?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim
Branched-chain amino acids (BCAAs) have been reported owning curative effects in early diabetic nephropathy. However, the mechanisms of its action have not been elucidated. The aim of this study is to investigate the effect of possible mechanism(s) of BCAAs on cultured rat mesangial cells (RMCs).
Methods
RMCs were treated with high glucose (30 mmol/L) and BCAAs (10 mmol/L) respectively. Cell proliferation was detected using an MTT assay. Expression of transforming growth factor (TGF)-β1 and gremlin mRNA was detected by semiquantitative reverse-transcription (RT) PCR. TGF-β1 and fibronectin (FN) protein levels were measured using enzyme-linked immunosorbent assays (ELISAs). Gremlin, bone morphogenic protein (BMP)-7, and Smad2/3 proteins were detected by immunofluorescence. Smad1/5/8 and phosphorylated (p)-Smad1/5/8 were detected by Western blotting.
Results
The proliferation rate of the RMCs in the high glucose group alone was 1.45-times of cells in the CON group, and it was reduced by 32% upon co-treatment with BCAAs. The expression of TGF-β1, gremlin, p-Smd2/3 and FN mRNA or protein in the HG group was higher than that in the CON group. In the BCAAs group, the corresponding levels were lower than that in HG group. The expression of BMP-7 and p-Smad1/5/8 were significantly lower in the HG group than in the CON group. Moreover, the expression of BMP-7 and p-Smad1/5/8 were higher in the BCAAs group than in the HG group.
Conclusion
BCAAs showed an antidiabetic effect via reducing TGF-β1-Smad2/3 pathway and Gremlin expression and upregulating BMP-7-Smad1/5/8 pathway in rat mesangial cells, consequently lessening ECM deposition in renal tissue.
Introduction
Diabetic nephropathy (DN) is one of the most serious complications of diabetes mellitus. It is an important reason for end-stage kidney disease. Abnormal proliferation of mesangial cells is the key pathological change involved in DN. Mesangial cells can secrete extracellular matrix (ECM) components, including fibronectin (FN), collagen IV, and laminin. Accumulation of ECM components in the kidney leads to glomerular mesangial expansion and fibrosis, which, in turn, is characteristic of DN.Citation1,Citation2
Transforming growth factor-β1 (TGF-β1) plays an important role in the physiological functions and pathological states of the kidney, and it is recognized as both a fibrogenic and an inflammatory cytokine.Citation3 Previous studies have demonstrated that the induction of TGF-β1 in diabetes accelerates fibrosis of renal mesangial cells. Some reports showed that renal mesangial cells (RMCs) exposed to high glucose stress respond by increasing TGF-β1 and FN expression in vitro.Citation4 Smad proteins participate in TGF β cell signal transduction. Smad proteins include BMP-smad (Smad1/5/8) and TGF-β/activin-smad (Smad2/3). TGF-β1 binding to its receptor can activate its major downstream mediators Smad2/3 to exert its biological functions.Citation5
Bone morphogenic protein (BMP)-7 is considered as a protective factor in DN.Citation6,Citation7 A number of studies show that BMP-7 plays protective roles in cultured renal cells. Maintenance of BMP-7 activity may result in blockade of ECM accumulation in mesangial cells.Citation8 Some study showed a reversibility of TGF-β-induced epithelial-to-myofibroblast transition (EMT) by BMP-7 in proximal tubular cells.Citation9
Moreover, recent study shows that BMP-7 binding to its receptor can affect Smad1/5/8 phosphorylation and plays function in the pathogenesis of hepatocellular carcinoma.Citation10 Gremlin is reported to be the only one of the three BMP-7 antagonists and the expression of which is increased in kidneys of diabetic rats,Citation11 moreover, gremlin has been reported regulating TGF-β in diabetic nephropathy.Citation12
Branched-chain amino acids (BCAA: leucine, isoleucine and valine), are essential amino acids and presently widely used in clinical treatment, such as liver disease.Citation13 Recently, Tajiri K found that BCAAs could promote endothelial dysfunction.Citation14 In our preliminary work, we found that BCAAs could protect streptozotocin-induced insulin secretion in pancreatic beta cells.Citation15 The present study show BCAAs could inhibit the TGF-β induced damage in HepG2 cells, suggesting a potential pharmacological significance of BCAAs supplementation to the patients with hepatic failure.Citation16 Branched-chain amino acids (BCAAs) have been reported owning curative effects in early diabetic nephropathy, acting on metabolic signal pathways directly or indirectly. In our previous study, we have proved that branched-chain amino acids could attenuate early kidney injury in diabetic rats.Citation17 However, there were few studies about the effect of BCAAs on the function of the renal mesangial cells. Therefore, the aim of this present study was to address whether BCAAs could protect RMCs from high-glucose-induced stress and also to elucidate the possible underlying mechanisms.
