Abstract
The aim of the study was to explore the possibility that propolis can control diabetes mellitus and prevent diabetic osteopathy in rats. The study compared 60 streptozotocin (STZ)-induced diabetic rats, with ten nondiabetic rats used as a negative control. The experimental design comprised seven groups (n = 10 rats per group): (1) nondiabetic, used as a negative control; (2) nontreated, used as a positive control; (3) treated with insulin alone; (4) treated with a single dose of propolis alone; (5) treated with a double dose of propolis; (6) treated with insulin and a single dose of propolis; and (7) treated with insulin and a double dose of propolis. After 6 weeks of treatment, the rats were sacrificed. Ratios of femur ash to femur weight and of femur weight to body weight (FW/BW) were calculated and calcium (Ca), phosphorus (P), and magnesium (Mg) concentrations in femur ash were estimated and analyzed. Fasting blood glucose (FBG), plasma insulin and glucagon, serum thiobarbituric acid reactive substances (TBARS), plasma parathyroid hormone (PTH), and calcitonin levels were also estimated and analyzed. There was significant reduction in FBG in all diabetic treated rats. Similarly, higher plasma insulin levels were observed in diabetic rats treated with propolis and insulin than in nontreated diabetic rats, although plasma insulin was not comparatively higher in diabetic rats treated with insulin alone. Serum TBARS was significantly lower in the propolis treated rats than the diabetic nontreated rats. No differences in PTH and calcitonin levels were observed among treatment groups. The FW/BW ratio was significantly higher in diabetic treated groups than in control groups. Furthermore, diabetic rats treated with propolis and insulin had significantly higher Ca, P, and Mg concentrations in femoral ash than nontreated diabetic rats and diabetic rats treated with insulin alone. In conclusion, propolis has a remarkable effect on glucose homeostasis and bone mineralization.
Introduction
Diabetes mellitus (DM) is the most common serious metabolic disorder in humans. It is characterized by hyperglycemia as a result of insulin shortage or insufficient insulin action, or both.Citation1 Insulinopenia, which occurs in type 1 diabetes (T1DM), is associated with decreased bone density and a state of low bone turnover.Citation2,Citation3
Hyperglycemia is an important factor responsible for the intense oxidative stress in diabetes, and the toxicity induced by glucose autoxidation is likely to be one of the important sources of reactive oxygen species.Citation4 Additionally, lipid peroxidation plays an important role in the production of free radicals and oxidative stress in diabetes. Citation5 Several intra- and extracellular antioxidant defense mechanisms counteract the destructive effects of free radicals by attenuating or omitting their activities.Citation6 However, in DM the oxidative stress exceeds the body’s antioxidant defense mechanisms. Although oxidative stress and free radicals have been reported to play a significant role in diabetic complicationsCitation7 and treatment with antioxidants has been reported to reduce these complications,Citation8 few studies have focused on diabetic osteopathy.
Recent studies have shown that propolis has hypoglycemic, hypolipidemic, and antioxidant activity,Citation9 which can be used to prevent or delay the appearance of diabetic complications. Its hypoglycemic activity has been attributed to inhibition of intestinal maltase activity, preventing rise of blood glucose following carbohydrate intake. Propolis has also been reported to enhance the antioxidant defense systemCitation10 and to protect pancreatic tissue.Citation9
In view of recent claims that propolis can cure streptozotocin (STZ)-induced DM,Citation9 the authors extend their studies to investigate the effect of propolis on the control of diabetes and the prevention of diabetic osteopathy in STZ-induced diabetic rats.
Material and methods
Adult male albino rats (obtained from the animal house at the University of Dammam, Dammam, Saudi Arabia) weighing 150–300 g were housed at a constant temperature (22°C) under a 12-hour light-dark cycle and were provided with standard rat food and water ad libitum. The University of Dammam ethics committee approved the protocol. The study compared 60 streptozotocin (STZ)-induced diabetic rats, with ten nondiabetic rats used as a negative control. The experimental design comprised seven groups (n = 10 rats per group): (1) nondiabetic, used as a negative control (GI); (2) nontreated, used as a positive control (GII-1); (3) treated with insulin alone (GII-2); (4) treated with a single dose of propolis (0.3 g/kg) alone (GII-3); (5) treated with a double dose of propolis (0.6 g/kg) (GII-4); (6) treated with insulin and a single dose of propolis (0.3 g/kg) (GII-5); and (7) treated with insulin and a double dose of propolis (0.6 g/kg) (GII-6).
