Abstract
Background
Diabetes is a major comorbidity in insomnia patients. The efficacy and safety of prolonged-release melatonin 2 mg in the treatment of glucose, lipid metabolism, and sleep was studied in 36 type 2 diabetic patients with insomnia (11 men, 25 women, age 46–77 years).
Methods
In a randomized, double-blind, crossover study, the subjects were treated for 3 weeks (period 1) with prolonged-release melatonin or placebo, followed by a one-week washout period, and then crossed over for another 3 weeks (period 2) of treatment with the other preparation. All tablets were taken 2 hours before bedtime for a period of 3 weeks. In an extension period of 5 months, prolonged-release melatonin was given nightly to all patients in an open-label design. Sleep was objectively monitored in a subgroup of 22 patients using wrist actigraphy. Fasting glucose, fructosamine, insulin, C-peptide, triglycerides, total cholesterol, high-density and low-density lipoprotein cholesterol, and some antioxidants, as well as glycosylated hemoglobin (HbA1c) levels were measured at baseline and at the end of the study. All concomitant medications were continued throughout the study.
Results
No significant changes in serum glucose, fructosamine, insulin, C-peptide, antioxidant levels or blood chemistry were observed after 3 weeks of prolonged-release melatonin treatment. Sleep efficiency, wake time after sleep onset, and number of awakenings improved significantly with prolonged-release melatonin as compared with placebo. Following 5 months of prolonged-release melatonin treatment, mean HbA1c (±standard deviation) was significantly lower than at baseline (9.13% ± 1.55% versus 8.47% ± 1.67%, respectively, P = 0.005).
Conclusion
Short-term use of prolonged-release melatonin improves sleep maintenance in type 2 diabetic patients with insomnia without affecting glucose and lipid metabolism. Long-term prolonged-release melatonin administration has a beneficial effect on HbA1c, suggesting improved glycemic control.
Introduction
Diabetes mellitus is a chronic age-related disease affecting an increasing number of patients worldwide and is currently reaching epidemic proportions.Citation1 Several studies have suggested a direct association between diabetes and sleep disturbances.Citation2–Citation7 Primary sleep disorders have been suggested to promote development of the metabolic syndrome that is strongly associated with increased type 2 diabetes and cardiovascular risk.Citation7 On the other hand, uncontrolled diabetes may adversely affect sleep quality nonspecifically, as a result of night-time thirst, a sensation of dryness, and nocturia, symptoms of hypoglycemia (sweating and tachycardia), stress, anxiety, and depression. All of these factors may impair good sleep at night.
Melatonin (N-acetyl-5-methoxytryptamine), the major hormone produced nocturnally by the pineal gland, is a sleep regulator and signal of darkness in humans. The circadian rhythm of synthesis and secretion of melatonin is closely associated with the sleep rhythm in both sighted and blind subjects.Citation8 Daytime administration of exogenous melatonin (when it is not present endogenously) promotes sleep in humansCitation8 and results in sleep-like brain activity patterns at specific areas such as the precuneus and hippocampus.Citation9,Citation10 Endogenous melatonin levels decrease with age,Citation11 and this decline may contribute to the common complaint of poor sleep quality in elderly people.Citation12 Abnormalities of the nocturnal melatonin profile have also been described in diabetic patients, mainly in those suffering from diabetic neuropathy.Citation13 Post mortem studies have indicated an association between diabetes mellitus and decreased melatonin secretion.Citation14 Melatonin deficiency deprives the brain of an important regulator of sleep and time cue to the internal circadian clock,Citation15 and may thus exacerbate sleep problems in diabetic patients.
There is also a growing body of evidence suggesting a link between disturbance in melatonin production and impaired insulin, glucose, lipid metabolism, and antioxidant capacity.Citation16–Citation19 Furthermore, melatonin has been found to influence insulin secretion both in vivo and in vitro,Citation18 and night-time melatonin levels are reportedly related to night-time insulin concentrations in patients with the metabolic syndrome.Citation19 In several recent studies, a single nucleotide polymorphism of the human melatonin receptor 1B has been described as being causally linked to increased risk of developing type 2 diabetes.Citation20–Citation22 All these data suggest that endogenous as well as exogenous melatonin may play a role in improving diabetic control.
