Abstract
Nowadays, obesity and related comorbidities like type 2 diabetes, hypertension, dyslipidaemia and obstructive sleep apnoea syndrome are considered one of the medical challenges of the 21st century. Even with the rise of bariatric and metabolic surgery, obesity and metabolic syndrome are reaching endemic proportions. Even in 2020, obesity is still a growing problem. There is increasing evidence that next to bariatric surgery, exercise interventions in the perioperative period could give extra beneficial effects. In this regard, effects on anthropometrics, cardiovascular risk factors and physical fitness. The aim of this review is to summarise effects of preoperative and postoperative exercise, tools for screening and directions for future research and implementations.
Introduction
Obesity and related comorbidities such as type 2 diabetes (T2DM), hypertension, dyslipidaemia and obstructive sleep apnoea syndrome (OSAS) are considered one of the medical challenges of the 21st century.Citation1,Citation2 Even with the rise of bariatric and metabolic surgery, obesity and metabolic syndrome are reaching endemic proportions and the proportion of the population with morbid obesity increases every year.Citation3 Dietary and exercise interventions alone are not entirely successful in reaching significant and longstanding weight loss.Citation4,Citation5 Bariatric surgery is the only treatment for patients with obesity that has a longstanding effect on weight loss and the (possible) remission of obesity-related comorbiditiesCitation4,Citation5 Despite these proven long-term effects, bariatric surgery is still a “tool” achieving long-term weight loss. Therefore, a significant amount of patients show weight regain after a few years.Citation6 Several studies have shown that the combination of bariatric surgery and dietary/exercise interventions could be beneficial in maintaining weight loss in the long term.Citation7,Citation8 In our opinion, a pivotal aspect is good and well-structured follow-up program.
In other surgical specialties, exercise training is often used to improve preoperative physical fitness a postoperative outcomes.Citation8–Citation11 Despite promising beneficial outcomes these perioperative exercise programs are barely used in bariatric surgical practice. There is a significant body of evidence that indicates that exercise is good for improving physical fitness, but also Quality of Life (QoL). A Cochrane review by Shaw et alCitation12 showed a reduction of 1.5 kg that was contributed to exercise. These findings were substantiated by a systematic review of Livhits et al.Citation13 They focused on physical training and obesity and found a 4% Excess Weight Loss (%EWL) contributable to the exercise intervention.Citation13 Similar findings were seen in the study by Egberts et al.Citation14 They found that exercise after bariatric surgery resulted in a body weight reduction of 3.6 kg.Citation14 However, there is still no consensus on how to structure exercise programs in bariatric surgery. Often only exercise advice is given without a structured program.Citation12 Assessing outcome measurements, without a structured program is problematic, since weight loss measurements are self-reported and are not objectively assessed. Secondly, there is no adequate description of physical activity and which exercises were done. Recently Pouwels et alCitation7 tried to summarise what kind of exercise is beneficial in the perioperative period (in bariatric surgery) and also when patients should exercise. They concluded that there is a positive effect of peri-bariatric exercise on anthropometrics, cardiovascular risk factors and physical fitness. However no unanimous conclusions could be drawn, since there was a high heterogeneity level among exercise programs. The aim of this review is to summarise effects of preoperative and postoperative exercise, tools for screening and directions for future research and implementations.
Screening
Surgery, in general, has considerable effects on patients with regards to complications, mortality and physical capacity/functioning. These risks are also present in patients with obesity and patient’s before/after bariatric and metabolic surgery. To improve perioperative practices, improve logistics in the Operating Room and finally reduce complications and length of hospital stay the Enhanced Recovery after Surgery (ERAS) pathway was developed in the 1990s.Citation15
These ERAS protocols were game changers in perioperative medicine and aimed to incorporate a multimodal and patient centred approach to surgical care. Initially developed for colorectal surgery, but it has been applied to all major surgical specialities.Citation16 Also in primary and revisional bariatric surgery, extensive research has been done and ERAS or ERABS (Enhanced Recovery After Bariatric Surgery) has been implemented in the majority of hospitals worldwide.Citation17–Citation20 These care pathways were designed to modify physiological and psychological responses to surgical trauma by integrating a range of evidence-based components into a standardised clinical pathway. The most important aspect is that all these reductions (e.g. reduction in complications, reduced length of hospital stay and healthcare cost reduction) were achieved without comprising the perioperative patient safetyCitation21 and were associated with lower healthcare costs.Citation22 Ljungqvist et alCitation16 wrote a landmark paper and they studied and defined the 24 core aspects of the ERAS protocol. Among these essential elements are smoking and alcohol cessation, a structured preoperative information and education regimen, preoperative carbohydrate treatment and prophylaxis against venous thrombo-embolisms and infections.Citation16
All these core ERAS aspects are implemented in the ERABS protocols and can also be easily used in perioperative exercise programs in patients scheduled for bariatric and metabolic surgery. These exercise interventions have similarities with a new concept of prehabilitation in other surgical practices. They have the goal of improving physical functioning of the patient to endure the upcoming surgical procedure.Citation23 These concepts were developed according to a wide variety of studies that indicate that a higher preoperative level of fitness is associated with a reduction in postoperative complications and an improved clinical outcome.Citation24–Citation26
In this context, Orange et alCitation27 and Minnella et alCitation28 investigated and summarised the essential point for preparing patients for a surgical procedure. Among them; optimisation of nutritional status, psychological status and of course improving exercise capacity and physical functioning. Topal et alCitation29 added one very important aspect, namely the improvement of comorbidities. This is essential since the bariatric surgical patients get older and have potentially more comorbidities with medication use. gives an overview of the modifiable patient-related factors.
