Abstract
Purpose
Dihydromyricetin (DHM), the main bioactive flavonoid in vine tea, exerts multiple health beneficial effects. This work aimed to identify whether a naturally derived flavonoid product, DHM, can significantly attenuate metabolic syndrome and improve insulin sensitivity.
Methods
10-week-old db/db mice were randomly assigned to receive the antidiabetic agent metformin (Met, 50 mg/kg BW), DHM (1.0 g and 0.5 g/kg BW) or placebo and were simultaneously fed a high-fat diet for 8 weeks. The general status of the animals was observed and recorded daily, body weight and blood glucose levels were measured weekly, during the experimental period. On day 55, the oral glucose tolerance test (OGTT) was performed. After OGTT, all animals were anesthetized and sacrificed by cervical decapitation. Blood samples were collected in tubes to detect plasma insulin and the biochemical parameters of lipid metabolism. Pancreas histological changes and islet fibrosis were demonstrated by H&E staining and Masson staining, respectively. Moreover, the expression of insulin receptor substrate-1 and phosphorylated insulin receptor substrate-1 in the insulin signaling pathway was detected by Western blot assay.
Results
The oral administration of DHM (1.0 g and 0.5 g/kg BW) reduced the fasting blood glucose, serum insulin, and glycated hemoglobin levels and the insulin resistance (HOMA-IR) index. Furthermore, DHM intervention decreased body weight and the serum lipid profile. In addition, DHM treatment also markedly decreased the relative abdominal fat weight. Western blot analysis indicated that DHM upregulated the IRS-1 (Y612) tyrosine phosphorylation, improving insulin resistance. Treatment with dihydromyricetin attenuated the progression of insulin resistance and pancreatic fibrosis in fatty db/db mice.
Conclusion
In summary, we determined the antimetabolic syndrome effect of DHM in db/db obese mice. DHM upregulates the IRS-1 (Y612) tyrosine phosphorylation, improving insulin resistance. Therefore, DHM is a promising therapeutic candidate for the control of metabolic syndrome.
Introduction
Currently, there is no agreed upon definition of metabolic syndrome (MetS). The most commonly used definition is the National Cholesterol Education Program (NCEP) definition, which defines MetS as a cluster of key cardiovascular risk factors for diabetes and cardiovascular diseases that includes central abdominal obesity, hypertension, dyslipidemia, and hyperglycemia.Citation1–Citation7 A recent report estimated that 20–25% of the adult population is affected by MetS worldwide. At the same time, the Asia-Pacific region has seen a dramatic increase in the prevalence of obesity, type 2 diabetes and cardiovascular disease.Citation8–Citation11 This rapid increase in MetS has been paralleled by the growing epidemic of type 2 diabetes, hypertension, and cardiovascular disease. Powerful and efficient strategies aimed at primary prevention are urgently needed to curb a further increase in the epidemic and to reduce the morbidity and mortality associated with MetS. The intrinsic causes of MetS include abdominal obesity, physical inactivity, genetic factors and aging.Citation1,Citation2 The most widely recognized metabolic risk factors are hypertension, dyslipidemia (high triglycerides and lower HDL), hyperglycemia and central obesity.Citation4,Citation5 MetS pathophysiology is complex, with insulin resistance and the abnormal regulation of lipid metabolism playing key roles.
Traditional Chinese medicine has played a key role in the healthcare system in the past.Citation12–Citation14 The screening of candidate compounds from Chinese medicine is a good strategy for new drug development.Citation15,Citation16 Vine tea, a traditional Chinese materia medica, is composed of the tender stem and leaves of Ampelopsis grossedentata (Hand.-Mazz) W. T. Wang. Vine tea therapy, as a folk treatment, has been applied widely in southern China. According to previous literature and traditional Chinese medicine records, vine tea can eliminate heat and dampness, promote diuresis and blood circulation, and remove blood stasis and channel obstructions.Citation17–Citation21 Dihydromyricetin (DHM), the main bioactive flavonoid in vine tea, exerts antioxidant, anti-inflammatory, antibacterial, anti-intoxication, and anticancer effects.Citation21–Citation33 In the recent report, DHM protects against memory impairment. This work aimed to confirm the antimetabolic syndrome effect by evaluating hypoglycemia, the regulation of lipid metabolism and antioxidant function in high fat diet-exposed metabolically abnormal obese mice.
Methods
Chemical Reagents
Dihydromyricetin was extracted from vine tea (Ampelopsis grossedentata) and was then purified by recrystallization (the chemical formula is shown in Supplementary Figure 1). The purity was above 95%, as analyzed by HPLC with a UV detector (Supplementary Figure 2). Blood glucose meter and single-use strips (One-Touch Ultra®, lot number: 3662321) were purchased from Shanghai Johnson & Johnson Medical Device Co., Ltd. (Shanghai, China). We used 0.5% carboxyl methyl cellulose solution as a vehicle in the animal trials. Metformin (50 mg/kg BW, suspended in 0.5% carboxyl methyl cellulose solution, Squib Pharmaceutical Co., Ltd., Shanghai, China) was applied as a positive reference drug for hypoglycemic activity measurement. Anti-IRS1(phosphor Y612) antibody (synthetic peptide corresponding to the epitope DGYMP with a single phosphorylation site Tyr 612, from human IRS1). Abcam plc (Cambridge Biomedical Campus, Cambridge, CB2 0AX, UK). The other chemical regents were obtained from Merck KGaA, (Darmstadt, Germany).
Figure 1 Relative abdominal fat weight and body weight of db/db mice. The relative abdominal fat weights of the model control, positive control, DHM (0.5 and 1.0 g/kg BW) groups were 9.79± 2.76, 8.91± 2.83, 8.98± 2.64, 8.20±1.61 g/100 g body weight, respectively. DHM intervention at the high dose (1.0 g/kg b.w) induced a significant difference from the model control group. ##p<0.05 compared with the normal group. **p<0.05 compared with the DM group.