Melatonin has a very short half-life of 40–50 minutes,Citation23 and is quickly eliminated from the circulation, but physiological levels are maintained throughout the night as a result of continuous secretion by the pineal gland. Prolonged-release melatonin 2 mg is a new drug licensed to treat primary insomnia in patients aged 55 years and older. It exerts its effects by mimicking the release pattern of endogenous melatonin in the brain. In randomized, placebo-controlled clinical trials, prolonged-release melatonin 2 mg significantly improved sleep latency, quality of sleep, and morning alertness, as compared with placebo in patients aged 55 years and older,Citation24–Citation27 and improved sleep maintenance assessed by actigraphy.Citation28
The aim of the current study was to investigate the effect of prolonged-release melatonin 2 mg administered at 9–11 pm for 3 weeks on glucose and lipid metabolism in community-dwelling diabetics suffering from insomnia. The effects of this treatment on sleep parameters were assessed in a subgroup of the patients using actigraphy. An extended period of 5 months of open-label, prolonged-release melatonin administration followed to evaluate the effects of prolonged-release melatonin on glycosylated hemoglobin (HbA1c) levels over a longer period of treatment, as an indicator of diabetic control.Citation29
Methods
The study was performed in accordance with the World Medical Assembly guidelines, ie, the latest version of the Declaration of Helsinki, and the standard operating procedures of Neurim Pharmaceuticals Ltd. All patients were given full details of the study in both verbal and written form by the investigator. Each patient gave their written informed consent for study participation according to Good Clinical Practice rules. The study protocol was approved by the Ethics Committee of the E Wolfson Medical Center, Holon, Israel.
Study design
In a randomized, double-blind, crossover design, the subjects were given tablets of either prolonged-release melatonin 2 mg (Circadin®, Rad Neurim Pharmaceuticals EEC Ltd) or an identical-looking placebo. The tablets were taken 2 hours before bedtime for a period of 3 weeks (period 1). This was followed by a washout period of 1 week and then by another 3-week period of treatment with the alternative preparation (period 2). Patients, investigators, and coworkers were blinded to the drug given during the crossover, double-blind treatment periods. Access to the randomization code was given to the pharmacist who prepared the tablets in containers. During the extension period of 5 months (period 3) prolonged-release melatonin was given nightly to all patients in an open-label manner. Treatment codes were opened after study completion and final entry of all study data.
Participants
Eligible patients were men and women diagnosed and treated for type 2 diabetes who also complained of insomnia. Patients with liver or renal disease (serum creatinine ≥1.5 mg/dL) were excluded. All concomitant medications were continued during the trial, and included metformin, sulfonylureas, glucosidase inhibitors, glitazones, insulin, statins, fibrates, angiotensin-converting enzyme inhibitors, calcium channel blockers, alpha-blockers, beta-blockers, antiplatelet agents, antiarrhythmic drugs, nitrates, phosphodiesterase inhibitors, bronchodilators, and antidepressants.
Evaluation of sleep parameters
A subset of patients in the study were assigned (based on availability of equipment) to undergo recording of their activity-rest patterns by wrist actigraphy (Somnitor™, Neurim Pharmaceuticals Ltd, Tel Aviv, IsraelCitation30) while sleeping at home. Motion recordings were analyzed as previously describedCitation24 to evaluate total sleep time (time spent asleep after sleep onset), sleep efficiency (total sleep time divided by time in bed multiplied by 100%), wake after sleep onset (sum of mid sleep arousal times after sleep onset), number of awakenings (between sleep onset and offset), and sleep latency (the lag period between entering bed and sleep onset). Changes in each parameter averaged over 3 consecutive nights from the placebo run-in period (baseline) to the end of 3 weeks of treatment were calculated for each patient.
Laboratory assessment
Fasting blood was withdrawn from all patients for routine hematologic and biochemistry evaluation on the morning before randomization, the morning following the last night of treatment periods 1 and 2, and the morning following the end of period 3. These tests included complete blood count, serum urea, creatinine, sodium, potassium, chloride, calcium, phosphate, total protein, albumin, globulin, bilirubin, alkaline phosphatase, glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, lactic dehydrogenase, and creatine phosphokinase. In the double-blind periods, glycemic control and lipid metabolism were evaluated by assessment of fasting glucose, fructosamine, HbA1c, insulin (for patients not treated with insulin), C-peptide, triglycerides, and cholesterol (total, high-density and low-density lipoprotein). Several antioxidants were also assessed in plasma, ie, malone dialdehyde, conjugated dienes, catalase, and glutathione peroxidase and reductase. The patients were then given a standard meal and postprandial blood sampling for serum glucose, insulin, and C-peptide was performed two hours later. Laboratory testing of HbA1c and postprandial glucose was performed again following 5 months of prolonged-release melatonin treatment.
