas were processed for semithin sectioning and stained with 1% Toluidine Blue according to our published protocol.Citation27 Sections were examined and photographed with a light microscope.
Histology and immunohistochemistry
Tissue samples from the pancreas were fixed in 4% paraformaldehyde solution, dehydrated in ascending graded ethanol, embedded in paraplast. Thin sections (3-5 μm thick) were sectioned by a Leica RM 2125RT microtome. For H&E (Roche) and immunostaining, paraffin sections (3-5 µm) from the pancreas were used. We detected insulin in pancreatic β-cells, according to our previous protocolCitation28 by using the specific primary antibodies (polyclonal anti-insulin) obtained from Chongqing Biospes Co., Ltd, China. Power-StainTM 1.0 Poly HRP DAB Kit for Mouse + Rabbit was obtained from Genemed Biotechnologies, Inc, San Francisco, CA USA.
Image J software was used for histological sections analysis as well as measuring of protein expression intensities.
Statistical analysis
The data were analyzed by means of one-way analysis of variance (ANOVA) and presented as mean ± standard error. Statistical analysis was done following Student’s t-test. A difference was considered significant when P<0.05.
Results
GB and MW protect the pancreatic structure against T2D
To investigate the effect of T2D on the rat pancreatic structure, we used paraffin sections stained with H&E. We found that diabetes-induced structural changes in the Endocrine portion (islets of Langerhans) and Exocrine (Acinus) portion as well when compared to control (Ctrl). The % size of islets of Langerhans and its cellular contents reflects the healthy condition of the endocrine pancreatic system. We observed that the size of islets of Langerhans was markedly decreased in the diabetic animals compared to Ctrl (, ). Furthermore, we found a marked decrease in the cellular contents of islets of Langerhans in diabetic rats compared to Ctrl (, , , ). Using Image J software, we measured the correlation of the whole islets of Langerhans size in Ctrl and other rat groups. In addition, with image J, we counted the cellular content of islets of Langerhans. The size of islets of Langerhans was significantly decreased in diabetic pancreas compared to Ctrl. Furthermore, the cell number of diabetic islets of Langerhans was markedly decreased compare to control (). Furthermore, we have noticed that the acidic staining of the pancreatic acini in diabetic rats was markedly decreased compared to Ctrl (, ). With Image J, we measured the intensity of the acinar acidic staining and we noticed a significant decrease in the diabetic rats compared to Ctrl (, ).
Figure 1 GB and MW protective effects against the diabetic nephrotoxic effect. (A) Paraffin sections stained against H&E. (A-D) islets of Langerhans (IL) (green bordered) was decreased in size in the diabetic pancreas and comparable to control in Diabetic+GB and Diabetic+MW. Scale bar 200 µm. Magnification of pancreatic acini (E-H). Image J measurements of acinar staining intensity (B), islets of Langerhans size and its cellular content in relation to Ctrl (C). *P<0.05, **P<0.01 and ***P<0.001 vs control group.
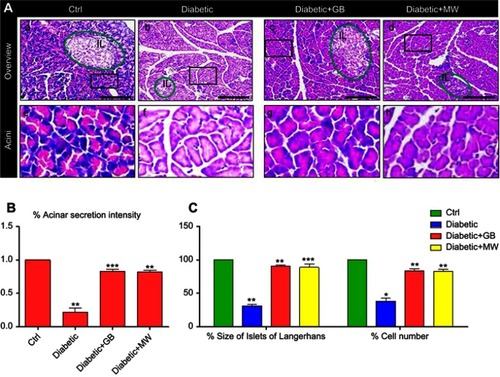
Figure 2 In the diabetic pancreas, cellular disturbances in islets of Langerhans rescued after GB and MW treatment. (A a-h) Semithin sections stained with Toluidine Blue. In diabetic rats, islets of Langerhans were smaller than Ctrl (A, B). The size of islets of Langerhans was comparable to Ctrl in GB and MW treated rats (C, D). The cells of islets of Langerhans were few in number and showed autophagy cytoplasmic vacuoles (asterisk). Scale bar 50 µm (A-D), 20 µm (E-H).