Materials and Methods
Materials
Trizol reagent, the reverse transcription (RT) kit, and semiquantitative real-time PCR kit were purchased from Takara (Takara, Japan). Rabbit polyclonal antibodies specific for BMP-7 (ab56023, Abcam, USA) and gremlin1 (ab140010, Abcam, USA), Smad1/5/8 (sc-7965, Santa Cruz, Canada), phosphorylated (p)-SMAD1/5/8 (SMAD1, 5-phospho-Ser463/465, and SMAD8 phospho-Ser 426/428 antibodies; 13820, Cell Signaling Technology, Canada), and p-Smad2/3 (YP0362, Immunoway Biotechnology, China) were used in this study. Secondary antibodies were obtained from Jackson (Jackson, USA). The TGF-β1 and FN enzyme-linked immunosorbent assay (ELISA) kits were purchased from Boster (EK0515, Boster, Wuhan, China). Low-glucose Dulbecco’s modified Eagle medium (DMEM) was purchased from Hyclone (Hyclone, USA). Penicillin/streptomycin and trypsin were obtained from North China Pharmaceutical (NCPC, China). BCAAs were purchased from Sigma-Aldrich Company (USA). Glucose was purchased from China Otsuka Pharmaceutical Company (Otsuka, China). Mannitol was purchased from Shandong JinYang Pharmaceutical (Shandong JinYang Pharmaceutical, China).
Cell Culture
RMCs were purchased from the China Center for Type Culture Collection (Wuhan, China), and were grown in low-glucose DMEM containing 10% fetal calf serum (FCS) and 100 Units/mL of penicillin/streptomycin. RMCs (104 cells/mL) were plated in 60-mm culture dishes. After 3 days of culture, the cells reached 80% confluence, whereby they were trypsinized, collected, centrifuged at 1000 rpm/min for 5 min. The supernatant was discarded, complete medium was added, and a cell suspension was prepared. The cell suspension was placed in new culture dishes.
BCAAs Processing
The BCAAs were diluted with distilled water to a final concentration of 10 mmol/L. The filtered solution was stored at 4°C until use.
Cell Processing
After RMCs adhered to the culture dishes (about 24 h), the medium was exchanged with low-glucose DMEM without FCS. After 24 h, the cells were divided into the following groups: The control (CON) group (cultured in complete medium containing 24.5 mmol/L mannitol), high-glucose (HG) group (cultured in complete medium containing 24.5 mmol/L glucose), and the BCAAs group (cultured in complete medium containing 24.5 mmol/L glucose and 10 mmol/L BCAAs). After 48 h, the cells were collected for assessments.
Cell Proliferation Assay
The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay was used to measure cell proliferation. Briefly, cells were seeded at 104 cells/well in 96-well plates. After starvation for 24 h in low-glucose medium, the cells we treated with DMEM contain 10% FCS in the presence of mannitol, high glucose, or high glucose with BCAAs (5 wells for each group). The optical density (OD) of the samples at wavelength of 490 nm was measured using a microplate reader. The viability of cells incubated in control medium was considered as 100% and the values for the other samples were normalized to that of the cells in the control medium.
