Abstract
Background
Using chemical agents in the treatment of diabetes mellitus type 2 may have some limitations due to frequent side effects. Some novel and natural agents may be promising alternatives in this case. This study was designed to evaluate the effects of oral Japanese sake yeast supplement, as a novel agent, on biochemical antioxidant and anti-inflammatory parameters in experimentally induced diabetic rats.
Materials and methods
After inducing diabetes (55 mg/kg intraperitoneal injection of streptozotocin), 120 male adult Wistar rats were randomly divided into 5 groups and each group received 0 (control), 15, 30, or 45 mg/kg of sake yeast or was considered a nondiabetic control. Then, the serum levels of tumor necrosis factor-α, IL-6, C-reactive protein, malondialdehyde, glutathione, total antioxidant status, glucose, cholesterol, triglycerides, and insulin were evaluated and compared to baseline measures.
Results
The results showed that oral administration of sake yeast at different concentrations reduced levels of malondialdehyde, glucose, cholesterol, and triglycerides and increased levels of insulin, glutathione, and total antioxidants (P<0.05). The best responses were observed in the nondiabetic control group.
Conclusion
Sake yeast supplement may be useful as a novel agent in the treatment of diabetes.
Introduction
Diabetes mellitus is known as one of the most common metabolic diseases that is accompanied by chronic complications including nephropathy, angiopathy, retinopathy, and peripheral neuropathy.Citation1 The diagnosis of diabetes mellitus is usually based on hyperglycemia and glucose intolerance.Citation2 Increased blood glucose, insulin resistance, and rising inflammatory markers have been reported in patients with diabetes mellitus.Citation3 Regarding inflammatory factors, a positive relation between levels of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and IL-6, and insulin resistance has been found.Citation4,Citation5 TNF-α is known to play a role in insulin resistance and acts as a mediator between obesity and inflammatory diseases such as heart disease and type 2 diabetes.Citation6 IL-6 and TNF-α are associated with declined glycemic control and endothelial disorder in diabetic patients.Citation7 C-reactive protein (CRP) has also been reported to have a role in the initiation and aggravation of the classical pathways and promotion of atherosclerosis in diabetic patients.Citation8 Imbalance between oxidant and antioxidant factors may result in oxidative stress which may increase the production of free radicals and reduces antioxidant factors.Citation9 It is known that antioxidants are capable of removing free radicals and ROS by preventing lipid peroxidation and reducing the side effects that are caused by ROS.Citation10 Synthetic agents have been widely used in the treatment of diabetes, but they may be confronted with major limitations due to their possible side effects.Citation5 Therefore, the use of safe and novel agents for the treatment of diabetes is promising.
Sake yeast, Japanese rice wine, has been used in the life and culture of Japanese people for a long time. Sake is a brewed alcoholic beverage, but the brewing process is more complex in comparison with other alcoholic beverages.Citation11 It is fermented from steamed white rice using koji and yeast. Studies have shown that sake is made up of water and ethanol, D-glucose, ethyl α-D-glucoside, glycerol, organic acids, and amino acids.Citation12 Kido et alCitation13 stated that high intake of alcoholic beverages with a meal may cause different responses in postprandial glucose and insulin concentrations. Studies showed that Japanese sake yeast enriched in adenosine analogs activates A2a receptors and its supplementation improved sleep quality.Citation14 Adenosine has several physiological and pharmacological roles in some diseases. These roles occur through four known adenosine receptor subtypes (A1, A2A, A2B, and A3). It has been reported that A2AAR agonist ameliorates histological and functional changes in kidneys induced by diabetes and reduced inflammation associated with diabetic nephropathy.Citation15 There is no study in this area and the effect of sake yeast on diabetes is unknown. Therefore, we aimed to study the effects of sake yeast supplement (GSP6) on hyperglycemia beside antioxidant and anti-inflammatory mechanisms. This study was thus conducted to evaluate how sake yeast supplement can influence antioxidant and anti-inflammatory parameters of diabetic rats as a novel therapeutic agent.
