Abstract
Background
Diabetic foot ulcer (DFU) is the main risk factor for nontraumatic lower-limb amputation. We hypothesized that by reversing the offending local tissue factors resulting from the low tissue supply of oxygen, inefficient fuel metabolism and acidosis, we can eradicate the infection and help to promote healing. This might be enhanced with the help of an innovated local preparation (PEDYPHAR®) through its enriched alkaline ointment base and the regenerating growth factors of Royal Jelly (RJ) plus the antimicrobial, immune-modulatory nutritional and other biochemical properties of RJ and Panthenol. We conducted this study to test the safety and efficacy of PEDYPHAR ointment as an adjuvant in limb salvage management for patients with limb-threatening diabetic foot wounds.
Methods
A prospective, randomized, controlled open-label study design with a mean follow-up period of 12 weeks. One hundred and nineteen eligible patients with diabetic foot wounds presenting to 3 outpatient clinics in Egypt were randomized to be treated with the local application of either PEDYPHAR or Panthenol ointment under dressing after conservative debridement of necrotic tissue and irrigation with warm normal saline.
Results
At the end of the 12-week follow-up period, PEDYPHAR showed a higher rate of complete healing of limb-threatening wounds in the intent-to-treat population, 11 of 34 (32.4%) in PEDYPHAR-treated group versus 3/25 (12%) in the Panthenol-treated (control) group (p=0.034* [*indicates it is statistically significant]).
Conclusion
We can conclude that PEDYPHAR could be an effective and safe conservative local adjuvant treatment for cases of diabetic foot infection.
Registration number in ClinicalTrials.gov: NCT01531517.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Diabetic patients have more than 25% lifetime risk of developing foot complicationsCitation1 and 30-fold higher lifetime risk of undergoing lower-limb amputations than nondiabetics.Citation2
Diabetic foot ulcers (DFU) are the main risk factor for nontraumatic lower-limb amputations, up to 60% of which occur in diabetics.Citation3
Peripheral neuropathy, macro/micro-angiopathy and infection often combine together to predispose an individual to develop diabetic foot ulceration and complications.Citation4,Citation5
Though evaluation of neuropathy, angiopathy and infection are standard procedures for DFU management, yet none of them can predict wound healing.Citation6
The only two validated methods to predict wound healing are the measured changes in ulcer area over 4 weeks of treatmentCitation7 and the measured tissue oxygenation level.Citation8
Whether the primary culprit is neuropathy, macrovascular or microvascular disease, tissues with low oxygenation levels are liable not to heal.
We assumed that the local tissue hypoxia with reduced aerobic metabolism will impair local energy production leading to local tissue acidosis and low tissue viability. This will provide the suitable local milieu for tissue ulceration, infection and nonhealing that could progress to limb threatening and amputation. Hence, we hypothesized that, by reversing these local offending factors in the wound, we can eradicate infection and promote healing. This might be facilitated through our enriched alkaline ointment base, the regenerating growth factors of Royal Jelly (RJ) and the antimicrobial, immuno-modulatory nutritional and biochemical properties of RJ and Panthenol.
Based on our hypothesis, a new patented local ointment PEDYPHAR® composed of natural RJ and Panthenol, in an innovated, enriched alkaline base, has been developed by our team and has shown effect in in-vitro and in pilot clinical studies.
RJ is a protein-rich excretion from the glands of worker honey-bees. It is highly nutritious to tissues and rich in sterols, phosphorus compounds, acetylcholine, growth factors and many other highly bioactive compounds.Citation9–Citation19 Panthenol is the alcohol analog of pantothenic acid and has the equivalent biological activity. It is metabolized in cells to pantothenic acid, which is a major constituent of coenzyme A.Citation20
Limb-threatening diabetic foot infection (DFI) is defined as cellulitis extending beyond 2 cm from the ulcer perimeter, as well as deep abscess, osteomyelitis or critical ischemia.Citation6
This study aimed to test the safety and efficacy of PEDYPHAR ointment as a limb salvage treatment in patients with limb-threatening diabetic foot wounds.