Semiquantitative Real-Time PCR
Total RNA was extracted from cells (collected as described in section 2.4) using Trizol reagent and processed with RNase-Free DNase before use in PCR. The RNA was reverse transcribed and semiquantitative RT-PCR was conducted using the Superscript RT-PCR kit (Invitrogen) for 40 cycles with the following
primers: TGF-β1-F: 5′-CTGCTGACCCCCACTGATAC-3′ and TGF-β1-R: 5′-CTGTATTCCGTCTCCTTGGTTC-3′; gremlin-F: 5′-CACCGCACTATCATCAATCGCTTCTCGAAAGAAGCGATTGATGATAGTGC-3′ and gremlin-R: 5′-AAAAGCACTATCATCAATCGCTTCTTTCGAGAAGCGATTGATGATAGTGC-3′; FN-F: 5ʹ-TGGAGAGACAGGAGGAAATAGC-3ʹ and FN-R:5ʹ-CAGTGACAGCATACAGGGTGAT-3ʹ; and GAPDH-F:5ʹ-ACCACAGTCCATGCCATC-3ʹ and GAPDH-R:5ʹ-TCCACCACCCTGTTGCTGTA-3ʹ using the LightCycler 480 system (Roche, USA). The 2 - ΔΔCt quantitative analysis method was used to analyze the results: GAPDH was sued as the internal reference gene, and the expression of TGF-β1, FN, and gremlin mRNA in the control group was used to calculate the relative levels of TGF-β1, Gremlin, and FN mRNA in the HG and BCAAs groups. The ΔΔCt value of the specific query genes was calculated as = (Ct value of query gene - Ct value of GAPDH) in the treatment group - (Ct value of query gene - Ct value of GAPDH) in the control group, with the relative amounts of the query mRNA = 2 - ΔΔCt.
ELISA
Protein levels of TGF-β1 and FN were determined using the ELISA kits, which employ the semiquantitative enzyme immunoassay technique. The absorbance of the resulting yellow product was measured at 450 nm.
Western Blotting
The cell samples (collected as described in section 2.4) were homogenized in radio immunoprecipitation assay buffer containing protease and phosphatase inhibitors, and the protein concentration was determined by the Bradford method. The proteins (30 μg per sample) were resolved on a 10% sodium dodecyl sulfate –polyacrylamide gel and transferred to a Hybond-P polyvinylidene difluoride membrane. The membranes were blocked in 5% nonfat milk at room temperature for 1 h and incubated with the primary antibodies overnight at 4°C. The primary antibodies were used at the following dilutions: BMP-7(1:500), Smad1/5/8(1:200), β-actin (1:10,000), and p-Smad1/5/8 (1:500). The membranes were washed with Tris-buffered saline containing Tween-20 (TBST) and incubated with either anti-rabbit or anti-mouse secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. The membranes were washed again with TBST before visualizing the protein-antibody complexes using enhanced chemiluminescence (Pierce Bio, USA).
Immunofluorescence Analysis
Briefly, chamber slides were rinsed in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde in TBS for 14 min at room temperature. After they were rinsed in TBS, the cells were permeabilized with 0.5% Triton X-100 for 3 min and then rinsed three times in TBS. The slides were incubated with primary antibodies specific for gremlin (1:50) and Smad2/3 (1:50) overnight at 4°C. The slides were rinsed and incubated with secondary antibodies conjugated to red or green (Beyotime, China) for 1 h at room temperature. Control incubation was included, with the secondary antibody alone. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Beyotime, China).
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was performed by one-way ANOVA with F-test. P value of < 0.05 was considered significant. The data were analyzed with SPSS II for Windows (release 11.0.1J, SPSS Japan Inc.). All experiments were performed at least three times.
Results
Proliferation of RMCs
The proliferation rate of the RMCs in the high-glucose group alone was 1.45-times that of cells in the CON group (0.6822±0.0944 vs 0.9858±0.2158, p=0.022) (). This higher proliferation rate was reduced by 32% upon co-treatment with BCAAs (0.9858±0.2158 vs 0.6744±0.1372, p=0.0264). These data indicate the BCAAs restore the cell proliferation rate of high-glucose-treated cells to that of the control cells.