Materials and methods
Animals
All experiments were approved by the Ethical Committee of the International Center for Intelligent Research-ICIR (ICIR-2018–185736). The experiments were conducted in accordance with the guideline of the National Institutes of Health (NIH Publication No. 85–23, revised 1996) for the care and use of laboratory animals. Wistar male rats with a weight of 200±10 g were purchased from Pastur Institute, Tehran, Iran. A total number of 120 Wistar rats were randomly divided into 5 groups and each group had 4 subgroups (n=6). Rats were hosted in well-ventilated and spacious stainless-steel cages for free movement and were fed with a standard food prepared in Javaneh Khorasan, Iran with free access to fresh water. Feed included a chow standard diet containing 3 kcal/kh energy, 20% protein, 3% fat, 4% ash, and 6% fiber. Hosting conditions included standard temperature (22±2 °C) and humidity (55±5%) and a light/dark cycle of 12 h. After inducing diabetes in rats, animals were treated with dried sake yeast powder (GSP6 as a gift from Dr Yuki Nagamori, Lion Corporation, Odawara-shi, Japan) as a food supplement for 4 weeks. The powder was dispersed in saline shortly before use and administered to animals in different doses including 0 (control), 15, 30, or 45 mg/kg by oral gavage. A nondiabetic group was also considered. The diabetic and nondiabetic control rats received saline alone and gavage solutions were refreshed daily. Body weight was measured initially and at the end of the study. The dose of sake yeast was chosen based on dose–response in the pilot study.
Pilot study
To select the optimum doses, 36 Wistar rats were deprived of food for 24 h and then were equally divided into 6 groups. Animals received the supplement in doses of 5, 10, 15, 20, 30, 45, and 60 mg/kg and were monitored for 48 h for any toxicity sign including convulsions, ataxia, hypoactivity and ventilation disorders, behavioral alteration, and related mortality. No toxicity sign was observed in this period. Finally, we selected 15, 30, and 45 mg/kg as experimental doses for further studies.
Induction of diabetes
To induce experimental diabetes, 55 mg/kg of streptozotocin (STZ; Sigma-Aldrich Co., St Louis, MO, USA) in 0.1 molar citrate buffer with pH 4.5 was intraperitoneally injected into each animal.Citation5 After 3 days, blood samples were evaluated from each rat using a glucometer (Accu-Check; Hoffman-La Roche Ltd., Basel, Switzerland). Animals with a glucose level >250 mg/dL were considered diabetic.Citation16
Blood sampling and blood variables
At the end of the trial and following 24 h of fasting, 60 mg/kg of sodium phenobarbital was injected to induce anesthesia. Blood samples were taken into tubes without any heparin. The samples were then centrifuged at 4000 RPM for 10 min at 4 °C and kept at −20 °C for further experiments. ELISA was applied for assessment of the levels of TNF-α and IL-6 following the instruction guide. The levels of TNF-α and IL-6 were evaluated using cytokine-specific monoclonal antibodies.Citation5 The thiobarbituric acid reactive substances procedure was applied to evaluate the serum malondialdehyde (MDA) as suggested by Biswas et al.Citation17 The serum concentration of glutathione (GSH) was evaluated using Ellman’s reagent (5,50-dithio-bis-2-nitrobenzoic acid).Citation18 We used the ferric reducing antioxidant power procedure to evaluate the total antioxidant status (TAS) as reported by Budin et al.Citation19 CRP was assessed using the CRP ELISA Kit (Thermo Fisher Scientific, Waltham, MA, USA). Commercially available kits (Pars Azmoon, Iran) were also used to examine the serum concentrations of glucose, cholesterol, triglycerides, and insulin.
Statistical analysis
Data were analyzed with GraphPad Prism statistical software (GraphPad Software, Inc., La Jolla, CA, USA). ANOVA and Dunnett’s Multiple Range Test were used to assess significance. The results are presented as mean±SD. P<0.05 was considered statistically significant.