Table 1 Overview of the Modifiable Patient-Related Factors
Nutritional Assessment
Malnutrition is a serious and impactful problem in surgical practice that affects approximately two out of three patients.Citation30,Citation31 We can state that malnutrition is a possibly undertreated condition that often leads to surgical and non-surgical complications, in both the lean population and in patients with obesity.Citation32–Citation34 The physiological response due to surgical stress triggers a cascade of processes from systemic inflammation, insulin and blood glucose abnormalities, significant changes in energy expenditure and secretion of hormones.Citation35,Citation36 This can lead to significant clinical sequelae like poor appetite, weight loss, and muscle mass wasting, and could lead to cachexia or sarcopenia.Citation35,Citation36 These symptoms or sequelae will eventually lead to a higher risk of postoperative complications, extended hospital stay, higher mortality and morbidity rates and finally an increase health-care costs.Citation35–Citation37
Especially in the surgical oncology literature, there seems to be a clear relationship between nutrition, age, cancer, surgery and physical functioning.Citation30,Citation31,Citation35,Citation36,Citation38 Therefore, nutritional assessment is an important part of bariatric surgical screening and prior to starting exercise programs.
Physical Fitness Assessment
The decrease in physical functioning due to obesity and the presence of comorbidities is an increasing worldwide problem.Citation39 Physical/cardiorespiratory fitness (CRF) is a constant indicating the ability to meet the increase in oxygen consumption during daily activities, exercise and stress conditions.Citation40–Citation42 This can be impaired by, for example, obesity, cancer, surgery, radiotherapy, chemotherapy, infection or hormone therapy.Citation7,Citation43,Citation44
A significant body of evidence has shown that the level of preoperative physical function is a significant predictor for postoperative complications and mortality.Citation45–Citation47 In light of this evidence, it has also been seen that a temporary functional decline is normal before, during and after hospitalization and after surgery.Citation48 As stated earlier when these periods of inactivity prolong it has effects on muscle mass and the cardiopulmonary system. Eventually, muscle wasting will occur combined with possible cardiopulmonary deconditioning which will increase the risk of postoperative complications, mortality and psychological distress. These findings were substantiated by several studies of Kortebein et al.Citation49–Citation51 They have studied the effects of prolonged periods of bed rest on muscle strength of major muscle groups and not surprisingly they found that prolonged periods of bed rest lead to a significant decrease in muscle strength. Decreasing muscle strength was also seen after short periods of bed rest. All of the earlier mentioned phenomena could result in significant morbidity after surgery and therefore need to be optimised.Citation50,Citation52-Citation54 In general physical capacity predicts postoperative recovery, but seems to be more effects in frail and elderly patients undergoing major surgery. Therefore, nowadays there is an increasing interest in investigating a variety of PET and postoperative rehabilitation strategies ton optimise the surgical outcomes in this specific population.Citation55–Citation58 However, there is no “one size fits all”, due to different patient characteristics, types of surgery and used programs (either PET or postoperative rehabilitation).Citation59–Citation61
We can now consider the effects of PET to treat/optimise perioperative care as a proof of concept.Citation44 However, we still do not understand the effects in different patient populations and of course the cellular and immunological processes that exercise interventions induce in this matter. In other words, according to several studies in different surgical specialities its effectiveness can be difference. Especially in patients scheduled for orthopaedic knee or hip replacement surgery, there are some controversies regarding the effects of perioperative exercise interventions, especially PET.Citation62
Regarding types of exercise in these exercise interventions, the evidence is far from conclusive. Most of the studies investigate effects of aerobic training,Citation63 however the combination of exercise types (for example, resistance and endurance training) can have even better effects. Unfortunately in perioperative medicine there is a lack of studies investigating these concepts, despite the cellular effects can be synergistic (with regards to tissue protein synthesis and aerobic capacity).Citation64–Citation66
Despite the fact that there is not much known about the physiological principles of exercise interventions in the perioperative period, clinicians should consider to get patients and exercise test prior to the start of an exercise intervention. Cardiopulmonary exercise testing (CPET) is considered to be the gold standard in exercise testing, however could also be costly and time consuming.Citation40,Citation67 CPET is essential in determining the exercise tolerance of patients by indicating/calculating them in METs (Metabolic Equivalents of Time)Citation40 One MET is defined as an oxygen use of 3.5 mL O2/kg/min, which indicates the energy used while being seated in a resting position.Citation40,Citation41 Climbing a flight of stairs or walking up a hill are activities associated with METs >4, and they are considered a “safe” threshold below which postoperative complications are more likely to occur.Citation68
Psychological Status and Improvement of Comorbidities
Psychological distress is an often-overlooked problem that can have a significant effect on the postoperative convalescence of patients. In general, there are high rates of anxiety, depression and associated disorders in the surgical population.Citation69 It is known that psychological distress in the form of anxiety and depressive disorders can have a negative impact on wound healing, infection rate, length of hospital stay and adherence to medical treatment, despite that the pathophysiological mechanism is still largely unknown.Citation70–Citation73 A study by Mitchell et al.Citation74 showed that anxiety disorders should be treated in the perioperative period to improve postoperative outcomes. Therefore, a psychologist should also be incorporated in the multidisciplinary team.