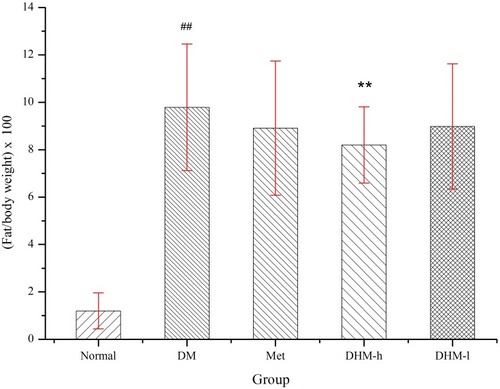
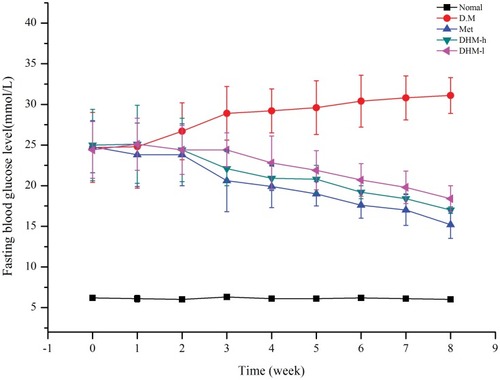
Figure 2 Fasting blood glucose levels of db/db mice from weeks 1 to 8. Fasting blood glucose levels of untreated db/db mice and db/db/mice treated with the oral administration of metformin and DHM (DHM-h, 1.0 g and 0.5 g/kg BW). DHM exhibited a time-dependent hypoglycemic effect with sustained administration. Normal: C57BL, DM: untreated metabolically abnormal obese db/db mice, Met: db/db mice treated with metformin, DHM-h, l: db/db mice treated with DHM at 1.0 g and 0.5 g/kg BW.
Experimental Animal
To confirm antimetabolic syndrome activity, high-fat diet-exposed db/db mice were used in this trial as metabolically abnormal obese animal models. C57BL/KsJ mice (8 weeks old, as normal control animal) were obtained from SLAC Laboratories Animal Co., Ltd. (Shanghai, China). All animal treatments were in accordance with ethical principles and corresponding standards. The experimental mice were evaluated and housed under controlled conditions (temperature, 23°± 2° C; relative humidity, 50% ± 10%; 12-h light/dark cycle) and allowed free access to the high-fat diet (45% fat, 20% protein and 35% carbohydrate) and deionized distilled water. The experimental protocol was reviewed and approved by the Animal Care and Use Committee of the Animal Resource Center, Institute of Basic Medicine, Chinese Academy of Medical Sciences (License No: SCXK 2016–0021). The animal welfare followed in this study was guideline for ethical review of animal welfare in the People’s Republic of China.
Experimental Protocol
Fasting blood glucose was determined after 2 weeks of adaptive feeding. The hyperglycemic mice (db/db) were randomly divided into five groups, each containing ten mice. The C57BL mice (normal control group) and the control metabolically abnormal obese db/db model mice (the placebo-treated model control group) received an equal volume of 0.5% carboxyl methyl cellulose solution. The positive control group (model + Met group) received the antidiabetic agent metformin (Met, 50 mg/kg BW, an equivalent dose). The DHM-treated mice (model + DHM groups) received DHM at a dose of 1.0 g and 0.5 g/kg BW. The 10-week-old db/db mice were fed a high-fat diet (45% fat, 20% protein and 35% carbohydrate) during an 8-week experimental period. As a functional food, the recommended dose of vine tea is 3 g per subject daily, or approximately 0.05 g/kg body weight for adults (average body weight of 60 kg). DHM is the most abundant component of vine tea, and the DHM content in vine tea is approximately 20–30%. The equivalent DHM dose in mice is 0.1–0.15 g/kg. Here, we used a ten-fold higher dose (1.0 g/kg) in mice as the high dose to evaluate the antimetabolic syndrome effect of DHM under experimental conditions and administered the treatment orally each day for 8 weeks. Then we use five-fold (0.5 g/kg) as the low dose correspondingly.
According to previous literature and traditional Chinese medicine records, vine tea (Ampelopsis grossedentata), as Chinese herbal medicine for treating disease, the recommended dose is 15–30 g per subject daily, or approximately 0.25–0.5 g/kg body weight for adults (average body weight of 60 kg). DHM content in vine tea is approximately 20–30%. As the botanical drug, the equivalent DHM dose in mice is 0.5–1.5 g/kg. All mice received the assigned treatment daily. The general status of the animals was observed and recorded daily. The body weight, food intake, and water intake of the mice were measured weekly and statistical analyzed.
To detect fasting blood glucose levels (FGB), we drew micro blood samples from the tail vein of the mice weekly. After placing fresh blood (approximately 50 μl) on single-use test strips, we used a validated One-Touch Basic Glucose Monitoring Set to detect the glucose content. The oral glucose tolerance test (OGTT) was performed on day 55. All animals were fasted overnight, FBG was detected and then the animals were given a dose of 20% glucose solution (3.0 g/kg BW). The plasma glucose levels were detected 30, 60, 120, and 180 min after the ingestion of a single high dose of glucose to calculate the area under the glucose response curve (AUC, min·mmol/L). After 8 weeks of antidiabetic drug or DHM intervention, the mice were fasted for 5 h and anesthetized with diethyl ether. Then, blood samples were collected for biochemical analysis. The liver tissue was harvested and prepared for tissue protein extraction and Western blot analysis.