To study the protective effect of GB and MW on the pancreas, we treated diabetic rats with GB and MW. We observed that the pancreatic phenotype was partially rescued in GB and MW treated rats compare to control (, , , ). Furthermore, the islets of Langerhans (size and cellular contents) in GB and MW treated groups were nearly comparable to control (, , ). In addition, with Image J we were able to confirm the comparable changes in the size of islets of Langerhans and the cellular contents in GB and MW groups compared to Ctrl (). Furthermore, we found that the pancreatic acinar staining intensity after GB and MW treatment was significantly increased compared with the diabetic rats ().
GB and MW maintain the pancreatic structure against T2D
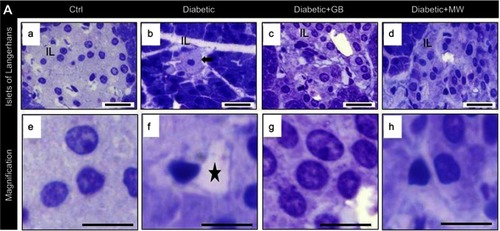
Histologically, the pancreas of the normal control group, which appears formed of an exocrine portion (pancreatic acini and ducts) and an endocrine portion (islets of Langerhans).The islets were randomly distributed amidst the pancreatic acini and were frequently neighboring the pancreatic ducts. The islets were formed of clusters or cords of cells of varying size and staining intensities. We found that, in the diabetic group, the frequency of occurrence and areas occupied by the islets of Langerhans were drastically reduced. Each islet contained few β-cells demonstrating a degenerative alteration as compared to Ctrl (, ). The degenerative changes were represented by a form of nuclear pyknosis and cytoplasmic vacuolization ( b arrow). The GB and MW treated groups (, ), however, showed more or less restoration of the normal morphology of the β- cells seen in the normal control with a comparatively better picture in GB treated group. Furthermore, the GB and MW treated groups showed less autophagy cytoplasmic vacuoles compared to the diabetic group (). Image analysis showed that the % size of islets of Langerhans and the number of ß-cells were significantly decreased in diabetic islets compared to Ctrl (). The GB and MW treated groups (, ), showed a marked protective effect which included an increase in the cell number and the % size of islets of Langerhans ().
GB and MW are able to rescue low levels of pancreatic insulin caused by T2D in rats
In order to investigate the pancreatic insulin after T2D induction, we stained against insulin in the pancreatic β-Cell. We found a marked decrease in insulin expression in diabetic pancreas compared to control ().
Figure 3 GB and MW treatment rescue insulin expression in islets of Langerhans. (A a-d) Paraffin sections stained with anti-insulin antibody. The expression of insulin was decreased in diabetic pancreas and back to almost normal after the use of GB and MW compare to Ctrl. Scale bar 100 µm. Image J analysis displayed a significant decrease of insulin expression intensity in diabetic islets of Langerhans compared to Ctrl. In Diabetic+GB and Diabetic+MW pancreas, insulin protein expression intensity was increased to be comparable with Ctrl (B). *P<0.05, **P<0.01 and ***P<0.001 vs control group.
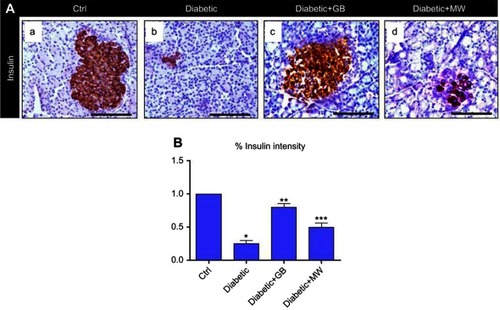
Image analysis using Image J showed that the intensity of the insulin expression was significantly decreased up to 10 folds in diabetic pancreas compared to Ctrl ().
Interestingly, insulin expression was almost comparable to control after GB and MW treatment (, ). Image J analysis confirmed the induction in the intensity of the insulin after GB and MW treatment ().
Altogether, GB and MW treatments were able to induce insulin expression in diabetic pancreas.
GB and MW treatment decreased diabetic effect on the pancreatic glutathione reductase and SOD2 protein expression
In order to investigate the pancreatic oxidative stress after T2D induction, we stained against glutathione reductase and SOD2 antibodies.
We found a marked increase in glutathione reductase and SOD2 expressions in diabetic pancreas compared to control (, , respectively).