With the increasing age of surgical patients, but also patients scheduled for bariatric surgery,Citation75 we need to take into account the presence of comorbidities. It is well known that extensive comorbid conditions are present in the majority of patients that undergo abdominal, bariatric, thoracic and cardiovascular surgery. High rates of coronary disease or congestive heart failure (>50%), hypertension (30–50%), COPD (30–40%) and chronic renal disease (5%) are seen in the surgical patient population.Citation76–Citation78 The presence of these comorbidities can have a significant impact on postoperative recovery and the occurrence of functional decline after surgery. Therefore, efforts should be made to optimize these conditions prior to surgery. This might be the case in patients with cancer, COPD and heart failure.Citation59,Citation60,Citation79
The burden of these morbidities should also be considered following bariatric surgery. A population-based study by Moonesinghe et al.Citation80 investigated the burden of postoperative morbidity on overall survival and they showed a significant impact in a variety of surgical specialties.Citation80 There must be a place for the optimisation of comorbid conditions (e.g. COPD, heart failure). Optimisation of medication use and/or lifestyle interventions could be incorporated in such a strategy.Citation10
Preoperative Exercise Therapy
In general, there are very few data on the effectiveness of specific preoperative exercise therapy programs on CRF and functional performance following surgery. Some of the literature has been summarised by Pouwels et al.Citation7 regarding its effects and perioperative timing.
In general for both the preoperative and postoperative there is no consensus what to do in terms of exercise interventions. Baillot et al.Citation81 studied a preoperative exercise program with 35 supervised exercise sessions in 12 weeks. In total seven patients completed the full program and five of them had their bariatric surgery before the end of the program. The corresponding attendance rates were a median of 57.3% (32.5–77.6%). Significant weight and BMI reduction, reduction in neck circumference and fat mass were achieved (respectively; before; 144.3 kg, after; 140.2, p=0.07; before; 51.4 kg/m2, after; 47.2 kg/m2, p=0.004; before; 42.2 cm, after; 41.0 cm, p=0.016 and before; 72.1 kg, after; 69.1 kg, p=0.026). After the exercise program, several physical fitness measures increased significantly (six-minute walk test distance, time of the half-squat test and number of flexion during the arm curl test)Citation81 This resulted in a high satisfaction with the intervention and the advice given by exercise professionals resulting in a significant improvement in the total Health-Related Quality Of Life (HRQOL) after the exercise intervention (p=0.012).Citation81
With regards to anthropometric measurements, the study by Funderburk et al.Citation82 showed similar results (a reduction in body weight after 12 weeks supervised aquatic exercises of 5.0 kg in the intervention group compared with 2.3 kg in the control group).Citation82 Six-minute walking test distance increased with 10.4m, but in the control group it increased with 40.2m (after 12 weeks of aquatic exercises).Citation82 HRQOL measurements were not significantly different between the intervention group and the control group. Bodily pain (as a sub-item of the Short Form 36 (SF-36) questionnaire) and depression scores (Beck Depression inventory) after 12 weeks decreased significantly (p<0.05).Citation82
Hickey et alCitation83 investigated a seven-day exercise training scheme consisting of 60 minute sessions once a day. They found no significant change in anthropometric variables (body weight and fat-free mass, but they did find a significant decrease in fasting plasma insulin (−41.7 pM). Strangely enough, there were not any corresponding changes in blood glucose levels and blood lipid concentrations.Citation83 Also, no significant differences were found in maximal oxygen uptake (which was measured by VO2 peak).Citation83
The findings of the study of Marcon et al.Citation84 corresponded with the tendency found in previous studies. They investigated 24 weeks supervised low intensity endurance training and found significant changes in anthropometric variables; body weight (- 5.3kg (p<0.001)) and BMI (−1.9 kg/m2 (p<0.001)) decreased significantly. Also, significant decrease in systolic and diastolic blood pressure (−23.8 mmHg (p=0.007) and −14.4 mmHg (p=0.001) respectively) was foundCitation84 Finally, significant improvements were seen in blood lipid and glucose concentration and in six minute walking test distance (+69.8m (p<0.0001)) after 24 weeks exercise intervention.Citation84
The Bari-Active trial from Bond et al.Citation85 showed that an exercise intervention in the period six weeks prior to bariatric surgery has a significant improvement on physical activity patterns compared to the standard care group. These results were supported by a randomized controlled trial by Marcon et al.Citation86 that showed a 4 month, twice weekly supervised program of low-intensity physical exercise can be valuable in the perioperative bariatric care.
Postoperative Exercise Therapy
In postoperative rehabilitation or postoperative exercise therapy in bariatric surgery, there are few studies that test the effectiveness of different exercise modes on outcomes. Further, the outcome measures appear to be heterogeneous.Citation7,Citation85,Citation87,Citation88
Stegen et al.Citation89 investigated a 16 weeks postoperative exercise program after Roux en Y Gastric Bypass (GB) surgery. In this study, postoperative patients that completed the exercise program (GB+E) were compared with patients that only had GB surgery. Both groups had the same extent of weight loss and changes in other anthropometrics after four months (GB = −26.6 ± 14.6 kg; GB+E = −22.7 ± 5.7 kg), BMI (GB = −8.3 ± 4.1 kg/m2; GB+E = −8.1 ± 2.5 kg/m2) and waist circumference (GB = −20.3 ± 11.6 cm; GB+E = −17.2 ± 8.1 cm).Citation89
In terms of exercise capacity, which was measured as ventilator anaerobic threshold (VAT) using a maximal bicycle ergometer, there were no significant differences. Both groups reached their VAT at the power (GB = 93 ± 24 W; GB+E = 90 ± 24 W) with an equal time of occurrence (GB = 270 ± 107 s; GB+E = 266 ± 133 s).Citation89 Both groups showed no differences in peak exercise parameters (preoperative versus postoperatively; respectively peak oxygen uptake of 17.4 ± 4.9 mL/kg/min (GB) and 17.6 ± 3.2 mL/kg/min (GB+E)).Citation89
However, the exercise training intervention showed promising effects regarding muscle loss after bariatric surgery. The untrained patients, who only had bariatric surgery showed a decrease in dynamic muscle strength (a decrease of 16% in quadriceps strength, 36% biceps strength and 39% triceps strength).Citation89 Patients who had an exercise program after gastric bypass surgery (GB+E) prevented this decrease.Citation89 Regarding static muscle strength (measured as handgrip strength, both groups had a decrease (respectively 18% and 7%).Citation89
Castello-Simoes et al.Citation90 investigated a three group 12 week post-bariatric exercise intervention, 1) a trained group (TG), 2) a eutrophic group (EG) and 3) a control group (CG).