Results
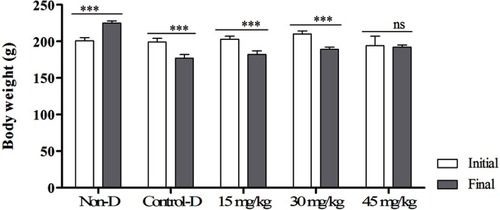
Effects of different doses of sake yeast supplementation on body weight are shown in . As the results show, body weight was increased in nondiabetic rats (P<0.05), but was decreased in diabetic control rats and rats administered 15 and 30 mg/kg supplement (P<0.05). There was no observed significant difference between initial and final weights in rats treated with 45 mg/kg of supplement (P<0.05).
Figure 1 Effects of different doses of the supplement (GSP6) on body weight (g) in diabetic rats.
Abbreviations: Control-D, control diabetic; Non-D, nondiabetic.
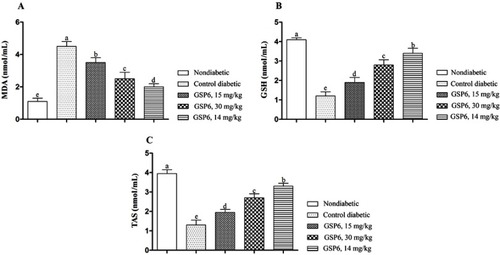
Effects of different doses of sake yeast supplementation on serum concentrations of oxidative stress and antioxidant markers are shown in . Results indicate that oral administration of sake yeast reduced the levels of the lipid peroxidation marker MDA (; P<0.05) and increased the levels of the antioxidants GSH () and TAS () in a dose-dependent manner (P<0.05). Nondiabetic rats showed better response in comparison to other groups.
Figure 2 Effects of different doses of the supplement (GSP6) on the antioxidant status in diabetic rats. (A) Malondialdehyde (MDA). (B) Glutathione (GSH). (C) Total antioxidant status (TAS).
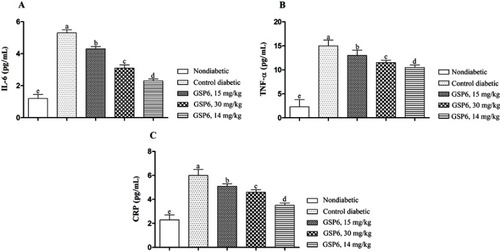
Next, the effects of experimental sake yeast treatment on levels of proinflammatory cytokines were analyzed and are shown in . Results revealed a dose-dependent and significant decrease of serum concentrations of IL-6 (), TNF-α (), and CRP () with increasing sake yeast supplementation (P<0.05). The lowest level of proinflammatory cytokines was observed by applying sake yeast at a concentration of 45 mg/kg and in the nondiabetic control (P<0.05).
Figure 3 Effects of different doses of the supplement (GSP6) on proinflammatory factors in diabetic rats. (A) IL-6. (B) Tumor necrosis factor-α (TNF-α). (C) C-reactive protein (CRP).
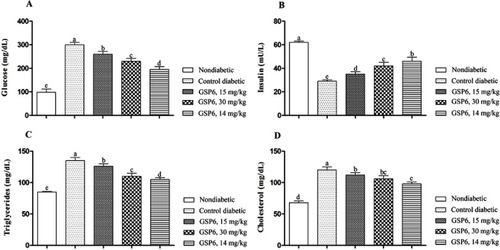
The effects of experimental sake yeast supplementation on blood biochemical parameters are shown (). The serum concentrations of glucose (), triglycerides (), and cholesterol () were significantly decreased with increasing sake yeast dosage (P<0.05). Remarkably, the serum concentration of insulin () increased in a dose-dependent manner. All treated groups showed better responses in comparison to the control group. The best responses were observed in the nondiabetic control.