Biochemical analysis: After fresh blood was collected, the blood samples were centrifuged at 3000 x g for 15 min, separated, removed and stored in an ultra-refrigerator (−60°C) for further plasma analysis. The plasma insulin levels were detected by radioimmunoassay immediately. We used commercial assay kits to estimate the plasma HbA1c level and the plasma lipid profile according to a standard validated assay.
The homeostasis model assessment-estimated insulin resistance index has been widely used to estimate insulin resistance in humans with high sensitivity and specificity.Citation34 Here, the HOMA-IR index was used to estimate the change in insulin resistance in metabolically abnormal obese db/db mice treated with DHM. The HOMA-IR index was calculated by multiplying fasting plasma insulin levels by fasting plasma glucose levels and then dividing by the constant 22.5, as shown in the following formula:
HOMA-IR=fasting blood glucose (mmol/l) × insulin (μU/mL)/22.5Citation34,Citation35
Protein Extraction And Western Blot Analysis
To analyze the expression of proteins involved in insulin resistance, liver tissue was harvested, cut and homogenized on ice in RIPA buffer solution [50 mM Tris (pH 7.6), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and 2 mM EDTA] containing protease inhibitor and phosphatase inhibitor. Then, the supernatants were separated and collected after centrifugation at 12,000 x g for 25 min at 4°C. A bicinchoninic acid assay was used to measure the protein concentration, and the protein was stored at −80°C for further Western blot analysis.
The above protein fractions were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes in Tris-glycine buffer at 110 V for 1 h. The membranes were blocked with 5% (w/v) skimmed milk powder in Tris-buffered saline containing 0.1% (v/v) Tween-20 (TBST) and then incubated overnight with appropriate primary antibodies at 4°C. Afterwards, they were washed twice with TBST and incubated with secondary antibodies for 2 h at 20–30°C. The results were visualized by enhanced chemiluminescence. The relative band intensity was determined using computerized densitometric analysis.
Histopathologic Examination
After collecting blood samples, we quickly removed the aorta between the aortic valve cusps and the iliac bifurcation and stored the samples in 4% paraformaldehyde for histological examination. Next, we cut consecutive cross-sections of the aorta and stained them with hematoxylin-eosin (HE). The pathological deterioration of the aorta inner wall was detected with an optical microscope (Leica Application Suite) and recorded.
Pancreatic tissue sections were fixed in formalin, stained with hematoxylin-eosin (HE), and observed under a light microscope using the Leica Application Suite. To prepare the harvested tissue for examination by light microscopy, we removed pancreatic sections, preserved them in 10% neutral phosphate-buffered formalin and processed them by routine paraffin sectioning and staining with hematoxylin-eosin (HE). Masson staining was performed to detect and analyze islet fibrosis. Staining was performed according to the manufacturers’ instructions. We observed pathological changes using an optical microscope.
Statistical Analysis
Data were recorded and collected properly, then statistically analyzed with SPSS software package (Version 19.0, IBM Inc., Armonk, New York, USA). All values are expressed as mean values and standard deviations. The quantitative data were normally distributed (as determined by the Kolmogorov and Smirnov test). For HOMA-IR, FBG, HbA1c, lipids, and AUC data, comparisons between groups were analyzed by one-way ANOVA, and group comparisons were analyzed using the Student-Newman-Keuls test. P values <0.05 were regarded as statistical significance.
Results
Decreased Body Weight And Relative Abdominal Fat Weight
The intake of a high-fat diet for 8 weeks led to a further body weight increase in metabolically abnormal obese db/db mice, and the final body weight of the model control was 69.6 ± 3.5 g. At the eighth week, the final body weights were 69.6 ± 3.5 g, 65.2 ± 3.7 g, 67.4 ± 3.6 g and 65.7 ± 4.4 g for the model control, positive control, and DHM (0.5 and 1.0 g/kg BW) groups, respectively ().
Table 1 Effect Of DHM On Body Weight In High-Fat Diet Induced db/db Mice
There was no significant difference in food or water consumption among the four groups (data not shown). Db/db mice were extremely obese and inactive. There was no difference in physical activity among the four groups. As shows, the relative abdominal fat weights for the model control, positive control, and DHM (0.5 and 1.0 g/kg BW) groups were 9.79± 2.76, 8.91± 2.83, 8.98± 2.64, 8.20 ±1.61 g/100 g body weight, respectively. Intervention with a high dose (1.0 g/kg BW) of DHM reduced the abdominal fat weight, with a significant difference compared to the model control group. As shows, the liver weight for the model control, positive control, and DHM (0.5 and 1.0 g/kg BW) groups were 2.99±0.24, 2.62±0.352.65±0.312.61± 0.33 g, respectively. Intervention with a high dose (1.0 g/kg BW) of DHM reduced the liver weight and liver weight/body weight, with a significant difference compared to the model control group.
Table 2 Effect Of DHM On Liver Weight And Liver Weight/body Weight In Mice
Decreased Fasting Blood Glucose Levels And HbA1c Levels And Improved Glucose Tolerance
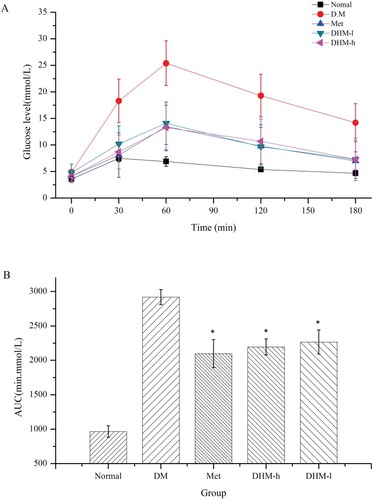
Intervention with DHM (1.0, 0.5 g/kg BW) or metformin (50 mg/kg) did not significantly affect food or water intake in db/db mice. As and shown, DHM intervention resulted in a dose-related reduction in FBG and HbA1c. As reported in the literature, metformin significantly lowered the levels of FBG and HbA1c ( and ) in metabolically abnormal obese db/db mice. In the OGTT, the metabolically abnormal obese db/db mice that received oral DHM treatment (1.0 and 0.5 g/kg) for 8 weeks demonstrated a significant suppressed of elevated plasma glucose levels 30, 60, 120, and 180 min after the ingestion of a single high dose of glucose (). Correspondingly, the area under the glucose response curve (AUC, min·mmol/L, ) in the DHM-treated groups was decreased significantly compared to that in the diabetic control group. The intervention of metformin significantly reduced the plasma glucose levels 30, 60, 120, and 180 min after the glucose ingestion, and the area under the glucose response curve.