Anthropometric changes were consistent with other studies and the TG and CG group showed significant weight reduction after 4 months. Also, both groups (TG and CG) showed a similar significant increase in the 6-minute walking test distance (6MWT)Citation90 Finally a significant increase of the predicted forced vital capacity (pFVC) was found (before: 94.0 ± 3.1; after: 101.0 ± 2.5) in TG and a significant reduction in dyspnoea scores was found in the same group 4 months after bariatric surgery (before: 5.8 ± 0.6; after 2.7 ± 0.8).Citation90 Shah et al.Citation91 found similar results regarding weight loss after a high volume exercise program in 20 patients for 12 weeks. Interestingly, theyfound no difference in cardiovascular risk factors after compared to the control group.Citation91
Berggren et al performed a study on skeletal muscle lipid oxidation before and after 10 days of endurance training in post-bariatric patients.Citation92 Not surprisingly, they found significant weight loss after the exercise intervention. (p <0.05).Citation92 Fatty acid oxidation in the muscle was not significantly different between patients with morbid obesity compared to the weight loss groups. Compared to lean individuals the oxidation of fatty acids was depressed (−45%; P<0.05). In contrast, ten days of exercise training increased fatty acid oxidation in the skeletal muscle of lean, obese and previously extreme obese subjects after weight loss (respectively +1.7-fold, +1.8-fold and +2.6-fold).Citation92 These data suggest that there is reversibility of cellular oxidation processes through exercise interventions in patients after bariatric surgery.
Marc-Hernandez et alCitation93 investigated the effects of a high-intensity exercise program has the ability to reduce weight regain three years after bariatric surgery. Twenty-one patients that had a sleeve gastrectomy three years ago were randomised in an exercise group that performed a 5-month supervised exercise program, compared to a control group that followed usual care.Citation93 Body anthropometrics, physical fitness and cardiovascular parameters were collected before and after the training program. After the program, the exercise group showed to have significant reductions in fat mass, glycaemia and blood cholesterol levels. The control group showed an increase in body weight and fast mass. Two months after finalising the exercise program, the patients who had the exercise intervention showed similar results as the control group during the study. In particular, an increase in body weight and fat mass and higher blood glucose and cholesterol levels, compared to directly after the exercise program.Citation93 This rebound effect might be due to exercise beliefs and exercise patterns that do not change after bariatric surgery. This was substantiated by a recent study done by Ouellette et al.Citation94 They found that in the postoperative period, moderate-to-vigorous physical activity (MVPA) patterns did not significantly change, despite patients expectations.Citation94 Is this context there might also be a behavioural component that can be treated.Citation94
The data of the mentioned studies show that there might be beneficial effects on physical activity measurements, quality of life and traditional risk factors. In addition, it is important for future studies to take into account the metabolic effects of the surgical procedures prior to exercise and lifestyle interventions.Citation6,Citation95 Hopefully, future initiatives will be able to answer these important questions.Citation96,Citation97
Discussion
In surgical practice, perioperative exercise interventions seem to get more and more attention, due to beneficial effects on complication rate and patient convalescence. Also with the introduction of prehabilitation programsCitation8–Citation11,Citation29 and their promising results in various surgical fields it is surprising that exercise interventions in bariatric surgery are so sparsely investigated. In the studies that investigate these perioperative exercise programs, there seems to be a beneficial effect on weight loss parameters, physical activity measurements and risk factors for cardiovascular diseases. However due to heterogeneous study designs, possible under powering and the lack of structured exercise training programs, no definitive conclusion can be drawn. All these factors hamper practical guidance and implementation in clinical practice.
gives an overview of the common findings in the studies assessing exercise interventions in bariatric surgery. gives a conceptual framework of perioperative exercise interventions in bariatric surgery. The heterogeneity of exercise programs makes comparisons difficult and according to several studies it is not evident as to what the optimal exercise programs should be and what the timing should be around bariatric surgery.Citation7
Table 2 Summary of Exercise Programs, Intensity, Duration, Supervision and Perioperative Timing
Figure 1 Conceptual framework of perioperative exercise interventions in bariatric surgery. Purple line: patients who do not receive any perioperative exercise intervention. Orange line: patients who only receive preoperative exercise training. Blue line: patients who receive preoperative and postoperative exercise training.
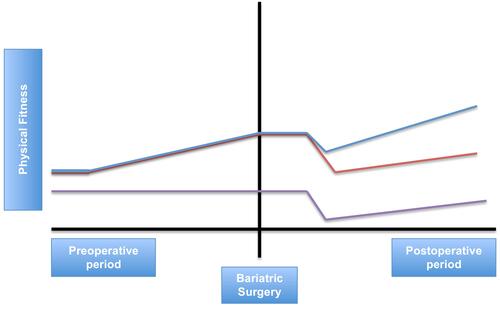
In general, it can be stated that exercise has beneficial total body effects. More specifically tremendous effects can be present in body composition changes, better blood pressure control, increase in insulin sensitivity, decrease of inflammation and presence of “inflammation” biomarkers and also subclinical carotid atherosclerosis (measured as Carotid Intima Media Thickness (CIMT)). The effects of exercise have been associated with physiological changes in the human cardiovascular system, for example removal of oxidized phospholipids from the vessel wall, stabilisation of atherosclerosis and positive changes in LDL cholesterol.Citation98
These physiological changes correlated strongly with improvement of vascular function and in improvement of atherosclerosis (e.g. decreased burden of atherosclerosis).Citation99
The evidence from the ARBITER 6 trial showed that exercise is superior in inducing these beneficial effects compared to medical treatment (with either statins or niacin).Citation100 In other words, exercise training should be a part of every medical intervention or perioperative rehabilitations program.Citation101
Despite a very large body of evidence that bariatric surgery is a longstanding intervention with great effects on the human body, it needs to be said that it also has consequences. One of them is muscle loss or otherwise called “muscle wasting”, which was showed in the study by Stegen et al.Citation89 This can be explained by the rapid weight loss after bariatric surgery. In the study by Stegen et al the untrained (GB) group lost approximately 7.6 kg of muscle mass, which is 29.7% of the total body weight lost (−26.6 kg)Citation89 Dieting, exercise interventions and also bariatric surgery results in the loss of fat-free mass. This was confirmed by studies done by Stiegler et al.Citation102 and Chaston et al.Citation103 They showed that there is a positive correlation between weight loss and fat-free mass loss (FFML). Very low caloric diets result in a greater FFML compared to moderate caloric diets. Bariatric surgery results in greater FFML than very low caloric diets.