Figure 4 GB and MW protective effect against oxidative stress induced by type 2 diabetes. (A a-d) Paraffin sections stained with anti-glutathione reductase antibody. The expression of glutathione reductase was increased in diabetic pancrease and back to almost normal after the use of GB and MW. Furthermore, (A e-h) Paraffin tissue sections stained with anti SOD2 antibody. SOD2 expression was intensively increased in diabetic pancreas while with the use of GB and MW was comparable with control. Scale bar 100 µm. Image J analysis displayed a significant increase of glutathione reductase and SOD2 intensities in diabetic pancreas compared to Ctrl. In Diabetic+GB and Diabetic+MW pancreas, glutathione reductase and SOD2 protein expression intensities were decreased to be comparable with Ctrl (B). *P<0.05, **P<0.01 and ***P<0.001 vs control group.
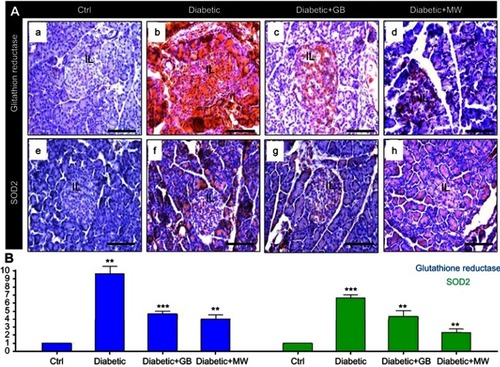
Image analysis using Image J showed that the intensity of the glutathione reductase expression was significantly increased up to 10 folds in STZ-treated pancreas compared to Ctrl (). Furthermore, we noticed a significant increase in SOD2 intensity up to 6.6 folds in diabetic pancreas compared to control ().
Interestingly, glutathione reductase (, ) and SOD2 (, ) expressions were almost comparable to control after GB and MW treatment. Image J analysis confirmed the reduction in the intensity of the glutathione reductase and SOD2 after GB and MW treatment ().
Altogether, GB and MW treatments were able to reduce the oxidative stress in the diabetic pancreas.
Discussion
In this study, we investigated the expected effects of the extract of GB and MW in the restoration of β-cell mass and amelioration of their functions after induction of T2D in rats. Our results verified a dramatic pancreatic (β-cells and acinar cells) failure in non-treated diabetic (positive control) rats. β-cells showed a marked pyknosis with signs of autophagy and apoptosis. These findings coincide with the data has been published previously,Citation29 it was reported that β-cell failure in T2D occurs when islets were unable to sustain β-cells compensation as a result of insulin resistance. Furthermore, the failure is progressive, particularly after hyperglycemia was established, where β-cells become poorly functioning, de-differentiated and apoptotic.Citation29
In the current study, diabetic rats showed a drastic decrease in acinar staining intensity reflecting the defect in the pancreatic digestive effect which could explain the decreased body weight of diabetic rats. Furthermore, diabetic rats showed a drastic decrease of β-cells masses and staining intensity of acinar cells. Moreover, insulin granules and their intensity in β-cells, as shown by immunohistochemistry, were downregulated in these animals. In this concern, it was reported that T2D starts by increasing insulin resistance and then β-cells will undergo apoptosis or necrosis.Citation14,Citation29,Citation30 In our diabetic rat, β-cells succumbed necrotic changes (cytoplasmic vacuolation and nuclear pyknosis) with a subsequent apoptosis and/or autophagy. Autophagy has been reported to play an important role in pancreatic β-cell dysfunction and insulin resistance in T2D.Citation31 Autophagy was stimulated at the beginning of T2D as a protective mechanism for β-cellCitation32 and then the accumulation of autophagosomes in β-cells will lead to cellular damage and apoptosis.Citation33,Citation34
The β-cell dysfunction and failure of insulin secretory capacity in T2D could be attributed to glucotoxicity, lipotoxicity and/or oxidative stress.Citation29,Citation35,Citation36 Our observation of hyperglycemia in diabetic rats,Citation21 could be attributed to exhaustion and failure of β-cells. A similar explanation has been suggested by Wang J & Wang H (2017), who mentioned that hyperglycemia leads to glucotoxicity to β-cells and induction of their apoptosis or necrosis. Reduction of serum glucose levels, however, has been supposed to increase survival of β-cells,Citation37,Citation38 a suggestion that agrees with our findings where GB and MW treatments reduced blood glucoseCitation21 and increased β-cells survivals.