Discussion
Results showed that body weight was reduced in diabetic rats and increased in the nondiabetic group. However, animals treated with a level of 45 mg/kg did not show a significant difference (P<0.05). Reduced body weight in diabetic animals was attributed to dehydration and catabolism of fats and proteins and increased catabolic reactions which result in muscle loss and finally weight loss in diabetic rats.Citation20 Maintaining the body weight in animals treated with 45 mg/kg of the supplement might be attributed to the control of hyperglycemia.
The results of the present study indicated an overall improvement of diabetes-related blood parameters induced by food supplementation with Japanese sake yeast. STZ-induced diabetic rats are normally characterized by reduced GSH and TAS as well as increased MDA levels, pointing to an imbalance toward peroxidation in this animal model.Citation5 Treatment with sake yeast supplement improved GSH and TAS levels which may be due to decreased production of free radicals and increased level of antioxidants. Previous studies have reported that STZ causes diabetes by elevating sensitivity to lipid peroxidation and oxidative stress, thus pointing to mechanisms probably involved in the pathogenesis of the disease.Citation9,Citation21 MDA has been reported as one of the products of lipid peroxidation that is rapidly mixed with biomolecules and therefore disturbs the glucose metabolism.Citation22 Sake yeast modulates the production of oxygen radicals which might be responsible for decreasing hyperglycemia, inflammation, and oxidative stress. Unfortunately, we could not find any study suggesting the effects of sake yeast on the antioxidant system. However, our study is the first to show that sake yeast, especially in higher doses, can increase the antioxidant factors and decrease lipid peroxidation (MDA). It could be concluded that sake yeast declines the level of MDA by increasing GSH and TAS.
Our findings indicated that sake yeast could decrease inflammatory factors in comparison to the control group. Inflammation is a basic biological process that is considered the background of many acute and chronic pathological conditions, and this action happens in response to the changes which restore tissue homeostasis by triggering different repair mechanisms. Appropriate regulation of such mechanisms is necessary to inhibit uncontrolled amplification of the initial inflammatory response which may otherwise lead the tissue repair process to collateral damage and development of diseases.Citation23 TNF-α is known to have adverse effects including increasing adipocyte lipolysis and alterations in the insulin signaling pathway by changing tyrosine/serine phosphorylation of insulin receptor substrates.Citation24 CRP is known as a main inflammatory factor which is regulated by IL-6, IL-1, and TNF-α and is produced in the liver in response to inflammation.Citation25 Chang et alCitation26 showed that diabetes mellitus can increase the level of nuclear factor-κB that is responsible for the formation of several proinflammatory cytokines. Importantly, our findings showed that supplementing with sake yeast decreased the inflammatory factors. Virgolici et alCitation27 illustrated elevated levels of inflammatory factors in relation to raised oxidative stress. Our findings showed that treatment with sake yeast improves the antioxidant status and may decrease inflammatory factors by improving antioxidant activity.
Increased triglycerides, cholesterol, and glucose and decreased insulin were observed in the control group compared to the diabetic and treated groups. Hypertriglyceridemia and hypercholesterolemia are known as common signs in diabetes.Citation28 Studies have also reported that STZ increases the sensitivity to lipid peroxidation.Citation9,Citation29 Decreased hepatic insulin sensitivity could be attributed to increased hepatic gluconeogenesis, postprandial hyperinsulinemia, and increased production of triglycerides in the liver cells. Hypertriglyceridemia, hypercholesterolemia, hyperglycemia, and hypoinsulinemia could be due to an increase in TNF-α. It steps up triglyceride production in plasmaCitation29 and cuts down glucose consumption in peripheral tissues by targeting insulin signaling pathways and the glucose transporter 4 (GLUT4).Citation3,Citation30 Parallel to our findings, Kido et alCitation13 have reported that high intake of alcoholic beverages with a meal causes different responses in postprandial glucose and insulin concentrations. Accordingly, sake reduces the levels of lipids and glucose through modulation in TNF-α. In addition, components of sake including ethyl α-d-glucoside, peptides, and amino acids are known to have hepatoprotective activity and hypotensive effects.Citation31,Citation32 Compounds of sake may therefore improve blood biochemical parameters.