Patients and methods
Design
A prospective, multicenter, open-label, randomized, controlled study with 12-week follow-up period.
Patients
The study protocol had been prepared by the research team and approved by the local research ethics committees of Alexandria Faculty of medicine (IRB00007555) and Green CRC (IRB00008268), in accordance with Helsinki declarations. It was registered in ClinicalTrials.gov with the registration number NCT01531517.
One hundred and nineteen consecutive eligible adult male or female patients presenting with limb-threatening diabetic foot ulcerations to 3 clinical study sites in Egypt, Alexandria main university hospital, Alexandria health insurance hospital and Green CRC, were included in the study after signing the informed consent. The main exclusion criteria were life-threatening extensive gangrenous lesions that needed immediate amputations; bad general condition; shock or unstable vital signs; critically ill with severe organ/system dysfunctions or advanced malignancy. Patients with low level of consciousness and those deemed to be incapable to comply with the instructions of treatment application and follow-up visits as well as those who refused to participate in the study or to sign the informed consent form were excluded from the study.
Procedures
All patients were subjected to full medical history taking and examination including assessment of wound severity grading using the Wagner wound classification system.Citation21 Laboratory investigations included complete blood count, erythrocyte sedimentation rate, liver and kidney function tests and monitoring for fasting and postprandial glucose for the control of blood glucose.
Wound infection was defined clinically, by the criteria of the International Working Group on Diabetic Foot last updated in 2019.Citation22 DFI definition is based on the presence of evidence of inflammation of any part of the foot, not just an ulcer or wound or findings of the systemic inflammatory response. Diagnosis of DFI is based on the presence of at least 2 of the following clinical criteria in absence of any other specific causes of skin inflammation: local swelling or induration; erythema >0.5 cm around the wound; local tenderness or pain; local increased warmth; purulent discharge.
Probe-to-bone test was done for each patient to test for osteomyelitis.Citation22 Lower-limb vascular insufficiency was diagnosed clinically on the basis of the absence of pedal pulses and/or an ankle-brachial pressure index of <0.9.Citation23 Presence of neuropathy was assessed by vibration (128 Hz) and pressure perception at great toe.Citation24
Interventions
We grouped the included patients according to the severity of lesions into 2 clinical severity groups: Group 1 (n=61), superficial ulcers (Wagner 0 and 1), and Group 2 (n=58), limb-threatening lesions with deep tissue infection or osteomyelitis (Wagner 2, 3 or 4). All patients were subjected to thorough wound debridement but with limited conservative excision of gangrenous and nonviable tissue. Patients were then randomized to either PEDYPHAR-treated group or Panthenol-treated (control) group by a centralized software generated block randomization technique and randomization sequence was concealed in sealed opaque envelopes. PEDYPHAR is manufactured by Pharco. Panthenol was prepared as dexpanthenol 5% in an ointment base. Both treatment groups were subjected to wound cleansing and irrigation with normal saline followed by the local application of sufficient covering amount of either ointment. Lesions were occluded with sterile dressings. Patients were then treated in an out-patient setting and asked to revisit the clinic at least 2 times weekly for the same local wound treatment to be repeated for a minimum period of 12 week follow-up. Patients were followed up with regular visits according to fixed time schedules and contacted by phone in case of any delays or noncompliance to time schedules. Hyperglycemia was controlled in both groups with insulin therapy.
No local antiseptics or antibiotics were used but systemic empirical antibiotics coverage in the form of ceftazidime + amoxicillin-flucloxacillin intramuscular injections was planned for any patient with manifestations of septicemia. Modifications of antibiotics according to the results of bacterial culture and sensitivity test in case of no clinical improvement with the empirical treatment.