Statistical analysis
The data collected were analyzed using SAS version 6.10 (SAS, Cary, NC). The effects of prolonged-release melatonin on each of the laboratory parameters were compared using a 2 × 2 mixed design analysis of covariance. The within-subject factor used was treatment (placebo versus prolonged-release melatonin) while the between-subject factor was order of administration (placebo then prolonged-release melatonin versus prolonged-release melatonin then placebo). The covariate used was the measure of the respective parameter at baseline (prestudy). The effect of prolonged-release melatonin on sleep parameters was evaluated using a 2 × 2 mixed design analysis of variance for repeated measurement. In addition, the interaction between the effects of prolonged-release melatonin and disease was assessed by analyses of covariance where the between-subject factor was insulin treatment for type 2 diabetes.
Results
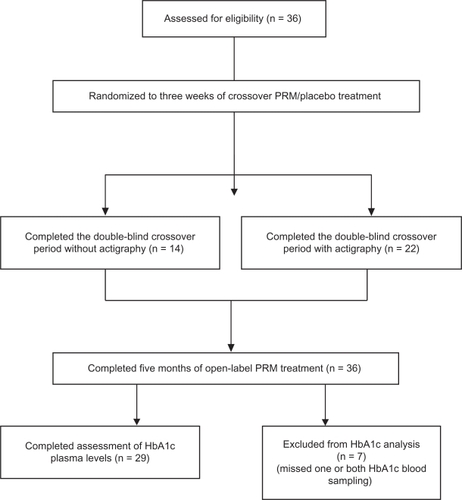
A total of 36 independently living type 2 diabetic patients (16 on oral hypoglycemic agents and 20 on insulin) who also complained of insomnia were entered into the study. Eleven were men and 25 were women, and mean age ± standard deviation was 63 ± 8 (range 46–77) years. All 36 patients completed the randomized crossover and extension parts of the study. Sleep quality was recorded by wrist actigraphy in a subgroup of 22 patients (seven men and 15 women). Statistical analyses of the efficacy of prolonged-release melatonin on sleep were therefore performed only in these 22 diabetic patients. One or both HbA1c measures were lacking in seven patients. Thus, statistical analyses of the long-term effects of prolonged-release melatonin on HbA1c were based on results obtained from 29 diabetic patients (10 men, 19 women), of whom 12 were treated with oral hypoglycemic agents and 17 were on insulin (see for full details).
Figure 1 Overall patient disposition. Analysis of the short-term period included eligible patients who completed three weeks of double-blind treatment with prolonged-release melatonin or placebo. Analysis of the long-term period included patients treated open-label with prolonged-release melatonin for 5 months. Analyses of actigraphic motion recordings included eligible patients who completed 3 weeks of treatment with prolonged-release melatonin and placebo and provided actigraphy data by the end of the treatment period.
A significant effect of order of administration (F(1,24) = 6.88, P < 0.05) and a significant interaction between treatment and order of administration (F(1,23) = 5.44, P < 0.05) were observed for fasting glucose; the mean serum glucose level on prolonged-release melatonin in the second crossover period was significantly lower than that during the first crossover period. No significant treatment effect on fasting glucose was found compared with placebo. No other significant treatment order effects in the laboratory parameters were found, nor any significant interactions between treatment and order.
The overall safety and tolerability results as well as diabetes status were similar in all treatment periods. Prolonged-release melatonin 2 mg had no significant effect on routine laboratory tests, glucose and lipid metabolism, or antioxidant levels. However, there was a significant decrease in HbA1c concentration from 9.13% ± 1.55% at baseline to 8.47% ± 1.67% following the five-month extension period (t(29) = 3.29, P = 0.005, t-test for dependent samples). Of the 29 patients in whom HbA1c data on both time points were available, HbA1c levels decreased by 1% or more in 11 and increased in 1; the mean decrease in HbA1c was 0.66% ± 1.15% regardless of the order of treatment in the randomization phase. A t-test for independent samples performed on the reduction in HbA1c from prestudy levels to those after 5 months on prolonged-release melatonin, indicated that the reduction in HbA1c was not significantly different in type 2 diabetics treated or not treated by insulin (P = 0.68).