Table 1 Proliferation of RMCs. CON, HG, and BCAAs (N=5)
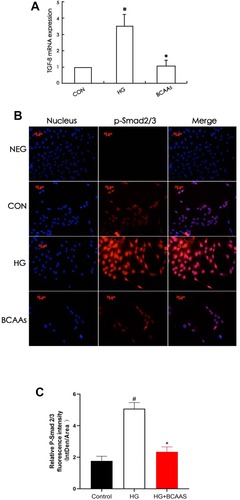
Expression of TGF-β1 and Smad2/3
TGF-β1 mRNA levels in RMCs were increased (to 350%) in the presence of high glucose stimulation, compared with that in the CON cells (), and the expression of TGF-β1 protein and p-Smd2/3 protein were also significantly higher than that in the CON group ( and and ). TGF-β1 mRNA levels were reduced (69.3%) when cells were co-treated with BCAAs and high glucose, compared with that in the HG group, and the protein levels exhibited a similar change.
Table 2 Expression of TGF-β1 (N=4)
Figure 1 (A) TGF-β1 mRNA levels were assayed using semiquantitative RT-PCR assay in CON group, HG group, BCAAs group, respectively. (B) Immunoflourescence staining for p-Smad2/3 in RMCs in CON group, HG group, BCAAs group, respectively. (C) Quantification of p-Smad2/3 fluorescence intensity (integrated density per stain area). #p <0.05 vs CON group, *p <0.05 vs HG group. Data were shown as the mean ± SD, with n = 5 samples in each group.
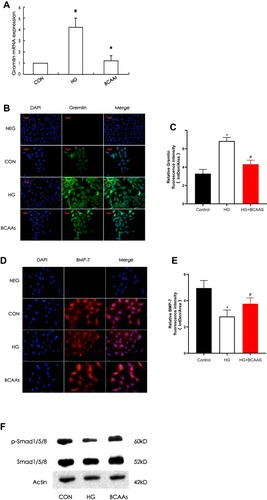
Expression of BMP-7, Gremlin, and Smad1/5/8
The expression of gremlin mRNA and protein in the HG group was significantly higher than that in the CON group, and in the BCAAs group, the expression of gremlin mRNA and protein was lower than that in the HG group (–). The expression of BMP-7 and p-Smad1/5/8 were significantly lower in the HG group than in the CON group, moreover, the expression of BMP-7 and p-Smad1/5/8 were higher in the BCAAs group than in the HG group (–).
Figure 2 (A) RT-PCR was performed to evaluate the expression of gremlin mRNA in CON group, HG group, BCAAs group, respectively. (B) Immunoflourescence staining was performed to evaluate the expression of gremlin in RMCs in three groups. (C) Quantification of Gremlin fluorescence intensity (integrated density per stain area). (D) Immunoflourescence staining for BMP-7 in RMCs in three groups. (E) Quantification of BMP-7 fluorescence intensity (integrated density per stain area). (F) Western blotting for p-Smad1/5/8 and total Smad1/5/8 in three groups. #p<0.05 vs CON group, *p<0.05 vs HG group.
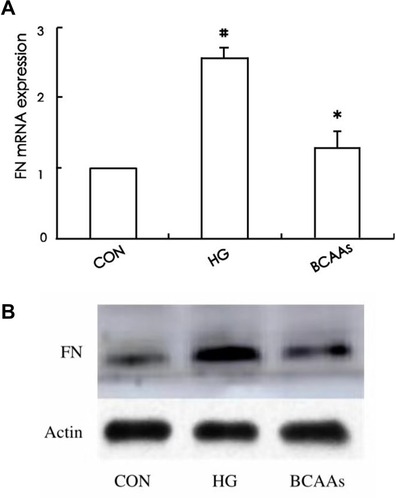
Expression of FN
The expression of FN mRNA and protein in the HG group was higher than that in the CON group; In the BCAAs group, the FN mRNA and protein levels were lower than that in HG group (, and ).