Figure 3 HbA1c levels in db/db mice at week 8. DHM treatment significantly reduced the HbA1c level. Normal: C57BL, DM: untreated metabolically abnormal obese db/db untreated mice, Met: db/db mice treated with metformin, DHM-h, l: db/db mice treated with DHM at 1.0 and 0.5 g/kg BW. ##p<0.05 compared with the normal group. **p<0.05 compared with the DM group.
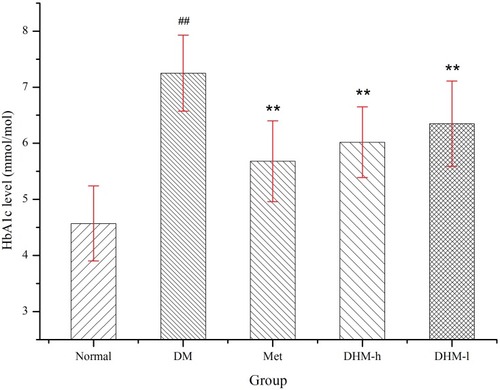
Figure 4 OGTT levels in db/db mice at week 8. Compared to diabetic control mice, metabolically abnormal obese db/db mice (groups 4 and 5) that received oral DHM (1.0 and 0.5 g/kg) intervention for 8 weeks exhibited a significant suppression of elevated plasma glucose levels 30, 60, 120, and 180 min after the ingestion of a single high dose of glucose (Figure 4A), as well as a decreased area under the glucose response curve (AUC, min·mmol/L, Figure 4B). Normal: C57BL, DM: untreated metabolically abnormal obese db/db mice, Met: db/db mice treated with metformin, DHM-h, l: db/db mice treated with DHM at 1.0 and 0.5 g/kg BW. *p<0.01 compared with the DM group.
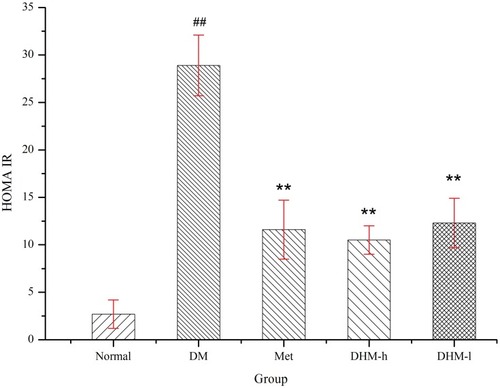
The serum insulin level increased significantly in the metabolically abnormal obese db/db mice compared to that in the normal control group. We observed significant differences in the HOMA insulin resistance (HOMA-IR) index between the DHM-treated (1.0 g and 0.5 g/kg BW), metformin-treated (50 mg/kg) and untreated metabolically abnormal obese db/db mice. As shows, DHM or metformin intervention led to a significant reduction in the HOMA-IR index compared with that of the untreated metabolically abnormal obese db/db mice.
Figure 5 HOMA-IR index in db/db mice at week 18. DHM treatment significantly reduced the HOMA-IR index. Normal: C57BL, DM: untreated db/db mice, Met: metabolically abnormal obese db/db mice treated with metformin, DHM-h and DHM-l: db/db mice treated with DHM at 1.0 and 0.5 g/kg BW. ##p<0.05 compared with the normal group. **p<0.05 compared with the DM group.
Reduced Blood Lipid Levels Regulated Inflammatory Mediators
The serum levels of TC and TG increased significantly, revealing dyslipidemia in the metabolically abnormal obese db/db mice. Compared with those of the obese control mice, serum the concentrations of TC and TG decreased significantly in the Met and DHM intervention groups (shown in ).
Table 3 Effect Of DHM On Lipid Profile In Mice
shows the parameters of redox behavior in metabolically abnormal obese mice. Compared to the model control group, the group treated with DHM for 8 weeks exhibited altered levels of oxidative stress markers, demonstrating the antioxidant effects of DHM. The level of malondialdehyde (MDA), the end-product of lipid peroxidation, increased significantly in the model group versus the normal controls. DHM intervention notably reduced MDA levels. Compared with that in the normal control group, the activity of the antioxidant enzyme superoxide dismutase (SOD) was significantly lower (P < 0.05) in the metabolically abnormal obese mice. Moreover, DHM treatment yielded significantly higher activity of antioxidant enzymes.
Table 4 Effect Of DHM On Biochemical Parameter In Mice
It has been reported previously in the literature that low-grade chronic inflammation play a key role in peripheral insulin resistance, which significantly increases the risk of developing T2DM.Citation36,Citation37 Controlling inflammation may be a core method for improving insulin sensitivity and subsequently reducing the risk of developing T2DM. This may also reduce the risk of cardiovascular disease in this high-risk group.Citation2,Citation38,Citation39 Our results indicated that DHM treatment significantly decreased the serum levels of tumor necrosis factor alpha (TNF-α) and IL-1β () compared with those in the metabolically abnormal obese group.