Outcome measures
Efficacy end point
The primary efficacy end point is comparison of the proportions of complete healing (in intent-to-treat analysis) at the 12th week visit between the two treatment groups. We defined clinical response as the followings: complete healing, defined as complete closure of the ulcer without signs of underlying bone infection; partial healing, defined as marked reduction of cellulitis and ≥50% incomplete closure of the ulcer or closure of the ulcer with persistent signs of infection; treatment failure, defined as the absence of significant clinical improvement or amputation due to aggravated/persistent infection (after 3 weeks) of treatment.
Safety endpoint
Any adverse event reported by patients or observed by investigators during the study visits or any deviation from a baseline normal laboratory test, occurring after administration of the first dose of study drugs until 30 days after the last dose, was considered a treatment-emergent adverse event. These were further evaluated by investigators for causality using the Uppsala Monitoring Center causality categorization. Adverse events deemed to have certain, probable or possible causality category were considered in analysis and graded according to seriousness (serious/nonserious) and severity using the Common Terminology Criteria for Adverse Events v3.0 into grades 1 (mild), 2 (moderate), 3 (severe), 4 (life threatening) or 5 (death related).
Well-trained clinical research associates were closely monitoring and double-checking all the data and source documents, photos of lesions were taken at each visit and the principal investigator review all data regularly.
Statistical methods
We presented our qualitative data as counts, proportions or percentage with confidence interval. For quantitative data, descriptive statistics are the arithmetic mean, the standard deviation, the median and the 95% confidence interval whenever found appropriate.
Sample sizes of 60 in each treatment group achieve 87% power to detect a difference between the group proportions of 0.204. The proportion in group one (PEDYPHAR group) is assumed to be 0.12 under the null hypothesis and 0.324 under the alternative hypothesis. The proportion in group two (Panthenol group) is 0.12. The test statistic used is the one-sided Mantel–Haenszel test. The significance level of the test was targeted at 0.05.
Results
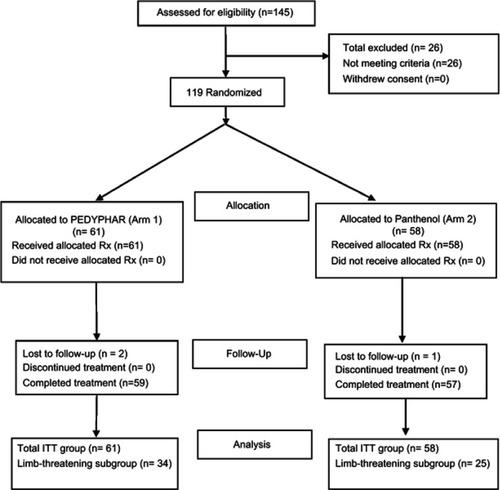
One hundred and nineteen eligible assessable patients were included in the study and randomized to 2 treatment groups. demonstrates the patients’ flowchart throughout the study.
The baseline characteristics of both groups were almost comparable ().
Table 1 Baseline characteristics
The rate of complete healing according to clinical severity in each treatment arm is presented in .
Table 2 Complete healing rate in each treatment group
Because the primary outcome measure (healing rate) is affected in both treatment arms by the natural healing process over time. This could alone contribute majorly to the healing of superficial ulcers (clinical severity group 1 (Wagner Grade 1)), who all (100%) showed treatment success in both treatment arms. This underestimates/confounds the pure salvaging effect size contributed by any experimental drug as compared to any comparator. While, comparison of efficacy in complete healing of cases with higher severity grades (clinical severity group 2) will be more sensitive to measure the salvaging effect of the experimental drug as compared to control. PEDYPHAR treatment was shown to be significantly superior to the comparator in the proportion of complete healing in the clinical severity group 2 (limb-threatening lesions (Wagner Grade 2 and above)). The one-sided tailed test of significance comparing the 2 groups for the rate of complete healing in the limb-threatening cases was statistically significant (p=0.034*) ().
Table 3 Comparison between treatments in the limb-threatening subgroups at 12 weeks
Safety data
No fatalities or serious adverse events attributable to the study drugs were reported throughout the study. Mild local irritation and pruritus were reported by 6 patients in PEDYPHAR group (9.8%) and 4 (6.9%) in the control group.