Table 3 Expression of FN
Discussion
Excess glucose and proteins become advanced glycosylation end products (AGEs), adding glaciated LDL and high glucose itself, can induce the expression of TGF-β1 on mesangial cells. TGF-β1 is just seemed as a biochemical marker for DN development in type 2 diabetic patients.Citation18 In vitro, high glucose can induce TGF-β1 and its receptor expression in tubular and mesangial cells.Citation19,Citation20 The high glucose induces serine/threonine protein kinase/protein kinase B (Akt/PKB) phosphorylation in a protein kinase C-β (PKC-β)-dependent manner resulting in the upregulation of TGF-β1 transcription.Citation21,Citation22 TGF-β1 is widely thought to be the most important cytokine in the ECM glomerular pathology. It is also a key fibrogenic factor that regulates epithelial to myofibroblast transition in renal tubular cells.Citation23,Citation24 It binds to a type II serine/threonine kinase receptor, which transphosphorylates and activates a type I receptor. This process is followed by modulation of the downstream-signaling molecules Smad, MAPK, and perhaps protein kinase A cellular pathways.Citation25 TGF- β1 binds to the TGF- β receptor II (T β RII) to result in phosphorylation of Smad2 and Smad3Citation26 to form a heterodimeric complex with Smad4, which translocate into the nucleus and regulates transcription of TGF-β1 target genes, such as collagen a 1 (I), PAI- 1, Jun B, c -Jun, and fibronectin.Citation27,Citation28
Bone morphogenetic protein-7 (BMP-7), a member of TGF-β superfamily, could reduce glomerular and tubulointerstitial fibrosis and protected the kidney from hyperglycemia-induced oxidative stress in diabetic nephropathy.Citation7,Citation29–Citation32 It has the distinguishing property of inhibiting TGF-β-dependent biological functions.Citation33 BMP-7 promotes the activating phosphorylation of Smad1/5/8. Phosphorylated Smad1/5/8 and phosphorylated Smad2/3 bind the Smad4 protein and regulate the transcription of target genes.Citation34 BMP-7-Smad1/5/8 pathway and TGF-β-Smad2/3 pathway keep balance in healthy kidney.
High glucose could affect BMP-7-Smad1/5/8 pathway through Gremlin, and BCAAs might decrease the expression of Gremlin and release the expression of BMP-7–Smad1/5/8. Gremlin belongs to a novel family of bone morphogenetic protein (BMP) antagonists.Citation35 Gremlin, an antagonist of bone morphogenetic protein 7(BMP-7),Citation36 it is overexpressed in adult diabetic nephropathy (DN).Citation37 Some experiments showed that both in animals and humans, the up-regulation of Gremlin in DN has been correlated with TGF-β expression. It has been suggested that Gremlin acting as a downstream mediator of TGF-β play a role in EMT. Expression of Gremlin in cultured kidney mesangial cells is induced by high glucose environment and TGF-β, so another name for gremlin is IHG-2 (induced in high glucose 2).Citation38 It plays distinct roles in the development and progression of kidney diseases.
Our experiments found that high glucose upregulated the expression of TGF-β1 and Gremlin while downregulate BMP-7-Smad1/5/8 pathway. It was in accordance with the mentioned findings. Our preliminary work has found that BCAAs could decrease the blood glucose level of diabetic rats and have anti-oxidative stress effect in diabetic nephropathyCitation15,Citation17 thereby alleviate diabetic kidney injury. We used rat glomerulus mesangial cells treated with high glucose model to find the possible mechanism of the BCAAs treated DN in vitro. In our experiments, we found that BCAAs could suppress the abnormal proliferation of RMCs. It also could downregulate the expression of TGF-β1 and Gremlin while upregulate BMP-7 and p-Smad1/5/8. In other words, high glucose disturbed the balance of TGF-β1 and BMP-7.
Conclusions
BCAAs displayed an anti-diabetic effect, via attenuation of the TGF-β1-Smad2/3 pathway and gremlin expression and upregulating the BMP-7-Smad1/5/8 pathway in RMCs, consequently reducing ECM deposition in renal tissue.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgment
This research was funded by grants from the Key Research and Development Plan of Shandong Province (2017G006006) and National Natural Science Foundation of China (81000325 and 81970700).
Disclosure
The authors report no conflicts of interest in this work.