Histological Profiles Of The Effect Of DHM On Lesion Severity In Mice
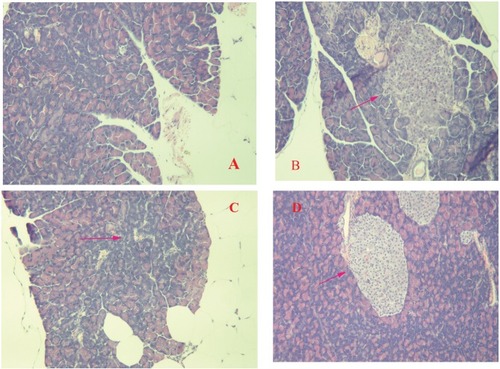
The effects of DHM as determined by histopathologic examination are shown in . The pancreas of the normal control group showed no significant histopathological changes and normal islet cell architecture (). The metabolically abnormal obese group exhibited evidence of severe damage characterized by a reduced number and area of islets, the transformation of the borders, vacuolation, and the degranulation of cells (). However, DHM intervention restored the damaged islets and obviously ameliorated their structure (D).
Figure 6 Histopathological changes in the pancreas of db/db mice. The diabetic control group showed evidence of severe damage characterized by a reduced number and area of islets, the transformation of borders, vacuolation, and the degranulation of cells (B), whereas the normal control group exhibited islets with typical histological structure in the pancreas (A). The administration of Met and DHM restored the damaged islets, obviously ameliorating their structure (C and D). Red arrow symbols mean pancreatic lesions.
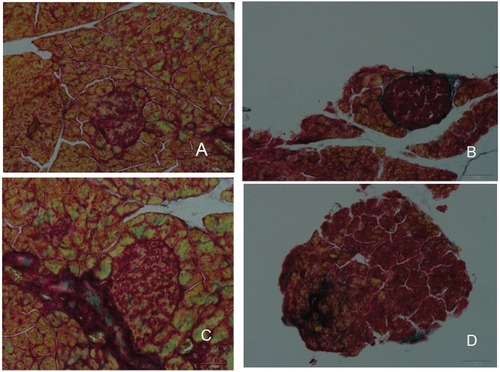
Islet fibrosis, as an important outcome of progressive β-cell failure, plays an important role in the progression and development of type 2 diabetes.Citation38,Citation40 The effects of DHM intervention on the attenuation of fibrosis are shown in . As demonstrated by Masson trichrome staining, islet fibrosis within the islets was significantly increased in the diabetic model mice (). However, it was dramatically reduced in the DHM-treated group (), as revealed by the percent area stained per islet section.
Figure 7 Histopathological changes in islet fibrosis in db/db mice. The diabetic control group showed evidence of severe damage characterized by the degranulation of β-cells and islet fibrosis (B), whereas the normal control group exhibited islets with typical histological structure in the pancreas (A). The administration of Met and DHM prevented islet fibrosis (C and D).
The pathological deterioration of the vessels was demonstrated by thicker and rough vessel inner walls, extensive arterial plaques containing foam cells, inflammatory cells, cholesterol crystals, and tissue calcification in the metabolically abnormal obese models. The pathological changes decreased visibly in the DHM-treated group, compared with the model group. The vessel walls were slightly thickened and rough, and there were fewer arterial plaques, in the DHM-treated mice.
Effect Of DHM On Insulin Receptor Substrate 1 (ISR-1) And Phospho-Insulin Receptor Substrate 1 (p-ISR-1Tyr612) Protein Expression In Liver Tissue
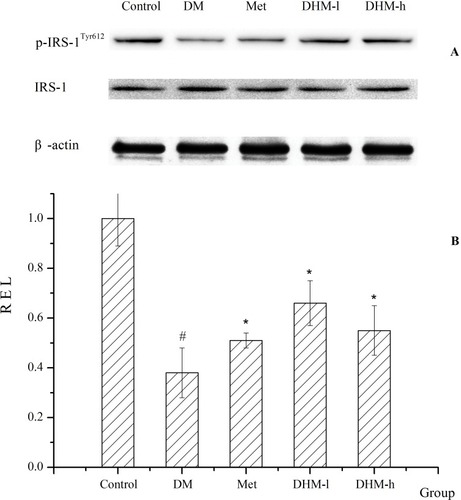
We examined the protein expression of insulin receptor substrate-1 and phosphorylated insulin receptor substrate-1 (p-ISR-1Tyr612, the active form) and other major constituents of the insulin signaling pathway to elucidate the molecular mechanism of the alleviation of insulin resistance and hyperglycemia (shown in ).Citation41–Citation43 As shown in , the level of phosphorylation of ISR-1Tyr612 was reduced significantly in the liver tissue of db/db mice compared with that of the normal control group. Increased p-ISR-1 Tyr612 level was observed in the DHM group. The administration of DHM induced a higher level of phosphorylation of ISR-1Tyr612 than that of the diabetic model group. There was statistically significant between groups. As expected, p-ISR-1Tyr612 level increased significantly in the Met group.
Figure 8 Protein expression of ISR-1 and p-ISR-1Tyr612. The protein expression of insulin receptor substrate 1 and phosphorylated insulin receptor substrate 1 in liver tissue are shown in (A). The quantitative analysis is shown in (B). The results indicate that the levels of phosphorylation of ISR-1 Tyr612 were markedly reduced in the db/db mice compared with the normal control group. Increased ISR-1 Tyr612 phosphorylation was observed in the liver tissue of the DHM group. The DHM-administered group showed higher ISR-1 Tyr612 phosphorylation than that in the diabetic model group, and the difference was statistically significant. As expected, ISR-1 Tyr612 phosphorylation increased significantly in the Met group. *p<0.05 compared with the DM group. # p<0.05 compared with the control group.