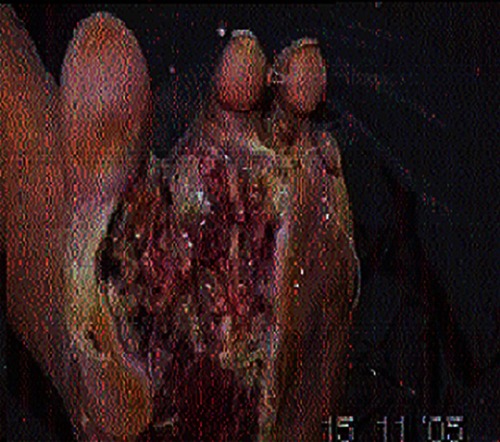
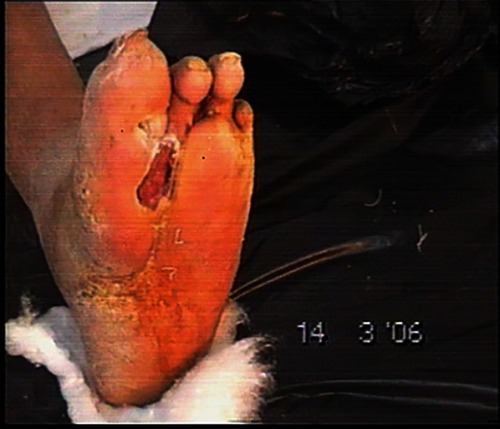
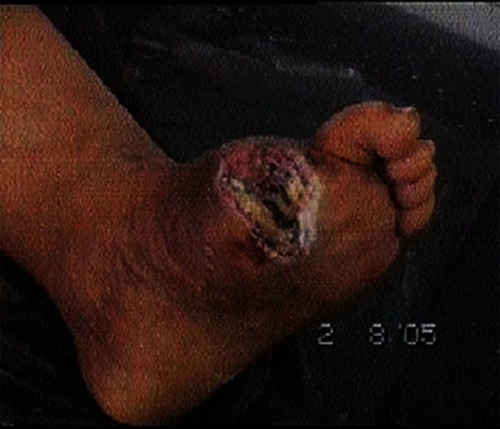
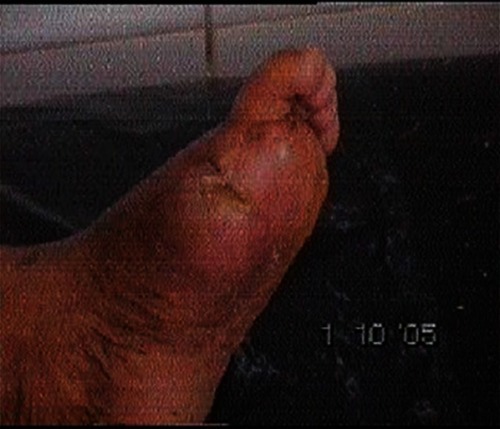
– demonstrate the foot lesions of 2 of our patients before and after treatment with PEDYPHAR ointment.
Discussion
In spite of the short follow-up duration, complete healing in the limb-threatening cases was observed in 32.4% of PEDYPHAR-treated group which was significantly higher than that in the control group (12%). Although there is conflicting data in human clinical trials for the efficacy of RJ as mono-component in the treatment of DFUs,Citation10–Citation13 the complementary/synergistic actions of PEDYPHAR multicomponents had been studied only in a single observational study with a high success rate.Citation13 There is a plethora of published studies in the literature that confirms the favorable wide spectral bioactivity of RJ and Panthenol.