T1DM was induced in the experimental ratsCitation11 by administering a single-dose intraperitoneal (IP) injection of STZ (60 mg/kg) (Sigma-Aldrich Co, St Louis, MO), dissolved in distilled water. Three days after the STZ injection, urine strips (Medi-Test Combi 10; Macherey-Nagel GmbH & Co, Düren, Germany) were used to detect glycosuria in rats (a dark-green color indicated blood glucose ≥ 500 mg/dL).Citation12 These 60 STZ-induced diabetic rats were randomly divided into the six subgroups, GII-1 to GII-6, in the study.
Treatment of all rats included in the study started daily at 7 am and continued for 6 weeks. Both the negative control and positive control groups (GI and GII-1, respectively) received a daily IP injection with normal saline and received 1 mL of water through a rat feeding needle (Kent Scientific Corporation, Torrington, CT).Citation13 Groups GII-2, GII-5, and GII-6 received an IP injection of insulin (5 IU/kg/day) (Humulin; Eli Lilly and Company, Indianapolis, IN).Citation14 Groups GII-3 and GII-5 received propolis to ingest (in aqueous solution, 0.3 g/kg)Citation15 and groups GII-4 and GII-6 received a double dose of propolis to ingest (in aqueous solution, 0.6 g/kg),Citation15 through an orogastric metallic needle.
At the end of the 6-week experimental period, the different treatment regimens were stopped, and food was stopped 12 hours before sacrificing the rats. Animals were weighed and then anesthetized with an IP injection of ketamine (50 mg/kg) (Alfasan International BV, Woerden, the Netherlands).Citation16 Blood was collected directly from the abdominal aorta in two tubes by means of a vacutainer. One tube was heparinized for separation of plasma for hormonal studies, while the other tube was kept plain for fasting blood glucose (FBG) and separation of serum to determine the antioxidant activities. Plasma and serum was separated from blood samples by centrifugation at 3000 rpm for 10 minutes at 4°C, collected, and stored at −80°C until the time of analysis.Citation15
Bone sampling
After drawing blood, the right femurs of all experimental rats were dissected out, cleansed of all soft tissue, washed with distilled water, and allowed to dry at room temperature for 24 hours. Each dried femur was then weighed, put in an oven at 100°C for 24 hours, and then put in a furnace at 800°C for 12 hours. The ash of each femur was collected separately, weighed, dissolved in 3 mL of 70% nitric oxide (Sigma-Aldrich), and centrifuged; the supernatant was separated for the measurement of calcium (Ca), phosphorus (P), and magnesium (Mg) by the standard colorimetric method.Citation17
Plasma insulin, glucagon, calcitonin, and parathyroid hormone
Plasma enzyme-linked immunosorbent assays (ELISAs) were used to estimate hormone levels. The Insulin ELISA kit (ALPCO Diagnostics, Salem, NH) used for quantitative determination of plasma insulin concentration in rats is a one-step sandwich enzyme immunoassay using two monoclonal antibodies.Citation18 Pancreatic glucagon levels were determined by a highly specific ELISA kit (Wako Pure Chemical Industries, Ltd, Richmond, VA), based upon a competitive ELISA using a highly specific antibody to glucagon.Citation19 The parathyroid hormone (PTH) kit (ALPCO Diagnostics, Salem, NH) is a two-site ELISA that quantitatively determines the rat bioactive intact PTH concentrations.Citation18 The calcitonin immunoassay kit (Phoenix Pharmaceuticals, Inc, Burlingame, CA) is a competitive enzyme immunoassay that detects calcitonin and its related peptides.Citation20
Serum FBG levels and oxidative status
FBG concentrations were determined by a glucometer (Accu-Chek Go, Roche Diagnostics GmbH, Indianapolis, IN).Citation6 The activity of superoxide dismutase (SOD) (Cayman Chemical, Ann Arbor, MI) was determined through ELISA technique.Citation21 Catalase (CAT) (Cayman Chemical) activity was measured using its peroxidative function.Citation22 A thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical) was used to measure the product of the reaction between malondialdehyde, a product of lipid peroxidation, and TBARS.Citation23
Statistical analysis
All values reported are expressed as mean plus or minus standard error of the mean. Differences among means were analyzed for significance by analysis of variance using SPSS software (v 10; SPSS Inc, Chicago, IL). Groups were then compared by Fisher’s least significant difference tests, and P < 0.05 was considered statistically significant.