Conclusion
Sake yeast supplement can decrease inflammatory parameters by increasing antioxidant factors. It also improved blood biochemical parameters compared to the control group. Although future studies need to be done in order to evaluate the effects of sake yeast on diabetes, our preliminary study showed that this supplement has potential for improving the treatment of diabetes. We recommend conducting human studies and the use of GSP6 as an oral supplement.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgment
The authors gratefully acknowledge Dr Yuki Nagamori for his assistance and providing the sake yeast powder GSP6.
Disclosure
The authors report no conflicts of interest in this work.
References
- Muriach M , Flores-Bellver M , Romero FJ , Barcia JM . Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev . 2014;2014:9 Article ID 102158. doi:10.1155/2014/102158
- Smyth S , Heron A . Diabetes and obesity: the twin epidemics. Nat Med . 2006;12:75–80. doi:10.1038/nm0106-75 16397575
- Tabibzadeh Dezfuli SA , Ehsani M , Lakzaei Azar O . Carvacrol alleviated negative effects of diabetes on inflammation and oxidation by modulation in gene expression of inflammatory and antioxidant system in diabetic rat model. GMJ Med . 2017;1(1):15–20. doi:10.29088/GMJM.2017.15
- Uysal KT , Wiesbrock SM , Marino MW , Hotamisligil GS . Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature . 1997;389:610–614. doi:10.1038/39335 9335502
- Mesbahzadeh B , Rajaei SA , Tarahomi P , et al. Beneficial effects of Spirogyra Neglecta Extract on antioxidant and anti-inflammatory factors in streptozotocin-induced diabetic rats. BioMol Concepts . 2018;9:184–189. doi:10.1515/bmc-2018-0015 30660132
- Coen PM , Flynn MG , Markofski MM , Pence BD , Hannemann RE . Adding exercise to rosuvastatin treatment: influence on C-reactive protein, monocyte toll-like receptor 4 expression, and inflammatory monocyte (CD14+CD16+) population. Metabolism . 2010;59:1775–1783. doi:10.1016/j.metabol.2010.05.002 20580035
- Mayadas TN . Regulation of Neutrophil Apoptosis. Molecular Basis for Microcirculatory Disorders. Paris: Springer; 2003 p. 271–287. doi:10.1007/978-2-8178-0761-4
- Hayden MR , Tyagi SC . Intimal redox stress: accelerated atherosclerosis in metabolic syndrome and type 2 diabetes mellitus. Cardiovasc Diabetol . 2002;1:3. doi:10.1186/1475-2840-1-3 12392600
- Samarghandian S , Farkhondeh T , Samini F , Borji A . Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem Res Int . 2016;2016:2645237. doi:10.1155/2016/7108261 26904286
- Abdulrahman L , Al-malki AL , El-Rabey HA . The antidiabetic effect of low doses of Moringaoleifera Lam. Seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Bio Med Res Int . 2015;2015:1–13 Article ID 381040. doi:10.1155/2015/381040
- Nakahara M , Mishima T , Hayakawa T . Effect of a sake concentrate on the epidermis of aged mice and confirmation of ethyl α-D-glucoside as its active component. Biosci Biotechnol Biochem . 2007;71(2):427–434. doi:10.1271/bbb.60489 17284832
- Tadenuma M . Sheishu no seibun wo megutte. Gendai Kagaku (in Japanese) . 1987;1:26–31.