Our suggestion that the damage of β-cells results from dyslipidemia and lipotoxicity was concomitant with what has been mentioned before.Citation38–Citation40 The latter authors added that elevation of triglycerides leads to elevation of free fatty acids which causes lipotoxicity that impairs the survivals of β-cells, insulin secretion and subsequently damages β-cells.
Even more, our previous observation of the marked improvement of dyslipidemic and high glucose status in diabetic rat,Citation21 could be attributed to our current findings of the ameliorating effects of GB and MW on the pancreatic β-cells. Similarly, it was reported that GB reduced hyperlipidemiaCitation41 that has been suggested to be due to its content of flavonoid components.Citation42 The MW has been also reported to have a powerful hypolipidemic action in T2D.Citation43 However, in our present study the improvement of both morphological picture of β-cells as well as serum glucose level and the lipid profile as revealed in our previous,Citation21 were comparatively better in GB treated rats than those treated with MW.
A possible factor for destroying β-cell function is the increasing free radical or oxidative stress that accompanies T2D.Citation39 Immunohistochemically, our findings demonstrated an overexpression of antioxidant enzymes; glutathione peroxidase and SOD2 in pancreatic islets of diabetic rat. These results coincide with Wang J & Wang H (2017), who reported that hyperglycemia, hyperlipidemia, hypoxia, and endoplasmic reticulum (ER) stress lead to ROS generation in β-cells. Hyperglycemia, in particular, can be directly associated with increased ROS generation.Citation39 In chronic hyperglycemia, β-cells are exposed to high glucose concentrations for long time, where the normal route of glycolysis gets saturated and excess glucose is shifted towards alternative ROS-forming pathways including glycosylation,Citation44 glucose autoxidation,Citation45,Citation46 and glucosamine pathway,Citation47 all of them lead to the accumulation of ROS and induction of oxidative stress. Furthermore, increase ROS production has been reported to decreases β-cell mass.Citation48
According to Mancini et al (2018), the total antioxidant capacity of the diet may play a role in reducing the risk of T2D as well as its complication. Our results demonstrated that treatment with GB or MW ameliorated antioxidant status in β-cells and hence downregulated antioxidants enzymes; glutathione and SOD2. We suggest that both treatments scavenge ROS with a subsequent decrease in the expression of both enzymes in pancreatic islets. In the same context, GB has been suggested to scavenge free radicals in vivo.Citation22 The currently increased survival of β-cells could be also attributed to the amelioration of the antioxidant status in these cells.
In conclusion, treatment with GB or MW protects pancreatic exocrine and endocrine systems against the damaging effect of T2D in rats.
Disclosure
The authors report no conflicts of interest in this work.
References
- Gaba R , Gambhire D , Uy N , et al. Factors associated with early relapse to insulin dependence in unprovoked A-beta+ ketosis-prone diabetes. J Diabetes Complications . 2015;29(7):918–922. doi:10.1016/j.jdiacomp.2015.04.013 26071380
- Hetta HF , Elkady A , Morsy KH , Mohamed IS , Ibrahim MA . Serum level of IL17a among cirrhotic hepatitis C virus infected patients with incidence of diabetes mellitus. Egypt J Immunol . 2017;24(1):79–88.29120580
- El-Deeb TS , Bakkar SM , Eltoony L , et al. The adipokine chemerin and fetuin-A serum levels in type 2 diabetes mellitus: relation to obesity and inflammatory markers. Egypt J Immunol . 2018;25(1):191–202.30243011