Results
Blood glucose, insulin, and glucagon
Significant differences in FBG, plasma insulin and glucagon, and insulin-glucagon (I/G) ratio were noted and are summarized in . Nontreated diabetic rats (coded NTD) had significantly higher mean FBG than all other groups. The mean blood glucose in all diabetic treated rats (including those treated with propolis only) was not significantly different from the negative control (coded C) group. With regard to plasma insulin, treated groups had significantly lower mean plasma insulin than the negative control group. Further, while the group treated with insulin only (insulin-treated diabetic rats, coded ITD) had plasma insulin comparable with the nontreated diabetic rats, the two groups treated with propolis plus insulin (propolis- and insulin-treated diabetic, coded PITD; double-dose propolis- and insulin-treated diabetic, coded DPITD) had significantly higher plasma insulin than the nontreated diabetic rats. Concerning plasma glucagon, the nontreated diabetic rats had significantly higher plasma glucagon than all other groups. In addition, all groups treated with propolis, with the exception of propolis-treated diabetic (coded PTD) rats, had mean plasma glucagon comparable with that of the negative control group. Treatment with double-dose propolis plus insulin was associated with significantly lower plasma glucagon than the two groups treated with either insulin or propolis alone. The results regarding I/G ratio were rather interesting. While the negative control group had a significantly higher I/G ratio than all other groups, the two groups given mixed treatment with insulin plus propolis had a significantly higher I/G ratio than the positive control group and those groups treated with either insulin or propolis alone.
Table 1 Fasting blood glucose (FBG), plasma insulin, plasma glucagon, and insulin-glucagon (I/G) ratio in the control and experimental groups of ratsTable Footnote*
Antioxidant parameters
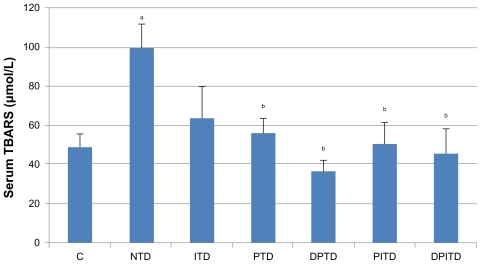
The mean serum TBARS levels (), an indicator of lipid peroxidation, were found to be significantly higher in the nontreated diabetic rats than in the negative control group. Furthermore, the mean serum TBARS levels of all diabetic groups treated with propolis (with or without insulin) were significantly lower than for the nontreated diabetic rats. Serum levels of the two antioxidant enzymes measured (CAT and SOD) were not shown to have significant difference among the studied groups.
Figure 1 Mean serum levels of thiobarbituric acid reactive substances (TBARS) in the control and experimental groups of rats.
Abbreviations: C, nondiabetic; DPITD, double-dose propolis- and insulin-treated diabetic; DPTD, double-dose propolis-treated diabetic; ITD, insulin-treated diabetic; NTD, nontreated diabetic; PITD, propolis- and insulin-treated diabetic; PTD, propolis-treated diabetic.