A study by Webster et al.Citation104 showed that there is a limit of FFML after bariatric surgery which is approximately 22% of the total weight loss. This is because of the functions of the muscle in resting metabolic rate, thermoregulation, oxidative capacity of the body and weight management. Exercise training can attenuate muscle atrophy and can maintain FFM during weight loss,Citation102,Citation103 but the value of a perioperative exercise program for bariatric surgery has not been investigated.
It is not clear whether or not there is a decrease of muscle strength contributes to poor health outcomes or risk after weight loss surgery. It is well known that obese individuals have higher absolute muscle strength compared to lean subjects, but there is lower relative muscle strength in terms of total body weight.Citation105–Citation108 This gives patients an impaired functional capacity, which results in the fact that more strength is needed to handle a heavier body. Therefore, it might be important to prevent a decrease in muscle strength after bariatric surgery.
Conclusion
We can state that perioperative exercise programs in bariatric surgery might have beneficial effects on weight loss parameters, physical activity measurements and risk factors for cardiovascular diseases. However, the heterogeneity of exercise programs makes comparisons difficult and according to several studies reviewed in this paper it is not evident as to what the optimal exercise programs should be and what the timing should be around bariatric surgery. Future studies focused on the effectiveness of exercise intervention mode, intensity and timing before or after bariatric surgery to maximize cardiorespiratory fitness, physical function and reductions in cardiometabolic risk appears evident.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 2000;894:i–xii,1–253.
- Tjepkema M. Adult obesity. Health Rep. 2006;17(3):9–25.
- Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121(7):492–496. doi:10.1016/j.puhe.2007.01.006
- Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13(41):1–190, 215–357, iii–iv.
- Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009;2:CD003641.
- Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg. 2014;24(8):1126–1135. doi:10.1007/s11695-014-1354-3
- Pouwels S, Wit M, Teijink JA, Nienhuijs SW. Aspects of exercise before or after bariatric surgery: a systematic review. Obes Facts. 2015;8(2):132–146. doi:10.1159/000381201
- Pouwels S, Stokmans RA, Willigendael EM, et al. Preoperative exercise therapy for elective major abdominal surgery: a systematic review. Int J Surg. 2014;12(2):134–140. doi:10.1016/j.ijsu.2013.11.018
- Pouwels S, Hageman D, Gommans LN, et al. Preoperative exercise therapy in surgical care: a scoping review. J Clin Anesth. 2016;33:476–490. doi:10.1016/j.jclinane.2016.06.032
- Pouwels S, Fiddelaers J, Teijink JA, Woorst JF, Siebenga J, Smeenk FW. Preoperative exercise therapy in lung surgery patients: a systematic review. Respir Med. 2015;109(12):1495–1504. doi:10.1016/j.rmed.2015.08.009
- Pouwels S, Willigendael EM, van Sambeek MR, Nienhuijs SW, Cuypers PW, Teijink JA. Beneficial effects of pre-operative exercise therapy in patients with an abdominal aortic aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2015;49(1):66–76.
- Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4):CD003817.