Discussions
Exploring new functional components from traditional medicine is a wise strategy.Citation12,Citation13 This paper aimed to evaluate the antidiabetic, lipid-lowering and antioxidative function of DHM and to explore the underlying mechanism. Pancreatic β-cell failure and apoptosis play a primary and pivotal role in the pathophysiologic progression and development of T2DM.Citation3,Citation36 We demonstrated that DHM has an antidiabetic effect in db/db mice, which was supported by its hypoglycemic and hypolipidemic effects, and its ability to protect islet cells and delay islet cell fibrosis. More importantly, the present study indicated that DHM treatment reduced insulin resistance, as evidenced by the decrease in the HOMA-IR index. This may partially explain the mechanism of the hypoglycemic effect.
In humans, β-cell mass expansion has been considered a response to the increased insulin demands imposed by insulin resistance. Excessive β-cell compensation leads to the dysfunction and destruction of fibrotic islets which are important features of pathological deterioration. Meanwhile, islet fibrosis may accelerate β-cell destruction, such as that in chronic pancreatitis, and induce the disruption of β-cell connectivity, consequently further impairing insulin secretion.
According to the previous literature, islet fibrosis aggravates pathological deterioration in the late stage of β-cell dysfunction during the progression of T2DM.Citation36,Citation40 Oxidative stress has been linked to obesity and associated cardiovascular diseases. Increased oxidative stress is considered a critical cause of insulin resistance, dyslipidemia, β-cell dysfunction, islet fibrosis, and impaired glucose tolerance and ultimately leads to T2DM.Citation44 We assessed the levels of inflammatory mediators and oxidative stress markers (malondialdehyde) and antioxidant enzyme (superoxide dismutase) activity. Oxidative stress has been recognized as a multifactorial etiological factor contributing to insulin resistance and the fibrosis of various organs in the body. Chronic islet inflammation, fibrosis and the loss of parenchymal cells in the pancreas synergistically contribute to the irreversibility of the progression of the disease. Pancreatic fibrosis severely impairs the exocrine and endocrine functions of the pancreas, diminishing the patient’s quality of life.
These results suggest that DHM may have protective effects against chronic inflammation and oxidative stress. In the animal trial, DHM exerted strong hypoglycemic and hypolipidemic effects, reduced insulin resistance, and decreased the relative abdominal fat weight. Treatment with DHM attenuated the progression of insulin resistance and pancreatic fibrosis in fatty db/db mice. This discovery brings hope to T2DM treatment and management since therapeutic intervention reduced pancreatic injury, protected islet cells and delayed islet cell fibrosis in the animals.
For the obesity control effect, the animal weight gain coincided with the abdominal fat raise. The lipid deposition in the arterial inner wall accelerated the progression pathological deterioration of aorta. This study showed that natural product DHM might protect the vascular endothelial function by lowering plasma lipid levels and blocking the oxidation of LDL.
Insulin resistance is a set of pathophysiological complications involved in the induction and worsening of many chronic diseases including T2DM. In the T2DM patient, insulin resistance is a pathological state in which the target cells in liver, skeletal muscle, and adipose tissue fail to respond properly to insulin, resulting in hyperglycemia. Serine/tyrosine phosphorylation of IRS-proteins has been implicated in the modulation of insulin signaling and may contribute to the development of insulin resistance.Citation41,Citation42,Citation45–Citation47 This study confirmed that DHM administration elevated IRS-1 Tyr612 protein phosphorylation in liver tissue when compared with that in the control, suggesting that DHM restored the impaired insulin signaling pathway. The results indicated that the phosphorylation of IRS-1 caused by DHM administration was strongly related to the improvement of insulin resistance.
We suggest that IRS-1 (Y612) tyrosine phosphorylation may play a major role in IR in T2DM, and p-IRS-1 protein activation may be a reasonable potential molecular target for attenuating the progression of metabolic syndrome.
Conclusions
In conclusion, our findings showed that DHM exerts a strong hypoglycemic effect and reduces insulin resistance. In addition, DHM treatment also markedly decreased the relative abdominal fat weight. Western blot analysis revealed that DHM upregulates the IRS-1 (Y612) tyrosine phosphorylation, resulting in intracellular transduction of the insulin signal, improving insulin resistance. Treatment with DHM attenuates the progression of insulin resistance and pancreatic fibrosis in fatty db/db mice. Overall, DHM may prove to be a promising approach for preventing metabolic syndrome.
Abbreviations
MetS, metabolic syndrome; DHM, dihydromyricetin; db/db, C57BL/KsJ-db/db, T2DM, type 2 diabetes mellitus; Met, metformin; Hb1Ac, glycated hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; IRS-1, insulin receptor substrate-1; TCM, traditional Chinese medicine; TC, serum total cholesterol; TG, triglyceride; MDA, malondialdehyde; SOD, superoxide dismutase; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor alpha; HDL-C, high density liptein cholesterol.
Acknowledgements
The authors gratefully acknowledge the financial assistance of the National Major Drug Discovery Projects (No: 2017ZX 09301025). The authors thank the scientific editors from American Journal Experts for providing professional English language editing of this paper.