- Hetta HF , Ez-Eldeen ME , Mohamed GA , et al. Visfatin serum levels in obese type 2 diabetic patients: relation to proinflammatory cytokines and insulin resistance. Egypt J Immunol . 2018;25(2):141–151.30600957
- Hetta HF , Elkady A , Meshaal AK . TH17/TH1 role in endocrine disorders among chronic HCV infected patients. Int J Curr Microbiol Appl Sci . 2017;6(8):2542–2551. doi:10.20546/ijcmas
- Hetta HF , Azza Elkady KH , Morsy IS , et al. Circulating IL17a and IFN-gamma serum levels in cirrhotic hepatitis C virus infected patients with autoimmune thyroiditis. Int J Curr Microbiol Appl Sci . 2017;6(3):1972–1983. doi:10.20546/ijcmas.2017.603.225
- Elkady H . Promising epigenetic approaches targeting TH17 in autoimmune diabetes among chronic hepatitis C infection. Int J Curr Microbiol Appl Sci . 2017;6(8):2362–2368. doi:10.20546/ijcmas.2017.608.279
- Kissebah AH , Vydelingum N , Murray R , et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab . 1982;54(2):254–260. doi:10.1210/jcem-54-2-254 7033275
- U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective diabetes study group. Diabetes . 1995;44(11):1249–1258.7589820
- Weyer C , Bogardus C , Mott DM , Pratley RE . The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest . 1999;104(6):787–794. doi:10.1172/JCI7231 10491414
- Kahn SE . The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia . 2003;46(1):3–19. doi:10.1007/s00125-002-1009-0 12637977
- Butler AE , Janson J , Bonner-Weir S , Ritzel R , Rizza RA , Butler PC . Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes . 2003;52(1):102–110. doi:10.2337/diabetes.52.1.102 12502499
- Hanley SC , Austin E , Assouline-Thomas B , et al. {beta}-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology . 2010;151(4):1462–1472. doi:10.1210/en.2009-1277 20176718
- Lim EL , Hollingsworth KG , Aribisala BS , Chen MJ , Mathers JC , Taylor R . Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia . 2011;54(10):2506–2514. doi:10.1007/s00125-011-2204-7 21656330
- Zahran AM , Sayed SK , Abd El Hafeez HA , Khalifa WA , Mohamed NA , Hetta HF . Circulating microparticle subpopulation in metabolic syndrome: relation to oxidative stress and coagulation markers. Diabetes Metab Syndr Obes . 2019;12:485–493. doi:10.2147/DMSO.S191750 31043798
- Anwar MM , Meki AR . Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp Biochem Physiol A Mol Integr Physiol . 2003;135(4):539–547. doi:10.1172/JCI29103 12890544
- Maritim AC , Sanders RA , Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol . 2003;17(1):24–38. doi:10.1002/jbt.10058 12616644
- Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 2005;59(7):365-373. doi: 10.1016/j.biopha.2005.07.002
- El-Missiry MA . Antioxidant enzyme. intechopen . 2012;7(ISBN978):51.
- Mancini FR , Affret A , Dow C , et al. Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia . 2018;61(2):308–316. doi:10.1007/s00125-017-4489-7 29119242
- Zayed AE , Saleh A , Gomaa AMS , et al. Protective effect of ginkgo biloba and magnetized water on nephropathy in induced type 2 diabetes in rat. Oxid Med Cell Longev . 2018;2018:1785614.29991974
- Raafat BM , Saleh A , Shafaa MW , Khedr M , Ghafaar AA . Ginkgo biloba and Angelica archangelica bring back an impartial hepatic apoptotic to anti-apoptotic protein ratio after exposure to technetium 99mTc. Toxicol Ind Health . 2013;29(1):14–22. doi:10.1177/0748233711433938 22294442
- Lee HJ , Kang MH . Effect of the magnetized water supplementation on blood glucose, lymphocyte DNA damage, antioxidant status, and lipid profiles in STZ-induced rats. Nutr Res Pract . 2013;7(1):34–42. doi:10.4162/nrp.2013.7.1.34 23423956
- Kadim KK . Effect of magnetic water on some physiological aspects of adult male rabbits. Proc Eleventh Vet Sci Conf . 2012;2012:120–126.