The effects of 3 weeks of prolonged-release melatonin compared with placebo on sleep parameters are depicted in . Statistically significant improvements in several sleep parameters were found for prolonged-release melatonin compared with placebo (). Of the 22 patients in whom sleep assessments were performed, 12 had a net improvement of more than 3% in sleep efficiency on prolonged-release melatonin as compared with placebo, and 15 had a net improvement of at least 25% in wake after sleep onset. In 12 patients, the number of awakenings was improved by at least 25%, while only seven showed little or no improvement in any of the sleep parameters on prolonged-release melatonin compared with placebo. No significant treatment order effects or interactions between treatment and order were found in the sleep parameters.
Table 1 Actigraphy-derived sleep parameters (mean ± standard deviation) following 3 weeks of administration of prolonged-release melatonin or placebo (crossover, n = 22)
Improvement in sleep parameters in the double-blind period did not predict change in HbA1c during the long-term period, and improvement in HbA1c levels was found both in patients in whom sleep quality was or was not improved.
No serious adverse events were reported. An adverse experience was reported seven times on prolonged-release melatonin treatment, ie, insomnia (n = 2), abnormal thoughts (n = 1), taste aversion (n = 1), and sexual dysfunction (n = 3), and five times on placebo treatment, ie, somnolence (n = 3), libido increase (n = 2), and sexual dysfunction (n = 1).
Discussion
These results show that prolonged-release melatonin is safe in diabetic patients, having no adverse effects on glucose and lipid metabolism or other routine biochemical tests, and no other adverse events during short-term (3 weeks) and long-term use. No interaction with any of the medications frequently used in diabetic patients was observed (ie, metformin, sulfonylureas, thiazolidinediones, peroxisome-proliferator activated receptors agonists, insulin, fibrates and other lipid-lowering agents, angiotensin-converting inhibitors, calcium antagonists, beta-blockers, anticoagulants, and serotonin reuptake inhibitors). Importantly, prolonged-release melatonin did not affect C-peptide levels, suggesting that it had no effect on the release of insulin in these patients.
It was shown that glucose tolerance and insulin sensitivity were both reduced as compared with placebo following a single oral administration of melatonin 1 mg in 22 postmenopausal nondiabetic women. The authors suggested that melatonin should be avoided in diabetes.Citation31 Our findings do not support this notion, and indicate that prolonged-release melatonin does not impair insulin action or glucose tolerance in diabetic patients, whether used in the short term or long term. On the contrary, we have shown improved glycemic control upon long-term use of prolonged-release melatonin.
In two other studies, the combination of melatonin and zinc acetate alone or in combination with metformin was found to improve fasting and postprandial glycemic control in type 2 diabetic patients.Citation32 Melatonin was not given alone in these studies, and it is therefore impossible to evaluate its specific effect on glycemic control. However, these results are in line with our data and support our findings regarding the safety of melatonin in diabetic patients.
Our findings of improved diabetic control with prolonged-release melatonin are also compatible with those of some animal studies. Long-term administration of time-release melatonin pellets (1.1 mg/day for 30 weeks) reduced the development of hypertriglyceridemia, hyperinsulinemia, and hyperleptinemia, and restored normal ratios of 20:3n–6/20:4n–6 phospholipids in a rat model of diabetes.Citation33 This finding suggests that long-term melatonin administration may slow down age-related deterioration in glucose and lipid metabolism. The decrease in HbA1c may be due to better compliance with diet and treatment as a result of participation in the study. Alternatively, it may be the result of an antistress effect of melatonin causing attenuation of glucose fluctuations.
Prolonged-release melatonin improved sleep maintenance in comparison with placebo, as indicated by improvements in sleep efficiency, wake after sleep onset, and number of awakenings. The effects were consistent with those seen in placebo-controlled studies of prolonged-release melatonin in patients suffering from insomnia.Citation24–Citation28 Sleep latency did not improve on prolonged-release melatonin as compared with placebo, perhaps because the mean sleep latency in this population (18.1 ± 13.2 minutes) was only slightly longer than 15 minutes (a value considered normal). We did not find any significant differences in glucose or lipid metabolism during the 3 weeks of treatment with prolonged-release melatonin. Therefore, the effect on sleep maintenance is probably directly linked to the specific sleep-promoting effects of melatonin rather than improvement in diabetes control. Because the improvement in sleep in diabetic patients was not predictive of reduction in HbA1c, the effect of prolonged-release melatonin on HbA1c is probably not related to the improvement in sleep, but rather reflects a mechanism that involves glucose metabolism per se. Of 13 patients who responded to prolonged-release melatonin with an increase in sleep efficiency of 3% or more, seven were concomitantly on metformin, five on sulfonylureas, five on both metformin and sulfonylureas, one on pioglitazone, and seven were on insulin. Hence, the soporific activity of prolonged-release melatonin is probably independent of concomitant antidiabetic drug effects.