- Livhits M, Mercado C, Yermilov I, et al. Exercise following bariatric surgery: systematic review. Obes Surg. 2010;20(5):657–665. doi:10.1007/s11695-010-0096-0
- Egberts K, Brown WA, Brennan L, O’Brien PE. Does exercise improve weight loss after bariatric surgery? A systematic review. Obes Surg. 2012;22(2):335–341. doi:10.1007/s11695-011-0544-5
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–617. doi:10.1093/bja/78.5.606
- Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–298. doi:10.1001/jamasurg.2016.4952
- Ruiz-Tovar J, Sanchez-Santos R, Martin-Garcia-Almenta E, et al. Enhanced recovery after bariatric surgery. Cir Esp. 2019;97(10):551–559. doi:10.1016/j.ciresp.2019.05.003
- Geubbels N, Evren I, Acherman YIZ, et al. Randomized clinical trial of an enhanced recovery after surgery programme versus conventional care in laparoscopic Roux-en-Y gastric bypass surgery. BJS Open. 2019;3(3):274–281. doi:10.1002/bjs5.50143
- Mannaerts GHH, Allatif REA, Al Hashmi FY, et al. First successful large-scale introduction of an Enhanced Recovery after Bariatric Surgery (ERABS) program in the Middle East: the results and lessons learned of Tawam Hospital/Johns Hopkins, a Tertiary Governmental Center in the UAE. Obes Surg. 2019;29(7):2100–2109. doi:10.1007/s11695-019-03841-4
- Trotta M, Ferrari C, D’Alessandro G, Sarra G, Piscitelli G, Marinari GM. Enhanced recovery after bariatric surgery (ERABS) in a high-volume bariatric center. Surg Obes Relat Dis. 2019;15(10):1785–1792. doi:10.1016/j.soard.2019.06.038
- Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29(4):434–440. doi:10.1016/j.clnu.2010.01.004
- Roulin D, Donadini A, Gander S, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg. 2013;100(8):1108–1114. doi:10.1002/bjs.9184
- Topp R, Ditmeyer M, King K, Doherty K, Hornyak J 3rd. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues. 2002;13:263–276. doi:10.1097/00044067-200205000-00011
- Wilson RJ, Davies S, Yates D, Redman J, Stone M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth. 2010;105(3):297–303. doi:10.1093/bja/aeq128
- Thompson AR, Peters N, Lovegrove RE, et al. Cardiopulmonary exercise testing provides a predictive tool for early and late outcomes in abdominal aortic aneurysm patients. Ann R Coll Surg Engl. 2011;93(6):474–481. doi:10.1308/003588411X587235
- Young EL, Karthikesalinam A, Huddart S, et al. A systematic review of the role of cardiopulmonary exercise testing in vascular surgery. Eur J Vasc Endovasc Surg. 2012;44(1):64–71. doi:10.1016/j.ejvs.2012.03.022
- Orange ST, Northgraves MJ, Marshall P, Madden LA, Vince RV. Exercise prehabilitation in elective intra-cavity surgery: a role within the ERAS pathway? A narrative review. Int J Surg. 2018;56:328–333. doi:10.1016/j.ijsu.2018.04.054
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol. 2018;44(7):919–926. doi:10.1016/j.ejso.2018.04.016
- Topal B, Smelt HJM, Van Helden EV, et al. Utility of preoperative exercise therapy in reducing postoperative morbidity after surgery; a clinical overview of current evidence. Expert Rev Cardiovasc Ther. 2019;17(6):395–412. doi:10.1080/14779072.2019.1625771
- Planas M, Alvarez-Hernandez J, Leon-Sanz M, Celaya-Perez S, Araujo K, Garcia de Lorenzo A. Prevalence of hospital malnutrition in cancer patients: a sub-analysis of the PREDyCES(R) study. Support Care Cancer. 2016;24(1):429–435. doi:10.1007/s00520-015-2813-7
- Blanc-Bisson C, Fonck M, Rainfray M, Soubeyran P, Bourdel-Marchasson I. Undernutrition in elderly patients with cancer: target for diagnosis and intervention. Crit Rev Oncol Hematol. 2008;67(3):243–254. doi:10.1016/j.critrevonc.2008.04.005
- Wells JC, Sawaya AL, Wibaek R, et al. The double burden of malnutrition: aetiological pathways and consequences for health. Lancet. 2019.
- Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2019.
- Raoult D. Microbiota, obesity and malnutrition. Microb Pathog. 2017;106:1–2. doi:10.1016/j.micpath.2016.02.001
- Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol. 2013;4(3):218–226. doi:10.1016/j.jgo.2013.04.001
- Maasberg S, Knappe-Drzikova B, Vonderbeck D, et al. Malnutrition predicts clinical outcome in patients with neuroendocrine neoplasia. Neuroendocrinology. 2017;104(1):11–25. doi:10.1159/000442983
- Heriot AG, Tekkis PP, Smith JJ, et al. Prediction of postoperative mortality in elderly patients with colorectal cancer. Dis Colon Rectum. 2006;49(6):816–824. doi:10.1007/s10350-006-0523-4
- Awad S, Lobo DN. What’s new in perioperative nutritional support? Curr Opin Anaesthesiol. 2011;24(3):339–348. doi:10.1097/ACO.0b013e328345865e
- Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med. 2009;43(1):1–2.
- Nelson N, Asplund CA. Exercise testing: who, when, and why? PM R. 2016;8(3 Suppl):S16–S23. doi:10.1016/j.pmrj.2015.10.019
- Leclerc K. Cardiopulmonary exercise testing: a contemporary and versatile clinical tool. Cleve Clin J Med. 2017;84(2):161–168. doi:10.3949/ccjm.84a.15013
- Mueller MJ, Maluf KS. Tissue adaptation to physical stress: a proposed “physical stress theory” to guide physical therapist practice, education, and research. Phys Ther. 2002;82(4):383–403. doi:10.1093/ptj/82.4.383
- Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–120. doi:10.1634/theoncologist.2010-0197
- Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10(6):598–605. doi:10.1016/S1470-2045(09)70031-2
- Thombs BD, Ziegelstein RC, Stewart DE, Abbey SE, Parakh K, Grace SL. Physical health status assessed during hospitalization for acute coronary syndrome predicts mortality 12 months later. J Psychosom Res. 2008;65(6):587–593. doi:10.1016/j.jpsychores.2008.06.004
- Tosteson AN, Gottlieb DJ, Radley DC, Fisher ES, Melton LJ 3rd Excess mortality following hip fracture: the role of underlying health status. Osteoporos Int. 2007;18(11):1463–1472. doi:10.1007/s00198-007-0429-6
- Cesari M, Onder G, Zamboni V, et al. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr. 2008;8:34. doi:10.1186/1471-2318-8-34
- Patel BK, Hall JB. Perioperative physiotherapy. Curr Opin Anaesthesiol. 2013;26(2):152–156. doi:10.1097/ACO.0b013e32835e8b34
- Functional impact of ten days of bed rest in healthy older adults. Nurs Older People. 2017;29(5):13. doi:10.7748/nop.29.5.13.s15
- Kortebein P. Rehabilitation for hospital-associated deconditioning. Am J Phys Med Rehabil. 2009;88(1):66–77. doi:10.1097/PHM.0b013e3181838f70
- Kortebein P, Symons TB, Ferrando A, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol a Biol Sci Med Sci. 2008;63(10):1076–1081. doi:10.1093/gerona/63.10.1076
- Bajotto G, Shimomura Y. Determinants of disuse-induced skeletal muscle atrophy: exercise and nutrition countermeasures to prevent protein loss. J Nutr Sci Vitaminol (Tokyo). 2006;52(4):233–247. doi:10.3177/jnsv.52.233
- Chetta A, Tzani P, Marangio E, Carbognani P, Bobbio A, Olivieri D. Respiratory effects of surgery and pulmonary function testing in the preoperative evaluation. Acta Biomed. 2006;77(2):69–74.