References
- Steffes MW , Mauer SM . Diabetic glomerulopathy in man and experimental animal models. Int Rev Exp Pathol . 1984;26:147–175.6400512
- Gilbert RE , Cooper ME . The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int . 1999;56:1627–1637. doi:10.1046/j.1523-1755.1999.00721.x 10571771
- Qi W , Chen X , Poronnik P , Pollock CA . Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int J Biochem Cell Biol . 2008;40:9–13. doi:10.1016/j.biocel.2007.01.006 17300978
- Kemler R , Hierholzer A , Kanzler B , et al. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development . 2004;131:5817–5824. doi:10.1242/dev.01458 15525667
- Wang D , Zhang G , Chen X , et al. Sitagliptin ameliorates diabetic nephropathy by blocking TGF-β1/Smad signaling pathway. Int J Mol Med . 2018;41(5):2784–2792. doi:10.3892/ijmm.2018.3504 29484381
- Feng Y , Jin MY , Liu DW , Wei L . Bone morphogenetic protein (BMP) 7 expression is regulated by the E3 ligase UBE4A in diabetic nephropathy. Arch Physiol Biochem . 2019;19:1–4. doi:10.1080/13813455.2018.1551905
- Yeh CH , Chang CK , Cheng MF , Lin HJ , Cheng JT . The antioxidative effect of bone morphogenetic protein-7 against high glucose-induced oxidative stress in mesangial cells. Biochem Biophys Res Commun . 2009;382(2):292–297. doi:10.1016/j.bbrc.2009.03.011 19275892
- Wang S , Hirschberg R . BMP7 antagonizes TGF-beta -dependent fibrogenesis in mesangial cells. Am J Physiol Renal Physiol . 2003;284:F1006–13. doi:10.1152/ajprenal.00382.2002 12676736
- Xu Y , Wan J , Jiang D , Wu X . BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition in human renal proximal tubular epithelial cells. J Nephrol . 2009;22:403–410.19557718
- Wang L , Ding Q , Zhao L , et al. Decreased BMP-7 and p-Smad1/5/8 expression, and increased levels of gremlin in hepatocellular carcinoma. Oncol Lett . 2018;16(2):2113–2118. doi:10.3892/ol.2018.8918 30008908
- Wang SN , Lapage J , Hirschberg R . Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J Am Soc Nephrol . 2001;12:2392–2399.11675415
- Wang XB , Zhu H , Song W , Su JH . Gremlin regulates podocyte apoptosis via transforming growth factor-β (TGF-β) pathway in diabetic nephropathy. Med Sci Monit. 2018;9(24):183–189. doi:10.12659/MSM.905758
- Tajiri K , Shimizu Y . Branched-chain amino acids in liver diseases. Transl Gastroenterol Hepatol. 2018;30(3):47. doi:10.21037/tgh.2018.07.06
- Zhenyukh O , González-Amor M , Rodrigues-Diez RR , et al. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J Cell Mol Med. 2018;22(10):4948–4962. doi:10.1111/jcmm.13759 30063118
- Lu M , Zhang X , Zheng D , Jiang X , Chen Q . Branched-chain amino acids supplementation protects streptozotocin-induced insulin secretion and the correlated mechanism. Biofactors. 2015;41(2):127–133. doi:10.1002/biof.1188 25359484
- Hagiwara A , Ishizaki S , Takehana K , et al. Branched-chain amino acids inhibit the TGF-beta-induced down-regulation of taurine biosynthetic enzyme cysteine dioxygenase in HepG2 cells. Amino Acids . 2014;46(5):1275–1283. doi:10.1007/s00726-014-1693-3 24553827
- Mi N , Zhang XJ , Ding Y , et al. Branched-chain amino acids attenuate early kidney injury in diabetic rats. Biochem Biophys Res Commun . 2015;466(2):240–246. doi:10.1016/j.bbrc.2015.09.017 26362188
- El Mesallamy HO , Ahmed H , Bassyouni AA , Ahmed AS . Clinical significance of inflammatory and fibrogenic cytokines in diabetic nephropathy. Clin Biochem . 2012;45:646–650. doi:10.1016/j.clinbiochem.2012.02.021 22421318
- Yamagishi S , Inagaki Y , Okamoto T , Amano S , Koga K , Takeuchi M . Advanced glycation end products inhibit de novo protein synthesis and induce TGF-beta overexpression in proximal tubular cells. Kidney Int . 2003;63:464–473. doi:10.1046/j.1523-1755.2003.00752.x 12631112