Melatonin may act at the level of the circadian clock in the suprachiasmatic nuclei of the hypothalamus to improve the robustness of the circadian system.Citation15 An increase in endogenous glucose production is a major contributor to fasting morning hyperglycemia in type 2 diabetes. Endogenous glucose production and gluconeogenesis display diurnal rhythms that drive fasting hyperglycemia and are absent in healthy control subjects.Citation34 Melatonin is closely related to endogenous glucose production, but its secretion is attenuated in diabetes.Citation34 Diurnal variations in endogenous glucose production in diabetes may be related to reduction in the robustness of the suprachiasmatic nuclei in the hypothalamus in diabetes that may be responsible, at least in part, for low melatonin production in these patients. Timing and dose-controlled exogenous hormone supplementation aimed at normalizing melatonin levels have been shown to affect the circadian pacemaker by modifying the internal clock in humans.Citation8 Melatonin treatment may thus reinforce circadian control of glucose metabolism and subsequently stabilize endogenous glucose production, reduce serum glucose, and eventually contribute to better glycemic control.
Long-term administration of low-dose, prolonged-release melatonin 2 mg/day was associated with a significant reduction in HbA1c in type 2 diabetic patients. Because a 0.5% HbA1c difference between successive results is considered a clinically relevant change, our finding of a mean decrease of 0.66% ± 1.15% in HbA1c is both statistically significant and of clinical importance.Citation35 It has been reported that each 1% reduction in HbA1c is associated with a risk reduction of 21% for any end point related to diabetes, suggesting that even a modest reduction in glycemia has the potential to prevent deaths from complications related to diabetes.Citation36 In the current study, more than one third of patients showed decreased HbA1c levels of 1% or more. Although no parallel placebo treatment was used during this period, this observation may suggest some antihyperglycemic activity for melatonin in humans.
Two major limitations of our study are that the long-term treatment was not placebo-controlled and the circadian rhythm of glucose production was not measured. Further studies to clarify the involvement of circadian modulation in improvement of diabetic control by prolonged-release melatonin, and the long-term nature of these effects, are warranted.
Disclosure
The study was an investigator-initiated trial and was funded by Neurim Pharmaceuticals Ltd. DG, MZ, and JW were the investigators and have no financial involvement with the company. ZM performed the laboratory assessments and has no financial connections with Neurim Pharmaceuticals Ltd. ML and NZ are employees of Neurim Pharmaceuticals Ltd.
References
- Narayan KM Gregg EW Fagot-Campagna A Engelgau MM Vinicor F Diabetes: a common, growing, serious, costly, and potentially preventable public health problem Diabetes Res Clin Pract 2000 50 Suppl 2 S77 S84 11024588
- Foley DJ Monjan A Simonsick EM Wallace RB Blazer DG Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years Sleep 1999 22 Suppl 2 S366 S372 10394609
- Chasens ER Understanding sleep in persons with diabetes Diabetes Educ 2007 33 3 435 436 17570874
- Gottlieb DJ Punjabi NM Newman AB Association of sleep time with diabetes mellitus and impaired glucose tolerance Arch Intern Med 2005 165 8 863 867 15851636
- Barone MT Menna-Barreto L Diabetes and sleep: a complex cause-and-effect relationship Diabetes Res Clin Pract 2011 91 2 129 137 20810183
- Knutson KL Van Cauter E Zee P Liu K Lauderdale DS Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study Diabetes Care 2011 34 5 1171 1176 21411507
- Eriksson AK Ekbom A Granath F Hilding A Efendic S Ostenson CG Psychological distress and risk of pre-diabetes and type 2 diabetes in a prospective study of Swedish middle-aged men and women Diabet Med 2008 25 7 834 842 18513304
- Cajochen C Krauchi K Wirz-Justice A Role of melatonin in the regulation of human circadian rhythms and sleep J Neuroendocrinol 2003 15 4 432 437 12622846