PTH, calcitonin, and bone mineralization
The results of plasma PTH, calcitonin, and FW/BW ratio are shown in . While, the nontreated diabetic rats had significantly higher mean plasma PTH than the negative control group, other groups did not. Moreover, mean serum PTH did not differ significantly between the treated diabetic subgroups. Mean plasma calcitonin was significantly higher in the nontreated diabetic group than in all other groups. In addition, no significant difference in plasma calcitonin among the diabetic treated groups was noted. As regards FW/BW ratio, no significant difference existed between the two control groups, negative and positive. However, the FW/BW ratio was significantly higher for all diabetic treated groups than for the positive control group. In addition, all groups treated with propolis (with or without insulin) had a higher FW/BW ratio than the negative control group. The concentration of ash in femur bones and the concentrations of Ca, P, and Mg provided interesting results, as summarized in . The bones of the negative control group and the two groups treated with insulin plus propolis had significantly higher ash contents than those of the positive control group. Treatment with either insulin or propolis alone did not significantly affect the concentration of ash in femur bones. The concentrations of Ca, P, and Mg in femur ash also showed significant differences among groups. The concentrations of these three minerals were significantly lower in the nontreated diabetic group than in all other groups. In addition, all groups treated with propolis (with or without insulin treatment, and with single- or double-dose propolis) had significantly higher ash Ca and Mg concentrations than the diabetic group treated with insulin only. Furthermore, double-dose propolis treatment (with or without insulin treatment) was associated with a significantly higher ash P concentration than insulin-only treatment.
Table 2 Plasma parathyroid hormone (PTH) and calcitonin levels and ratio of femur weight to body weight (FW/BW) in the control and experimental groups of ratsTable Footnote*
Table 3 Ratio of femur ash to femur weight (FA/FW) and calcium (Ca), phosphorous (P), and magnesium (Mg) concentrations in the control and experimental groups of ratsTable Footnote*
Discussion
This study on adult male albino rats confirms the earlier reportsCitation9 that propolis could almost control the hyperglycemia in the STZ-induced diabetic rat model.Citation24 The glycemic control achieved by propolis treatment could be due to the stimulation of glucose uptake by peripheral tissues, inhibition of its release in circulation,Citation25 or reduced glucose absorption in the gut.Citation10
However, the present study shows that propolis treatment in STZ-induced diabetic rats is associated with high plasma insulin and low glucagon levels. These findings suggest that decreased glucose output by the liver and increased glucose uptake by peripheral tissues were the probable mechanisms through which propolis achieved glycemic control.
However, the recovery of insulin secretion in diabetic rats is a partial restoration only, because insulin levels in all diabetic rats treated with propolis were significantly lower than in the negative control group. The source of insulin in propolis-treated diabetic rats could be the β cells of the pancreas and therefore two possibilities are suggested: either propolis induces regeneration or it prevents further deterioration of β cells. Others have reported similar findings on the ability of propolis to induce regenerative effects on β cells.Citation10,Citation11,Citation15
As already noted, lipid peroxidation is the most potent oxidative defect that damages β cells in T1DM.Citation26 All diabetic rats in the study treated with propolis alone or with insulin were observed showing significantly lowered lipid peroxidation levels nearing normal control values, which suggests that propolis prevents deterioration of β-cell function. This finding confirms the earlier report that propolis causes partial restoration of β-cell function.Citation11 However, the authors could not confirm whether the glycemic control achieved by propolis was also achieved by the inhibition of intestinal glucose absorption,Citation10 as the rats were deprived of food for 12 hours before they were sacrificed.
However, propolis administration in the present study could not raise plasma insulin to the levels achieved by exogenous insulin administration, although it significantly lowered blood glucose to reach normal control values. This suggests that propolis administration causes inhibition of glucose release from the liver and/or improvement of peripheral tissue insulin sensitivity.
The study found that a decline in glucagon concentration in propolis-treated rats with elevation of the I/G ratio, again suggesting that decline in liver glucose output is an important mechanism in glycemic control by propolis. The study also found that a significant decline of glucagon concentrations in all propolis-treated diabetic rats compared with nontreated diabetic rats is due to insulin-induced inhibition of pancreatic α cells. This is because high insulin levels were found to be associated with significantly low glucagon levels in diabetic rats treated with both propolis and insulin. Additionally, compared with single-dose propolis-treated rats, double-dose propolis-treated rats showed low glucagon levels that were nearing negative control group levels, despite the insulin dose being the same for both groups (propolis-treated diabetic and double-dose propolis-treated diabetic [coded DPTD] rats). However, a direct inhibitory effect of propolis on α cells cannot be excluded and requires further investigation.