- Kido M , Asakawa A , Koyama KK , et al. Acute effects of traditional Japanese alcohol beverages on blood glucose and polysomnography levels in healthy subjects. PeerJ . 2016;4:e1853. doi:10.7717/peerj.1853 27069795
- Monoi N , Matsuno A , Nagamori Y , et al. Japanese sake yeast supplementation improves the quality of sleep: a double‐blind randomised controlled clinical trial. J Sleep Res . 2016;25:116–123. doi:10.1111/jsr.12336 26354605
- Awad AS , Huang L , Ye H , et al. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol . 2006;290:828–837. doi:10.1152/ajprenal.00310.2005
- Nasirian F , Dadkhah M , Moradi-Kor N , Obeidavi Z . Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab Syndr Obes . 2018;11:375–380. doi:10.2147/DMSO.S172104 30104892
- Biswas D , Banerjee M , Sen G , et al. Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol Appl Pharmacol . 2008;230:57–66. doi:10.1016/j.taap.2008.02.003 18377941
- Beutler E , Duron O , Kelly BM . Improved method for the determination of blood glutathione. J Lab Clin Med . 1963;61:882–888.13967893
- Budin SB , Othman F , Louis SR , Bakar MA , Das S , Mohamed J . The effects of palm oil to cotrienol rich fraction supplementation on biochemical variables, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics (Sao Paulo) . 2009;64:235–244. doi:10.1590/s1807-59322009000300015 19330251
- Rajkumar L , Srinivasan N , Balasubramanian K , Govindarajulu P . Increased degradation of dermal collagen in diabetic rats. Indian J Exp Biol . 1991;29:1081–1083.1816088
- Yazdanparast R , Ardestani A , Jamshidi S . Experimental diabetes treated with Achilleasantolina: effect on pancreatic oxidative parameters. J Ethnopharmacol . 2007;112:13–18. doi:10.1016/j.jep.2007.01.030 17336007
- Sivaraman K , SenthilKumar GP , Sankar P , Bobby Z . Attenuation of oxidative stress, inflammation and insulin resistance by Allium sativum in fructose-fed male rats. J Clin Diagn Res . 2013;7:1860–1862. doi:10.7860/JCDR/2013/6924.3334 24179882
- Goldszmid RS , Trinchieri G . The price of immunity. Nat Immunol . 2012;13:932–938. doi:10.1038/ni.2422 22990891
- El-Abhar HS , Schaalan MF . Topiramate-induced modulation of hepatic molecular mechanisms: an aspect for its anti-insulin resistant effect. PLoS One . 2012;7(5):e37757. doi:10.1371/journal.pone.0037757 22649556
- Nicklas BJ , You T , Pahor M . Behavioral treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ . 2005;172:1199–1209. doi:10.1503/cmaj.1040769 15851714
- Chang CC , Chang CY , Huang JP , Hung LM . Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1 diabetic rats. Chin J Physio . 2012;55(3):192–201. doi:10.4077/CJP.2012.BAA012
- Virgolici B , Mohora M , Gaman L , et al. Relation between inflammation and oxidative stress markers in diabetic foot patients. Romanian. J Biophys . 2008;18(4):273–282.
- Khan BA , Abraham A , Leelamma S . Hypoglycemic action of Murraya koenigii (curry leaf) and Brassica juncea (mustard): mechanism of action. Indian J Biochem Biophys . 1995;32(2):106–108.7642200
- Sun X , Han F , Yi J , Lina H , Ben W . Effect of aspirin on the expression of hepatocyte NF-κB and serum TNF-α in streptozotocin-induced type 2 diabetic rats. Korean Med Sci . 2011;26:765–770. doi:10.3346/jkms.2011.26.6.765
- Mostafa AM , Mohamed WS , Serwah AHA , Serwah MA . Effect of diclofenac on plasma glucose level, insulin resistance, inflammatory markers and hepatocytes in diabetic albino rats. The Egyptian J Hospital Med . 2014;54:117–128. doi:10.12816/0002438
- Saito Y , Wanezaki K , Kawato A , Imayasu S . Antihypertensive effects of peptide in sake and its by-products on spontaneously hypertensive rats. Biosci Biotechnol Biochem . 1994;58:812–816. doi:10.1271/bbb.58.812 7764971
- Izu H , Hizume K , Goto K , Hirotsune M . Hepatoprotective effects of a concentrate and components of sake against galactosamine (GalN)-induced liver injury in mice. Biosci Biotechnol Biochem . 2007;71:951–957. doi:10.1271/bbb.60613 17420605