- Wang S , Skorczewski J , Feng X , Mei L , Murphy-Ullrich JE . Glucose up-regulates thrombospondin 1 gene transcription and transformin0g growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem . 2004;279:34311–34322. doi:10.1074/jbc.M401629200 15184388
- Hills CE , Bland R , Bennett J , Ronco PM , Squires PE . High glucose up-regulates ENaC and SGK1 expression in HCD-cells. Cell Physiol Biochem . 2006;18:337–346. doi:10.1159/000097611 17170520
- Wu D , Peng F , Zhang B , et al. PKC-beta1 mediates glucose-induced Akt activation and TGF-beta1 upregulation in mesangial cells. J Am Soc Nephrol . 2009;20:554–566. doi:10.1681/ASN.2008040445 19211711
- Böttinger EP , Bitzer M . TGF-beta signaling in renal disease. J Am Soc Nephrol . 2002;13:2600–2610. doi:10.1097/01.ASN.0000033611.79556.AE 12239251
- Fan JM , Ng YY , Hill PA , et al. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int . 1999;56:1455–1467. doi:10.1046/j.1523-1755.1999.00656.x 10504497
- Ziyadeh FN . Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol . 2004;15:S55–7. doi:10.1097/01.ASN.0000093460.24823.5B 14684674
- Lan HY . Transforming growth factor-beta/Smad signalling in diabetic nephropathy. Clin Exp Pharmacol Physiol . 2012;39:731–738. doi:10.1111/j.1440-1681.2011.05663.x 22211842
- Leask A , Abraham DJ . TGF-beta signaling and the fibrotic response. FASEB J . 2004;18:816–827. doi:10.1096/fj.03-1273rev 15117886
- Shi Y , Massague J . Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell . 2003;113:685–700. doi:10.1016/S0092-8674(03)00432-X 12809600
- Yeh CH , Chang CK , Cheng MF , Lin HJ , Cheng JT . Decrease of bone morphogenetic protein-7 (BMP-7) and its type II receptor (BMP-RII) in kidney of type 1-like diabetic rats. Horm Metab Res . 2009;41:605–611. doi:10.1055/s-0029-1220736 19440953
- Hatano R , Takano F , Fushiya S , et al. Water-soluble extracts from Angelica acutiloba Kitagawa enhance hematopoiesis by activating immature erythroid cells in mice with 5-fluorouracil-induced anemia. Exp Hematol . 2004;32:918–924. doi:10.1016/j.exphem.2004.07.003 15504547
- Lehmann R , Schleicher ED . Molecular mechanism of diabetic nephropathy. Clin Chim Acta . 2000;297:135–144. doi:10.1016/S0009-8981(00)00240-0 10841915
- Miyazono K , Kusanagi K , Inoue H . Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol . 2001;187:265–276. doi:10.1002/jcp.1080 11319750
- Derynck R , Zhang YE . Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature . 2003;425:577–584. doi:10.1038/nature02006 14534577
- Hensey C , Dolan V , Brady HR . The Xenopus pronephros as a model system for the study of kidney development and pathophysiology. Nephrol Dial Transplant . 2002;17:73–74. doi:10.1093/ndt/17.suppl_9.73
- Michos O , Panman L , Vintersten K , Beier K , Zeller R , Zuniga A . Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development . 2004;131:3401–3410. doi:10.1242/dev.01251 15201225
- Lappin DW , McMahon R , Murphy M , Brady HR . Gremlin: an example of the re-emergence of developmental programmes in diabetic nephropathy. Nephrol Dial Transplant . 2002;17:65–67. doi:10.1093/ndt/17.suppl_9.65 12386293
- McMahon R , Murphy M , Clarkson M , et al. IHG-2, a mesangial cell gene induced by high glucose, is human gremlin. Regulation by extracellular glucose concentration, cyclic mechanical strain, and transforming growth factor-beta1. J Biol Chem . 2000;275:9901–9904. doi:10.1074/jbc.275.14.9901 10744662
- Yanagita M . BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev . 2005;16:309–317. doi:10.1016/j.cytogfr.2005.02.007 15951218