Evidence for a direct link between insulin and bone formation in vivo is scant. Recent studies explain the potential role of insulin as an anabolic agent in osteoblastogenesis.Citation27 Researchers have shown that bone regeneration is impaired by insulin deficiency but that it can be restored by insulin treatment, even with modrate hyperglycemia, indicating a primary role for insulin in bone formation.Citation28 Hence, in diabetic states, bone formation rather than bone resorption is affected, leading to bone loss.Citation29
The nontreated diabetic group showed serum insulin levels at about 10% of normal control values, exhibiting pronounced bone loss, reflected in a low ratio of femur ash to femur weight (FA/FW), as well as low bone concentrations of Ca, P, and Mg compared with the negative control group. This finding suggests that bone regeneration is impaired by insulin deficiency.
Despite the marked bone loss, FW/BW ratio showed a nonsignificant decline compared with the corresponding ratio from the negative control group. When compared with body weight in the nontreated diabetic group, masking of this bone loss may be secondary to the associated reduction in body weight.
Interestingly, Ca concentration in ash was reduced by almost 50% and that of P was reduced by about 30% only in nontreated diabetic rats, compared with the ones in the control group. Similar findings have been reported in insulin deficiencyCitation30 being associated with a decrease in both ash content and ratio of Ca to P in tibia ash. In another study in insulin deficiency,Citation31 a significant decrease (>50%) of bone volume fraction in the tibia and femur of mice was demonstrated. Consistent loss of bone mass was detected by cytophotometry, due to a deficit in mineralized surface area in an untreated insulin-deficient state.Citation32
In the present study, diabetic rats treated with propolis (with or without insulin) showed an increased FW/BW ratio compared with nontreated diabetic rats and the negative control group, reflecting an increased bone mass. On the other hand, only diabetic rats treated with propolis plus insulin showed a significant increase in the FA/FW ratio when compared with nontreated diabetic rats. However, the most striking feature was that all groups of diabetic rats treated with propolis (with or without insulin) showed Ca and Mg values significantly higher than nontreated diabetic rats and those diabetic rats treated with insulin alone, reflecting improvement of bone mineral content. Specifically, the double-dose propolis-treated groups (with or without insulin) showed Ca concentration levels in femur ash closer to those of the negative control group; Ca levels in nontreated diabetic rats were about 50% of the negative control.
The diabetic group also showed a significant rise of serum PTH and calcitonin levels as compared with the negative control group. PTH elevation is another factor for inducing bone loss, through both bone osteolysis and resorption.Citation33 This increase of serum PTH levels in diabetic rats could be due to an increase in Ca excretion accompanying glycosuria and a decreased Ca absorption secondary to deficiency of vitamin D.Citation34 The mechanism behind the double rise in calcitonin concentration in diabetic rats and their return to normal levels by the different treatment regimen may provide a protective effect, ameliorating the bone loss effects of insulin deficiency and PTH elevation. However, this is not clear and needs further investigation.
In the present study, propolis showed remarkable effects on bone minerals, and therefore on bone mass, especially when administered with insulin to STZ-induced diabetic rats. Previous studies have reported propolis to have exerted an inhibitory action on osteoclasts, leading to attenuation of osteoclastogenesis, compared with insulin and its anabolic effects on bone osteoblasts.Citation35 Another experimental study has reported that propolis inhibits late stages of osteoclast maturation, including fusion of osteoclast precursors to form giant cells.Citation36 At the molecular level, an earlier study has documented that propolis has dual effects on osteoclasts: it inhibits osteoclastogenesis and it induces apoptosis.Citation37 It has also been reported that hyperglycemia contributes to diabetic bone complications through a variety of mechanisms including increasing the reactive oxygen species,Citation37,Citation38 polyol pathway activity,Citation39 and protein kinase C activity.Citation40 In addition, hyperglycemia can induce an osmotic response in cells such as osteoblasts, leading to suppression of their activity.Citation41
In this study, the authors observed that propolis administration through its glycemic control may play an important role in prevention of bone loss associated with the hyperglycemic state. In particular, the double propolis dose combined with insulin treatment could almost return bone mineralization to normal control levels. Therefore, the authors propose that when administered together, insulin, with its potent effect on bone formation, and propolis, with its inhibitory effect on bone resorption, can provide a promising therapy to protect against bone loss in T1DM.
Conclusion
In conclusion, experimental treatment of STZ-induced diabetic rats with propolis and insulin was found to effectively control blood glucose level, improve function of the pancreatic islets, eliminate the oxidative stress, and protect bone from diabetic osteopathy. Clinical application of this combined therapy in humans requires further investigation and evaluation of its effectiveness and safety in T1DM patients.