- Saddala RR , Thopireddy L , Ganapathi N , Kesireddy SR . Regulation of cardiac oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with aqueous extract of Pimpinella tirupatiensis tuberous root. Exp Toxicol Pathol . 2013;65(1–2):15–19. doi:10.1016/j.etp.2011.05.003 21640568
- Al-Saffar SF , Amer N , Zaki LS , et al. Effect of magnetized water on histological structure of heart, lung and spleen of albino rats. J Al-Nahrain Uni Sci . 2013;16(4):152–160. doi:10.22401/JNUS.16.4.18
- Kotb AM , Abd-Elkareem M , Abou Khalil NS , Sayed AEH . Protective effect of Nigella sativa on 4-nonylphenol-induced nephrotoxicity in clarias gariepinus (Burchell, 1822). Sci Total Environ . 2018;619–620:692–699. doi:10.1016/j.scitotenv.2017.11.131
- Kotb AM , Simon O , Blumenthal A , et al. Knockdown of apoL1 in zebrafish larvae affects the glomerular filtration barrier and the expression of nephrin. PLoS One . 2016;11(5):e0153768. doi:10.1371/journal.pone.0153768 27138898
- Prentki M , Nolan CJ . Islet beta cell failure in type 2 diabetes. J Clin Invest . 2006;116(7):1802–1812. doi:10.1172/JCI29103 16823478
- Wang J , Wang H . Oxidative stress in pancreatic beta cell regeneration. Oxid Med Cell Longev . 2017;2017:1930261. doi:10.1155/2017/1930261 28845211
- Yang JS , Lu CC , Kuo SC , et al. Autophagy and its link to type II diabetes mellitus. Biomedicine (Taipei) . 2017;7(2):8. doi:10.1051/bmdcn/2017070201 28612706
- Rivera JF , Costes S , Gurlo T , Glabe CG , Butler PC . Autophagy defends pancreatic beta cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest . 2014;124(8):3489–3500. doi:10.1172/JCI71981 25036708
- Fujitani Y , Kawamori R , Watada H . The role of autophagy in pancreatic beta-cell and diabetes. Autophagy . 2009;5(2):280–282. doi:10.4161/auto.5.2.7656 19158492
- Las G , Shirihai OS . The role of autophagy in beta-cell lipotoxicity and type 2 diabetes. Diabetes Obes Metab . 2010;12(Suppl 2):15–19. doi:10.1111/j.1463-1326.2010.01268.x 21029295
- Chang-Chen KJ , Mullur R , Bernal-Mizrachi E . Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord . 2008;9(4):329–343. doi:10.1007/s11154-008-9101-5 18777097
- Demirtas L , Guclu A , Erdur FM , et al. Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus. Indian J Med Res . 2016;144(4):515–524. doi:10.4103/0971-5916.200887 28256459
- Ramos S , Martin MA , Goya L . Effects of cocoa antioxidants in type 2 diabetes mellitus. Antioxidants (Basel) . 2017;6(4):84. doi: 10.3390/antiox6040084
- Weir GC , Bonner-Weir S . Islet beta cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci . 2013;1281:92–105. doi:10.1111/nyas.12031 23363033
- Gerber PA , Rutter GA . The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal . 2017;26(10):501–518. doi:10.1089/ars.2016.6755 27225690
- Morgan D , Rebelato E , Abdulkader F , et al. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology . 2009;150(5):2197–2201. doi:10.1210/en.2008-1149 19147679
- Ibrahim AE , Fadhil DH , Jawad AH , et al. Ginkgo biloba for reducing hyperlipideamia case study. Preprints . 2016; 2016110134. doi: 10.20944/preprints201611.0134.v1
- Jung IH , Lee YH , Yoo JY , et al. Ginkgo biloba extract (GbE) enhances the anti-atherogenic effect of cilostazol by inhibiting ROS generation. Exp Mol Med . 2012;44(5):311–318. doi:10.3858/emm.2012.44.5.035 22282402
- Ali Ebrahim S . biological effects of magnetic water on human and animals. Biomed Sci . 2017;3(4):78. doi:10.11648/j.bs.20170304.12
- Baynes JW . Role of oxidative stress in development of complications in diabetes. Diabetes . 1991;40(4):405–412. doi:10.2337/diab.40.4.405 2010041
- Hunt JV , Smith CC , Wolff SP . Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes . 1990;39(11):1420–1424. doi:10.2337/diab.39.11.1420 2227114
- Wolff SP , Dean RT . Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J . 1987;245(1):243–250. doi:10.1042/bj2450243 3117042
- Kaneto H , Xu G , Song KH , et al. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem . 2001;276(33):31099–31104. doi:10.1074/jbc.M104115200 11390407
- Solaini G , Baracca A , Lenaz G , Sgarbi G . Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta . 2010;1797(6–7):1171–1177. doi:10.1016/j.bbabio.2010.02.011 20153717