- Gorfine T Assaf Y Goshen-Gottstein Y Yeshurun Y Zisapel N Sleep-anticipating effects of melatonin in the human brain Neuroimage 2006 31 1 410 418 16427787
- Gorfine T Zisapel N Late evening brain activation patterns and their relation to the internal biological time, melatonin, and homeostatic sleep debt Hum Brain Mapp 2009 30 2 541 552 18095278
- Sharma M Palacios-Bois J Schwartz G Circadian rhythms of melatonin and cortisol in aging Biol Psychiatry 1989 25 3 305 319 2914154
- Leger D Laudon M Zisapel N Nocturnal 6-sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapy Am J Med 2004 116 2 91 95 14715322
- O’Brien IA Lewin IG O’Hare JP Arendt J Corrall RJ Abnormal circadian rhythm of melatonin in diabetic autonomic neuropathy Clin Endocrinol (Oxf) 1986 24 4 359 364 3742831
- Sandyk R Anastasiadis PG Anninos PA Tsagas N Is the pineal gland involved in the pathogenesis of endometrial carcinoma Int J Neurosci 1992 62 1–2 89 96 1342018
- Zisapel N Sleep and sleep disturbances: biological basis and clinical implications Cell Mol Life Sci 2007 64 10 1174 1186 17364142
- Nishida S Metabolic effects of melatonin on oxidative stress and diabetes mellitus Endocrine 2005 27 2 131 136 16217126
- Korkmaz A Reiter RJ Topal T Manchester LC Oter S Tan DX Melatonin: an established antioxidant worthy of use in clinical trials Mol Med 2009 15 1–2 43 50 19011689
- Peschke E Melatonin, endocrine pancreas and diabetes J Pineal Res 2008 44 1 26 40 18078445
- Robeva R Kirilov G Tomova A Kumanov P Melatonin-insulin interactions in patients with metabolic syndrome J Pineal Res 2008 44 1 52 56 18078448
- Prokopenko I Langenberg C Florez JC Variants in MTNR1B influence fasting glucose levels Nat Genet 2009 41 1 77 81 19060907
- Tam CH Ho JS Wang Y Common polymorphisms in MTNR1B, G6PC2 and GCK are associated with increased fasting plasma glucose and impaired beta-cell function in Chinese subjects PLoS One 2010 5 7 e11428 20628598
- Mussig K Staiger H Machicao F Haring HU Fritsche A Genetic variants in MTNR1B affecting insulin secretion Ann Med 2010 42 6 387 393 20597807
- Waldhauser F Waldhauser M Lieberman HR Deng MH Lynch HJ Wurtman RJ Bioavailability of oral melatonin in humans Neuroendocrinology 1984 39 4 307 313 6493445
- Wade A Downie S Prolonged-release melatonin for the treatment of insomnia in patients over 55 years Expert Opin Investig Drugs 2008 17 10 1567 1572
- Luthringer R Muzet M Zisapel N Staner L The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia Int Clin Psychopharmacol 2009 24 5 239 249 19584739
- Wade AG Ford I Crawford G Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety BMC Med 2010 8 51 20712869
- Wade AG Crawford G Ford I Prolonged release melatonin in the treatment of primary insomnia: evaluation of the age cut-off for short- and long-term response Curr Med Res Opin 2011 27 1 87 98 21091391
- Garfinkel D Laudon M Nof D Zisapel N Improvement of sleep quality in elderly people by controlled-release melatonin Lancet 1995 346 8974 541 544 7658780
- Coniff RF Shapiro JA Robbins D Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM. A placebo-controlled dose-comparison study Diabetes Care 1995 18 6 817 824 7555508
- Wrist activity based monitoring of nocturnal sleep: validation of a novel scoring algorithm. Ninth annual meeting of the Associated Professional Sleep Societies, Nashville, TE 1995
- Cagnacci A Arangino S Renzi A Influence of melatonin administration on glucose tolerance and insulin sensitivity of post-menopausal women Clin Endocrinol (Oxf) 2001 54 3 339 346 11298086
- Hussain SA Khadim HM Khalaf BH Ismail SH Hussein KI Sahib AS Effects of melatonin and zinc on glycemic control in type 2 diabetic patients poorly controlled with metformin Saudi Med J 2006 27 10 1483 1488 17013468
- Nishida S Segawa T Murai I Nakagawa S Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of delta-5 desaturase activity J Pineal Res 2002 32 1 26 33 11841597
- Radziuk J Pye S Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia 2006 49 7 1619 1628 16752180
- Lenters-Westra E Slingerland RJ Hemoglobin A1c point-of-care assays; a new world with a lot of consequences! J Diabetes Sci Technol 2009 3 3 418 423 20144277
- Stratton IM Adler AI Neil HA Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study BMJ 2000 321 7258 405 412 10938048