Acknowledgments
All authors contributed to the design and coordination of the research, analysis of data, and writing of the manuscript. All authors read and approved the final manuscript, and all authors share responsibility for the final contents of this article. The authors wish to express their appreciation to King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia, for financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
- Zimmet PZ McCarty DJ de Courten MP The global epidemiology of non-insulin-dependent diabetes mellitus and the metabolic syndrome J Diabetes Complications 1997 11 2 60 68 9101389
- López-Ibarra PJ Pastor MM Escobar-Jiménez F Bone mineral density at time of clinical diagnosis of adult-onset type 1 diabetes mellitus Endocr Pract 2001 7 5 346 351 11585369
- Ishida H Seino Y Taminato T Circulating levels and bone contents of bone gamma-carboxyglutamic acid-containing protein are decreased in streptozocin-induced diabetes: possible marker for diabetic osteopenia Diabetes 1988 37 6 702 706 3260200
- Giugliano D Ceriello A Paolisso G Oxidative stress and diabetic vascular complications Diabetes Care 1996 19 3 257 267 8742574
- Halliwell B Gutteridge JM Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy Lancet 1994 1 8391 1396 1397 6145845
- Afshari AT Shirpoor A Farshid A The effect of ginger on diabetic nephropathy, plasma antioxidant capacity and lipid peroxidation in rats Food Chem 2007 101 148 153
- Hamada Y Fujii H Kitazawa R Yodoi J Kitazawa S Fukagawa M Thioredoxin-1 overexpression in transgenic mice attenuates streptozotocin- induced diabetic osteopenia: a novel role of oxidative stress and therapeutic implications Bone 2009 44 5 936 941 19146996
- Yilmaz HR Uz E Yucel N Altuntas I Ozcelik N Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver J Biochem Mol Toxicol 2004 18 4 234 238 15452882
- El-Sayed el-SM Abo-Salem OM Aly HA Mansour AM Potential antidiabetic and hypolipidemic effects of propolis extract in streptozotocin-induced diabetic rats Pak J Pharm Sci 2009 22 2 168 174 19339227
- Matsui T Ebuchi S Fujise T Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3,4,5-tri-O-caffeoylquinic acid Biol Pharm Bull 2004 27 11 1797 1803 15516726
- Noorafshan A Esmail-Zadeh B Bahmanpour S Poost-Pasand A Early stereological changes in liver of Sprague-Dawley rats after streptozotocin injection Indian J Gastroenterol 2005 24 3 104 107 16041101
- Wang NZ Li D Effect of combined propolis-ethanol-extract and Shaoyao-Gancao-tang on blood sugar levels in rabbits with alloxan induced experimental diabetes Asia Pac J Clin Nutr 2004 13 Suppl S66
- Baker H Lindsey J Weisbroth S Biology and Disease The Laboratory Rat II: Research Applications Orlando, FL Academic press 1980 276
- Xing Y Sonner J Laster MJ Insulin decreases isoflurane minimum alveolar anesthetic concentration in rats independently of an effect on the spinal cord Anesth Analg 2004 98 6 1712 1717 15155333
- Matsushige K Basnet P Hase K Kodota S Tanaka K Namba T Propolis protects pancreatic beta-cells against the toxicity of streptozotocin (STZ) Phytomedicine 1996 3 2 203 209
- Lei H Nwaigwe CI Williams H Dunn JE Effects of ketamine and ketamine-xylazine anesthesia on cerebral blood flow in rat observed using arterial spin tagging perfusion imaging Proc Int Soc Mag Reson Med 2001 9 1488
- Norazlina M Ima-Nirwana S Abul Gapor MT Abdul Kader Khalid B Tocotrienols are needed for normal bone calcification in growing female rats Asia Pac Clin Nutr 2002 11 3 194 199
- Findlay JW Dillard RF Appropriate calibration curve fitting in ligand binding assays AAPS J 2007 9 2 E260 E267 17907767
- Jaspan JB Rubenstein AH Circulating glucagon: plasma profiles and metabolism in health and disease Diabetes 1977 26 9 887 904 330295