Disclosure
Dr Long Cheng reports a grant from National Major Drug Discovery Projects (No: 2017ZX 09301025), during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- Rayyan Assi H , Ziv A , Dankner R . The metabolic syndrome and its components are differentially associated with chronic diseases in a high‐risk population of 350 000 adults: A cross‐sectional study. Diabetes Metab Res Rev . 2019;35:e3121. doi:10.1002/dmrr.v35.4 30600581
- Lent-Schochet D , McLaughlin M , Ramakrishnan N , Jialal I . Exploratory metabolomics of metabolic syndrome: a status report. World J Diabetes . 2019;10:23–36. doi:10.4239/wjd.v10.i1.23 30697368
- Daskalopoulos G , Karkanaki A , Piouka A , et al. Excess metabolic and cardiovascular risk is not manifested in all phenotypes of polycystic ovary syndrome: implications for diagnosis and treatment. Curr Vasc Pharmacol . 2015;13:788–800.25600030
- Mogre V , Salifu ZS , Abedandi R . Prevalence, components and associated demographic and lifestyle factors of the metabolic syndrome in type 2 diabetes mellitus. J Diabetes Metab Disord . 2014;13:80. doi:10.1186/2251-6581-13-80 25054102
- Haffner SM . Risk constellations in patients with the metabolic syndrome: epidemiology, diagnosis, and treatment patterns. Am J Med . 2006;119:S3–S9. doi:10.1016/j.amjmed.2006.01.008
- Punthakee Z , Goldenberg R , Katz P . C. Diabetes Canada clinical practice guidelines expert. Can J Diabetes . 2018;42(Suppl 1):S10–S15. doi:10.1016/j.jcjd.2017.10.003 29650080
- Engin A . The Definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol . 2017;960:1–17. doi:10.1007/978-3-319-48382-5_1 28585193
- Kim M , Kim IH , Lim MK , Kim Y , Park B . Increased prevalence of metabolic syndrome in adult cancer survivors: asian first report in community setting. Cancer Epidemiol . 2019;58:130–136. doi:10.1016/j.canep.2018.12.006 30576983
- Kim JH , Yoo JY , Kim HS . Metabolic syndrome in South Korean patients with chronic obstructive pulmonary disease: a focus on gender differences. Asian Nurs Res (Korean Soc Nurs Sci) . 2019;13:137–146. doi:10.1016/j.anr.2019.03.002 30910730
- Misra A , Bhardwaj S . Obesity and the metabolic syndrome in developing countries: focus on South Asians. Nestle Nutr Inst Workshop Ser . 2014;78:133–140. doi:10.1159/000354952 24504214
- Misra A , Soares MJ , Mohan V , et al. Body fat, metabolic syndrome and hyperglycemia in South Asians. J Diabetes Complications . 2018;32:1068–1075. doi:10.1016/j.jdiacomp.2018.08.001 30115487
- Zhang F , Xie JM , Zhang YY , Kong LL , Li SC . What is important during the selection of Traditional Chinese Medicine (TCM) in a health care reimbursement or insurance system? Critical issues of assessment from the perspective of TCM Practitioners. Value Health Reg Issues . 2013;2:141–146. doi:10.1016/j.vhri.2013.01.004 29702843
- Xu J , Yang Y . Traditional Chinese medicine in the Chinese health care system. Health Policy . 2009;90:133–139. doi:10.1016/j.healthpol.2008.09.003 18947898
- Zou L , Zhang Y , Sasaki JE , et al. Wuqinxi Qigong as an Alternative Exercise for Improving Risk Factors Associated with Metabolic Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int J Environ Res Public Health . 2019;16.
- Patel S . Functional food red yeast rice (RYR) for metabolic syndrome amelioration: a review on pros and cons. World J Microbiol Biotechnol . 2016;32:87. doi:10.1007/s11274-016-2035-2 27038957
- Song HP , Wu SQ , Qi LW , et al. A strategy for screening active lead compounds and functional compound combinations from herbal medicines based on pharmacophore filtering and knockout/knockin chromatography. J Chromatogr A . 2016;1456:176–186. doi:10.1016/j.chroma.2016.06.009 27320377
- Fan L , Zhao X , Tong Q , et al. Interactions of dihydromyricetin, a flavonoid from vine tea (Ampelopsis grossedentata) with gut microbiota. J Food Sci . 2018;83:1444–1453. doi:10.1111/1750-3841.14128 29660761
- Wan W , Jiang B , Sun L , Xu L , Xiao P , Peterson JM . Metabolomics reveals that vine tea (Ampelopsis grossedentata) prevents high-fat-diet-induced metabolism disorder by improving glucose homeostasis in rats. PLoS One . 2017;12:e0182830. doi:10.1371/journal.pone.0182830 28813453
- Gao Q , Ma R , Chen L , et al. Antioxidant profiling of vine tea (Ampelopsis grossedentata): off-line coupling heart-cutting HSCCC with HPLC-DAD-QTOF-MS/MS. Food Chem . 2017;225:55–61. doi:10.1016/j.foodchem.2016.11.122 28193433
- Wu J , Wang C , Huang G , et al. Biotransformation of vine tea (Ampelopsis grossedentata) by solid-state fermentation using medicinal fungus Poria cocos. J Food Sci Technol . 2016;53:3225–3232. doi:10.1007/s13197-016-2297-6 27784917
- Ye L , Wang H , Duncan SE , Eigel WN , O’Keefe SF . Antioxidant activities of vine tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem . 2015;172:416–422. doi:10.1016/j.foodchem.2014.09.090 25442572
- Chen T , Zhu S , Lu Y , et al. Probing the interaction of anti-cancer agent dihydromyricetin with human serum albumin: a typical method study. Anticancer Agents Med Chem . 2012;12:919–928.22292768
- Dong S , Ji J , Hu L , Wang H . Dihydromyricetin alleviates acetaminophen-induced liver injury via the regulation of transformation, lipid homeostasis, cell death and regeneration . Life Sci . 2019.