RJ had been demonstrated to exhibit a powerful antibacterial activity which was reported to be 20 times more potent than natural pure honey.Citation14 Through its content of “Royalisin”, it is effective against Gram +ve bacteria including methicillin-resistant Staphylococcus aureus at very low concentrations. Whereas, the 10-hydroxydecenoic acid, which is another bioactive constituent in RJ, had demonstrated strong antimicrobial, neurogenic and angiogenic activities.Citation14,Citation15
RJ was shown to facilitate in-vitro the differentiation of all types of nerve cells (neurons, astrocytes and dendrocytes) and increases the mRNA expression of the potent glial cell line-derived neurotrophic factor.Citation16 Through its active compound, “AMP N1-oxide”, RJ facilitates neurogenesis by stem cells through activation of signal transducer and activator of transcription 3.Citation17 RJ stimulates the production of type I collagen and bone formation through action on osteoblasts.Citation18 The expression of vascular endothelial growth factor gene was found to be stimulated by subcutaneous injection of RJ in experimental animals.Citation19
Panthenol enhanced oxidative phosphorylation of pyruvate and fatty acid carnitine esters and increased the activity of carnitine palmitoyltransferase.Citation20
We acknowledge the limitations of the small sample size and the short follow-up duration. But this proof of concept study could be a foundation to invite other investigators to conduct further studies. Further large-sized studies with longer follow-up durations and including the quality of life as an outcome measure are recommended.
Conclusion
We can conclude from this study that PEDYPHAR could be an effective and safe conservative local adjuvant treatment for cases of DFI.
Data sharing statement
Authors are willing to share some of the unidentified photos of some patients’ lesions before and after treatment. This could be uploaded to the journal site.
Author contributions
Yakoot M and Abdelatif M conceived the idea and designed the study. Helmy S formulated the study medication. Yakoot M and Abdelatif M from Alexandria Faculty of Medicine collected the clinical data. Yakoot M prepared the first draft of the paper. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The product PEDYPHAR used in this study has been manufactured by Pharco. We acknowledge the help of coworkers from Alexandria University in collecting the data.
Disclosure
MY had conducted clinical studies for Pharco. SH is employeed by and holds stocks in Pharco. The authors report no other conflicts of interest in this work.
References
- Singh N , Armstrong DG , Lipsky BA . Preventing foot ulcers in patients with diabetes. JAMA . 2005;293:217–228. doi:10.1001/jama.293.2.217 15644549
- Lavery LA , Ashry HR , van Houtum W , Pugh JA , Harkless LB , Basu S . Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care . 1996;19:48–52. doi:10.2337/diacare.19.1.48 8720533
- Lavery LA , Armstrong DG , Wunderlich RP , Mohler MJ , Wendel CS , Lipsky BA . Risk factors for foot infections in individuals with diabetes. Diabetes Care . 2006;29(6):1288–1293. doi:10.2337/dc05-2425 16732010
- De Lalla F , Pellizzer G , Strazzabosco M , et al. Randomized prospective controlled trial of recombinant granulocyte colony-stimulating factor as adjunctive therapy for limb-threatening diabetic foot infection. Antimicrob Agents Chemother . 2001;45(4):1094–1098. doi:10.1128/AAC.45.4.1094-1098.2001 11257020
- Young MJ , Boulton AJ , MacLeod AF , Williams DR , Sonksen PH . A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia . 1993;36(2):150–154.8458529
- American Diabetes Association. Consensus development conference on diabetic foot wound care: 7–8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care . 1999;22(8):1354–1360. doi:10.2337/diacare.22.8.1354 10480782