- Porstmann T Kiessig ST Enzyme immunoassay techniques: an overview J Immunol Methods 1992 150 1–2 5 12 1613258
- McCord JM Fridovich I Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein) J Biol Chem 1969 244 22 6049 6055 5389100
- Armstrong D Browne R The analysis of the free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory Adv Exp Med Biol 1994 366 43 58 7771281
- Rungby J Ernst E Experimentally induced lipid peroxidation after exposure to chromium, mercury and silver: interactions with carbon tetrachloride Pharmacol Toxicol 1992 70 3 205 207 1579547
- Thulesen J Orskov C Holst JJ Poulsen SS Short-term insulin treatment prevents the diabetogenic action of streptozotocin in rats Endocrinology 1997 138 1 62 68 8977386
- Lee ES Uhm KO Lee YM CAPE (caffeic acid phenethyl ester) stimulates glucose uptake through AMPK (AMP-activated protein kinase) activation in skeletal muscle cells Biochem Biophys Res Commun 2007 361 4 854 858 17689496
- Okutan H Ozcelik N Yilmaz RH Uz E Effects of caffeic acid phenethyl ester on lipid peroxidation and antioxidant enzymes in diabetic rat heart Clin Biochem 2005 38 2 191 196 15642285
- Akune T Ogata N Hoshi K Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts J Cell Biol 2002 159 1 147 156 12379806
- Thrailkill KM Lumpkin CKJr Bunn RC Kemp SF Fowlkes JL Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues Am J Physiol Endocrinol Metab 2005 289 5 E735 E745 16215165
- Botolin S Faugere MC Malluche H Orth M Meyer R McCabe LR Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice Endocrinology 2005 146 8 3622 3631 15905321
- Einhorn TA Boskey AL Gundberg CM Vigorita VJ Devlin VJ Beyer MM The mineral and mechanical properties of bone in chronic experimental diabetes J Orthop Res 1988 6 3 317 323 3258636
- Martin LM McCabe LR Type I diabetic bone phenotype is location but not gender dependent Histochem Cell Biol 2007 128 2 125 133 17609971
- Bachrach LK Dual energy X-ray absorptiometry (DEXA) measurements of bone density and body composition: promise and pitfalls J Pediatr Endocrinol Metab 2000 13 Suppl 2 983 988 11086651
- Iida-Klein A Hahn TJ Insulin acutely suppresses parathyroid hormone second messenger generation in UMR-106-01 osteoblast-like cells: differential effects on phospholipase C and adenylate cyclase activation Endocrinology 1991 129 2 1016 1024 1855451
- Fitzpatrick LA Secondary causes of osteoporosis Mayo Clin Proc 2002 77 5 453 468 12004995
- Ha J Choi HS Lee Y Lee ZH Kim HH Caffeic acid phenethyl ester inhibits osteoclastogenesis by suppressing NF kappaB and downregulating NFATc1 and c-Fos Int Immunopharmacol 2009 9 6 774 780 19285574
- Pileggi R Antony K Johnson K Zuo J Shannon Holliday L Propolis inhibits osteoclast maturation Dent Traumatol 2009 25 6 584 588 19843135
- Ang ES Pavlos NJ Chai LY Caffeic acid phenethyl ester an active component of honeybee propolis attenuates osteoclastogenesis and bone resorption via the suppression of RANKL-induced NF-kappaB and NFAT activity J Cell Physiol 2009 221 3 642 649 19681045
- Hunt JV Smith CC Wolff SP Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose Diabetes 1990 39 11 1420 1424 2227114
- Inaba M Nishizawa Y Shioi A Morii H Importance of sustained high glucose condition in the development of diabetic osteopenia: possible involvement of the polyol pathway Osteoporos Int 1997 7 Suppl 3 S209 S212 9536334
- Ceolotto G Gallo A Miola M Protein kinase C activity is acutely regulated by plasma glucose concentration in human monocytes in vivo Diabetes 1999 48 6 1316 1322 10342822
- Thomas DM Maher F Rogers SD Best JD Expression and regulation by insulin of GLUT 3 in UMR 106-01, a clonal rat osteosarcoma cell line Biochem Biophys Res Commun 1996 218 3 789 793 8579592