- Huang H , Hu M , Zhao R , Li P , Li M . Dihydromyricetin suppresses the proliferation of hepatocellular carcinoma cells by inducing G2/M arrest through the Chk1/Chk2/Cdc25C pathway. Oncol Rep . 2013;30:2467–2475. doi:10.3892/or.2013.2705 24002546
- Li H , Yu F , Sun X , Xu L , Miu J , Xiao P . Dihydromyricetin ameliorates memory impairment induced by acute sleep deprivation. Eur J Pharmacol . 2019;853:220–228. doi:10.1016/j.ejphar.2019.03.014 30876981
- Li Q , Wang J , Zhu X , et al. Dihydromyricetin prevents monocrotaline-induced pulmonary arterial hypertension in rats. Biomed Pharmacother . 2017;96:825–833. doi:10.1016/j.biopha.2017.10.007 29078260
- Peng J , Zhang J , Zhang L , Tian Y , Li Y , Qiao L . Dihydromyricetin improves vascular hyporesponsiveness in experimental sepsis via attenuating the over-excited MaxiK and KATP channels. Pharm Biol . 2018;56:344–350. doi:10.1080/13880209.2018.1478430 30003825
- Ren Z , Yan P , Zhu L , et al. Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacology . 2018;235:233–244. doi:10.1007/s00213-017-4761-z 29058041
- Shen Y , Lindemeyer AK , Gonzalez C , et al. Dihydromyricetin as a novel anti-alcohol intoxication medication. J Neurosci . 2012;32:390–401. doi:10.1523/JNEUROSCI.4639-11.2012 22219299
- Wang YC , Liu QX , Zheng Q , et al. Dihydromyricetin Alleviates Sepsis-Induced Acute Lung Injury through Inhibiting NLRP3 Inflammasome-Dependent Pyroptosis in Mice Model. Inflammation . 2019.
- Ye X , Pang Z , Zhu N . Dihydromyricetin attenuates hypertrophic scar formation by targeting activin receptor-like kinase 5. Eur J Pharmacol . 2019;852:58–67. doi:10.1016/j.ejphar.2019.02.039 30807748
- Zhang YS , Ning ZX , Yang SZ , Wu H . Antioxidation properties and mechanism of action of dihydromyricetin from ampelopsis grossedentata. Yao Xue Xue Bao . 2003;38:241–244.12889119
- Zuo Y , Xu Q , Lu Y , et al. Dihydromyricetin induces apoptosis in a human choriocarcinoma cell line. Oncol Lett . 2018;16:4229–4234. doi:10.3892/ol.2018.9220 30214558
- Matthews DR , Hosker JP , Rudenski AS , Naylor BA , Treacher DF , Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia . 1985;28:412–419. doi:10.1007/bf00280883 3899825
- Wallace TM , Levy JC , Matthews DR . Use and abuse of HOMA modeling. Diabetes Care . 2004;27:1487–1495. doi:10.2337/diacare.27.6.1487 15161807
- Alejandro EU , Gregg B , Blandino-Rosano M , Cras-Meneur C , Bernal-Mizrachi E . Natural history of β-cell adaptation and failure in type 2 diabetes. Mol Aspects Med . 2015;42:19–41. doi:10.1016/j.mam.2014.12.002 25542976
- Goldenberg R , Punthakee Z . C. Canadian Diabetes Association clinical practice guidelines expert. Can J Diabetes . 2013;37(Suppl 1):S8–S11. doi:10.1016/j.jcjd.2013.01.011 24070969
- Petrie JR , Guzik TJ , Touyz RM . Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol . 2018;34:575–584. doi:10.1016/j.cjca.2017.12.005 29459239
- Katakami N . Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus.. J Atheroscler Thromb . 2018;25:27–39. doi:10.5551/jat.RV17014 28966336
- Nelson L , Turkel S , Shulman I , Gabbe S . Pancreatic-islet fibrosis in young infants of diabetic mothers. Lancet . 1977;2:362–363. doi:10.1016/s0140-6736(77)91531-8
- Alipourfard I , Datukishvili N , Mikeladze D . TNF-α downregulation modifies Insulin Receptor Substrate 1 (IRS-1) in metabolic signaling of diabetic insulin-resistant hepatocytes. Mediators Inflamm . 2019;2019:1–6. doi:10.1155/2019/3560819
- Rashidi B , Azizy L , Najmeddin F , Azizi E . Prevalence of the insulin receptor substrate-1(IRS-1) Gly972Arg and the insulin receptor substrate-2(IRS-2) Gly1057Asp polymorphisms in PCOS patients and non-diabetic healthy women. J Assist Reprod Genet . 2012;29:195–201. doi:10.1007/s10815-011-9693-7 22205343
- Burgos-Ramos E , Gonzalez-Rodriguez A , Canelles S , et al. Differential insulin receptor substrate-1 (IRS1)-related modulation of neuropeptide Y and proopiomelanocortin expression in nondiabetic and diabetic IRS2-/- mice. Endocrinology . 2012;153:1129–1140. doi:10.1210/en.2011-1278 22210743
- Tangvarasittichai S . Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes . 2015;6:456–480. doi:10.4239/wjd.v6.i3.456 25897356
- Evert M , Sun J , Pichler S , Slavova N , Schneider-Stock R , Dombrowski F . Insulin receptor, insulin receptor substrate-1, Raf-1, and Mek-1 during hormonal hepatocarcinogenesis by intrahepatic pancreatic islet transplantation in diabetic rats. Cancer Res . 2004;64:8093–8100. doi:10.1158/0008-5472.CAN-04-2040 15520221
- Fernandez-Twinn DS , Alfaradhi MZ , Martin-Gronert MS , et al. Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol Metab . 2014;3:325–333. doi:10.1016/j.molmet.2014.01.007 24749062
- Badin PM , Louche K , Mairal A , et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes . 2011;60:1734–1742. doi:10.2337/db10-1364 21498783