- Sheehan P , Jones P , Caselli A , Giurini JM , Veves A . Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care . 2003;26(6):1879–1882. doi:10.2337/diacare.26.6.1879 12766127
- Khaodhiar L , Dinh T , Schomacker KT , et al. The use of medical hyperspectral technology to evaluate microcirculatory changes in diabetic foot ulcers and to predict clinical outcomes. Diabetes Care . 2007;30(4):903–910. doi:10.2337/dc06-2209 17303790
- Kodai T , Umebayashi K , Nakatani T , Ishiyama K , Noda N . Compositions of royal jelly II. Organic acid glycosides and sterols of the royal jelly of honeybees (Apis mellifera). Chem Pharm Bull . 2007;55(10):1528–1531. doi:10.1248/cpb.55.1528 17917301
- Maier HM , Ilich JZ , Kim JS , Spicer MT . Nutrition supplementation for diabetic wound healing: a systematic review of current literature. Skinmed . 2013;11(4):217–224.24053007
- Siavash M , Shokri S , Haghighi S , Shahtalebi MA , Farajzadehgan Z . The efficacy of topical royal jelly on healing of diabetic foot ulcers: a double-blind placebo-controlled clinical trial. Int Wound J . 2015;12(2):137–142. doi:10.1111/iwj.12063 23566071
- Siavash M , Shokri S , Haghighi S , Mohammadi M , Shahtalebi MA , Farajzadehgan Z . The efficacy of topical Royal Jelly on diabetic foot ulcers healing: A case series. J Res Med Sci . 2011;16(7):904–909.22279458
- Abdelatif M , Yakoot M , Etmaan M . Safety and efficacy of a new honey ointment on diabetic foot ulcers: a prospective pilot study. J Wound Care . 2008;17(3):108–110. doi:10.12968/jowc.2008.17.3.28667 18376651
- Salazar-Olivo LA , Paz-González V . Screening of biological activities present in honeybee (Apis mellifera) royal jelly. Toxicol In Vitro . 2005;19(5):645–651. doi:10.1016/j.tiv.2005.03.001 15893447
- Hattori N , Nomoto H , Fukumitsu H , Mishima S , Furukawa S . Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed Res . 2007;28(5):261–266.18000339
- Hashimoto M , Kanda M , Ikeno K , et al. Oral administration of royal jelly facilitates mRNA expression of glial cell line-derived neurotrophic factor and neurofilament h in the hippocampus of the adult mouse brain. Biosci Biotechnol Biochem . 2005;69(4):800–805. doi:10.1271/bbb.69.800 15849420
- Hattori N , Nomoto H , Fukumitsu H , Mishima S , Furukawa S . AMP N1-oxide potentiates astrogenesis by cultured neural stem/progenitor cells through STAT3 activation. Biomed Res . 2007;28(6):295–299.18202519
- Narita Y , Nomura J , Ohta S , et al. Royal jelly stimulates bone formation: physiologic and nutrigenomic studies with mice and cell lines. Biosci Biotechnol Biochem . 2006;70(10):2508–2514. doi:10.1271/bbb.60240 17031045
- Miyata T . Pharmacological basis of traditional medicines and health supplements as curatives. J Pharmacol Sci . 2007;103(2):127–131.17287592
- Naruta EE , Egorov AI , Omel’ianchik CN , Buko VU . The influence of panthotenic acid mitochondrial oxidation and oxidative phosphorylation in liver of rats with alimentary obesity. Vopr Pitan . 2004;73(4):3–7.
- Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle . 1981;2:64–122. doi:10.1177/107110078100200202 7319435
- Lipsky BA , Senneville E , Abbas ZG , et al. IWGDF Guideline on the diagnosis and treatment of foot infection in persons with diabetes. Diabetes Metab Res Rev . 2019; in press. Available online from: https://iwgdfguidelines.org/wp-content/uploads/2019/05/05-IWGDF-infection-guideline-2019.pdf. Accessed May 25, 2019.
- Apelqvist J , Castenfors J , Larsson J , Stenström A , Agardh CD . Prognostic value of systolic ankle and toe blood pressure levels in outcome of diabetic foot ulcer. Diabetes Care . 1989;12(6):373–378. doi:10.2337/diacare.12.6.373 2731457
- Davis EA , Jones TW , Walsh P , Byrne GC . The use of biothesiometry to detect neuropathy in children and adolescents with IDDM. Diabetes Care . 1997;20(9):1448–1453. doi:10.2337/diacare.20.9.1448 9283795