- Walton-Geer PS. Prevention of pressure ulcers in the surgical patient. AORN J. 2009;89(3):538–48; quiz 49–51. doi:10.1016/j.aorn.2008.12.022
- Arozullah A, Khuri S, Henderson W, Daley J. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–857. doi:10.7326/0003-4819-135-10-200111200-00005
- Biccard B. Relationship between the inability to climb two flights of stairs and outcome after major non-cardiac surgery: implications for the pre-operative assessment of functional capacity. Anaesthesia. 2005;60:588–593. doi:10.1111/j.1365-2044.2005.04181.x
- Brutsche M, Spiliopoulos A, Bolliger C, Licker M, Frey J, Tschopp J. Exercise capacity and extent of resection as predictors or surgical risk in lung cancer. Eur Respir J. 2000;15:828–832. doi:10.1034/j.1399-3003.2000.15e03.x
- Michota F, Frost S. The preoperative evaluation: use the history and physical rather than routine testing. Cleve Clin J Med. 2004;71:63–70. doi:10.3949/ccjm.71.1.63
- Halloway S, Buchholz SW, Wilbur J, Schoeny ME. Prehabilitation interventions for older adults: an integrative review. West J Nurs Res. 2015;37(1):103–123. doi:10.1177/0193945914551006
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi:10.1164/rccm.201309-1634ST
- Valkenet K, van de Port I, Dronkers J, de Vries W, Lindeman E, Backx F. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil. 2011;25:99–111. doi:10.1177/0269215510380830
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi:10.1152/physrev.00031.2007
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi:10.1249/MSS.0b013e3181a0c95c
- Wilkinson SB, Phillips SM, Atherton PJ, et al. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586(15):3701–3717. doi:10.1113/jphysiol.2008.153916
- Philipson TJ, Snider JT, Lakdawalla DN, Stryckman B, Goldman DP. Impact of oral nutritional supplementation on hospital outcomes. Am J Manag Care. 2013;19(2):121–128.
- Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol. 2005;568(Pt 1):283–290. doi:10.1113/jphysiol.2005.093708
- American Thoracic Society. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi:10.1164/rccm.167.2.211
- Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. 1999;159(18):2185–2192. doi:10.1001/archinte.159.18.2185
- Jefford M, Karahalios E, Pollard A, et al. Survivorship issues following treatment completion–results from focus groups with Australian cancer survivors and health professionals. J Cancer Surviv. 2008;2(1):20–32. doi:10.1007/s11764-008-0043-4
- Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14(7):397–405. doi:10.5435/00124635-200607000-00002
- Mavros MN, Athanasiou S, Gkegkes ID, Polyzos KA, Peppas G, Falagas ME. Do psychological variables affect early surgical recovery? PLoS One. 2011;6(5):e20306. doi:10.1371/journal.pone.0020306
- Kitagawa R, Yasui-Furukori N, Tsushima T, Kaneko S, Fukuda I. Depression increases the length of hospitalization for patients undergoing thoracic surgery: a preliminary study. Psychosomatics. 2011;52(5):428–432. doi:10.1016/j.psym.2011.03.010
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi:10.1001/archinte.160.14.2101
- Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14(8):721–732. doi:10.1016/S1470-2045(13)70244-4
- Versteegden DPA, Buise MP, Nienhuijs SW. Shift towards older bariatric patients. Obes Surg. 2018;28(2):555–556. doi:10.1007/s11695-017-3039-1
- Karthikesalingam A, Holt PJ, Vidal-Diez A, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet. 2014;383(9921):963–969. doi:10.1016/S0140-6736(14)60109-4
- Pol RA, Zeebregts CJ, van Sterkenburg SM, Reijnen MM. Thirty-day outcome and quality of life after endovascular abdominal aortic aneurysm repair in octogenarians based on the Endurant Stent Graft Natural Selection Global Postmarket Registry (ENGAGE). J Vasc Surg. 2012;56(1):27–35. doi:10.1016/j.jvs.2011.12.080
- Visser L, Pol RA, Tielliu IF, van den Dungen JJ, Zeebregts CJ. A limited and customized follow-up seems justified after endovascular abdominal aneurysm repair in octogenarians. J Vasc Surg. 2014;59(5):1232–1240. doi:10.1016/j.jvs.2013.11.070
- Asrar Ul Haq M, Goh CY, Levinger I, Wong C, Hare DL. Clinical utility of exercise training in heart failure with reduced and preserved ejection fraction. Clin Med Insights Cardiol. 2015;9:1–9. doi:10.4137/CMC.S21372
- Moonesinghe SR, Harris S, Mythen MG, et al. Survival after postoperative morbidity: a longitudinal observational cohort study. Br J Anaesth. 2014;113(6):977–984. doi:10.1093/bja/aeu224
- Baillot A, Mampuya W, Comeau E, Meziat-Burdin A, Langlois M. Feasibility and impacts of supervised exercise training in subjects with obesity awaiting bariatric surgery: a pilot study. Obes Surg. 2013;23(7):882–891. doi:10.1007/s11695-013-0875-5
- Funderburk JA, Callis S. Aquatic intervention effect on quality of life prior to obesity surgery: a pilot study. Annu Ther Recreation. 2010;18:66–78.
- Hickey MS, Gavigan KE, McGammon MR. Effects of 7 days of exercise training on insulin action in morbidly obese men. Clin Exerc Physiol. 1999;1:24–28.
- Marcon ER, Gus I, Neumann CR. Impact of a minimum program of supervised exercises in the cardiometabolic risk in patients with morbid obesity. Arq Bras Endocrinol Metabol. 2011;55:331–338. doi:10.1590/S0004-27302011000500006
- Bond DS, Vithiananthan S, Thomas JG, et al. Bari-active: a randomized controlled trial of a preoperative intervention to increase physical activity in bariatric surgery patients. Surg Obes Relat Dis. 2015;11(1):169–177. doi:10.1016/j.soard.2014.07.010
- Marcon ER, Baglioni S, Bittencourt L, Lopes CL, Neumann CR, Trindade MR. What is the best treatment before bariatric surgery? Exercise, Exercise and Group Therapy, or Conventional Waiting: a randomized controlled trial. Obes Surg. 2017;27(3):763–773.
- Bond DS, Phelan S, Wolfe LG, et al. Becoming physically active after bariatric surgery is associated with improved weight loss and health-related quality of life. Obesity. 2009;17(1):78–83. doi:10.1038/oby.2008.501
- Bond D. Bari-active: a preoperative intervention to increase physical activity. Obes Surg. 2011;21(8):1042. doi:10.1007/s11695-010-0204-1
- Stegen S, Derave W, Calders P, Van Laethem C, Pattyn P. Physical fitness in morbidly obese patients: effect of gastric bypass surgery and exercise training. Obes Surg. 2011;21(1):61–70. doi:10.1007/s11695-009-0045-y
- Castello-Simoes V, Polaquini Simoes R, Beltrame T, et al. Effects of aerobic exercise training on variability and heart rate kinetic during submaximal exercise after gastric bypass surgery–a randomized controlled trial. Disabil Rehabil. 2013;35(4):334–342. doi:10.3109/09638288.2012.694575
- Shah M, Snell PG, Rao S, et al. High-volume exercise program in obese bariatric surgery patients: a randomized, controlled trial. Obesity (Silver Spring). 2011;19(9):1826–1834. doi:10.1038/oby.2011.172
- Berggren JR, Boyle KE, Chapman WH, Houmard JA. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab. 2008;294(4):E726–E732. doi:10.1152/ajpendo.00354.2007
- Marc-Hernandez A, Ruiz-Tovar J, Aracil A, Guillen S, Moya-Ramon M. Effects of a high-intensity exercise program on weight regain and cardio-metabolic profile after 3 years of bariatric surgery: a randomized trial. Sci Rep. 2020;10(1):3123. doi:10.1038/s41598-020-60044-z
- Ouellette KA, Mabey JG, Eisenman PA, et al. Physical activity patterns among individuals before and soon after bariatric surgery. Obes Surg. 2020;30(2):416–422. doi:10.1007/s11695-019-04186-8
- Nguyen N, Champion JK, Ponce J, et al. A review of unmet needs in obesity management. Obes Surg. 2012;22(6):956–966. doi:10.1007/s11695-012-0634-z
- Villa-Gonzalez E, Barranco-Ruiz Y, Rodriguez-Perez MA, et al. Supervised exercise following bariatric surgery in morbid obese adults: CERT-based exercise study protocol of the EFIBAR randomised controlled trial. BMC Surg. 2019;19(1):127. doi:10.1186/s12893-019-0566-9
- Soriano-Maldonado A, Martinez-Forte S, Ferrer-Marquez M, et al. Physical Exercise following bariatric surgery in women with morbid obesity: study protocol clinical trial (SPIRIT compliant). Medicine. 2020;99(12):e19427. doi:10.1097/MD.0000000000019427
- Bergmark C, Dewan A, Orsoni A. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi:10.1194/jlr.M800174-JLR200
- Ahmadi N, Tsimikas S, Hajsadeghi F. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am J Cardiol. 2010;105:459–466. doi:10.1016/j.amjcard.2009.09.052
- Ahmadi N, Eshaghian S, Huizenga R, Sosnin K, Ebrahimi R, Siegel R. Effects of intense exercise and moderate caloric restriction on cardiovascular risk factors and inflammation. Am J Med. 2011;124(10):978–982. doi:10.1016/j.amjmed.2011.02.032
- Taylor AJ, Villines TC, Stanek EJ. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–2122. doi:10.1056/NEJMoa0907569
- Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36:239–262. doi:10.2165/00007256-200636030-00005
- Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond). 2007;31:743–750. doi:10.1038/sj.ijo.0803483
- Webster JD, Hesp R, Garrow JS. The composition of excess weight in obese women estimated by body density, total body water and total body potassium. Hum Nutr Clin Nutr. 1984;38(4):299–306.
- Maffiuletti NA, Jubeau M, Munzinger U. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007;101:51–59. doi:10.1007/s00421-007-0471-2
- Blimkie CJ, Sale DG, Bar-Or O. Voluntary strength, evoked twitch contractile properties and motor unit activation of knee extensors in obese and non-obese adolescent males. Eur J Appl Physiol Occup Physiol. 1990;61:313–318. doi:10.1007/BF00357619
- Pescatello LS, Kelsey BK, Price TB. The muscle strength and size response to upper arm, unilateral resistance training among adults who are overweight and obese. J Strength Cond Res. 2007;21:307–313. doi:10.1519/R-22236.1
- Hulens M, Vansant G, Lysens R, Claessens AL, Muls E, Brumagne S. Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach. Int J Obes Relat Metab Disord. 2001;25:676–681. doi:10.1038/sj.ijo.0801560
