Abstract
Purpose
Metabolic syndrome (MetS), characterized by a constellation of insulin resistance, central obesity, hypertension, and hyperlipidemia, is a global health threat. High-sensitivity C-reactive protein (hs-CRP) has been shown to be associated with type 2 diabetes and cardiovascular disease; however, its association with incident MetS is less known. Therefore, the aim of this study was to examine the prospective association between hs-CRP and MetS among a Chinese population in a 5-year follow-up study.
Patients and Methods
The levels of hs-CRP were measured using serum samples collected at baseline recruitment in 2012 from 886 participants without MetS. Follow-up interviews were conducted in 2018, and MetS was diagnosed by 2017 criteria from the Chinese Diabetes Society. Multivariate logistic regression models were used to assess the overall and sex-specific associations between hs-CRP and incident MetS. The odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were computed with adjustment for demographic, socioeconomic, clinical, and lifestyle factors.
Results
After a mean follow-up duration of 5.40 ± 0.56 years, 116 (13.3%) participants developed MetS. In the total study population, increased hs-CRP levels were associated with a higher risk of MetS (OR comparing extreme quartiles of hs-CRP: 4.06 [95% CI: 1.91–8.65]) in the fully-adjusted model. When stratified by sex, the positive association was only observed in women (OR: 4.82 [1.89–12.3]) but not in men (OR: 3.15 [0.82–12.1]; P-interaction = 0.039).
Conclusion
In this study of a Chinese population, a positive association between hs-CRP and incident MetS was found only in women and not in men. Sex-specific prediction and intervention of MetS using hs-CRP as a target should be further evaluated.
Introduction
According to the World Health Organization,Citation1 metabolic syndrome (MetS) is a global health epidemic;Citation2 it is characterized by a constellation of interrelated cardiac risk factors consisting of insulin resistance, central obesity, hypertension, and hyperlipidemia. In 2010, one in three adults suffered from MetS in the USA,Citation1 and a similar prevalence was observed in China (33.9%).Citation3 Moreover, the prevalence of MetS has skyrocketed in the past few decades due to the sharp increase in obesity worldwide, and it is predicted to continue to increase at an alarming rate.Citation1 In addition, MetS has been shown to be associated with increased risks of subsequent development of diabetes mellitus, cardiovascular disease, and mortality.Citation4 Faced with the immense health burden, prediction and prevention of MetS is of utmost importance at the global level.
Chronic low-grade inflammation has been suggested as a major factor for both MetS and subsequent clinical outcomes.Citation5 Furthermore, high-sensitivity C-reactive protein (hs-CRP), a known biomarker for acute and chronic inflammation,Citation6 has been shown to be correlated with MetS in various cross-sectional studies.Citation7–Citation15 However, the temporal association between hs-CRP and MetS cannot be inferred from cross-sectional studies, and prospective cohort studies are warranted. Although numerous prospective studies and meta-analyses have been conducted to evaluate the associations between hs-CRP and type 2 diabetesCitation16–Citation19 and cardiovascular disease,Citation14,Citation15,Citation20,Citation21 only a few studies have assessed the association between hs-CRP and incident MetS in Finland,Citation22,Citation23 Mexico,Citation24 and Korea;Citation25 to date, no prospective study has evaluated the association among a Chinese population. Moreover, the results from prior studies have not been consistent:Citation20,Citation22,Citation24,Citation25 some prospective studies have found a positive association between hs-CRP and MetS in both women and men,Citation22,Citation23,Citation25 while one study reported no such association in men.Citation24 Furthermore, the potential sex difference also has been reported in cross-sectional studies between hs-CRP and MetS, with a stronger correlation in women than in men.Citation26–Citation29 According to a national representative survey among 31 provincial-level administrative units in China, the prevalence of MetS was also higher in women compared to men (36.8% vs 31.0%). Therefore, it is of scientific interest to examine whether the sex difference between hs-CRP and MetS is also observed in China. This information would be vital for the sex-specific prevention and prediction of MetS. However, no prospective studies have been conducted in China to examine the potential sex heterogeneity.
Therefore, the aim of this study was to examine the association between hs-CRP and incident MetS and the potential sex dissimilarity in a 5.4-year prospective follow-up study among a representative population of Chinese adults.
Materials and Methods
Study Population
The detailed design of the current cohort study has been described previously.Citation30–Citation35 Briefly, in 2012, 2142 southern Chinese adults aged ≥18 years old from Wanzai Town (Zhuhai City, China) were recruited by the stratified random sampling method. At recruitment, trained research coordinators used structured questionnaires to collect information on sociodemographic, clinical, and lifestyle factors; in addition, they measured the waist circumference (WC) and blood pressure of the participants. In 2018, follow-up interviews were conducted. This study was approved by the Ethics Committee of the Third Affiliated Hospital of Southern Medical University (IRB approval number: 201708011). All subjects provided their written informed consent at recruitment.
Ascertainment of Outcome: MetS
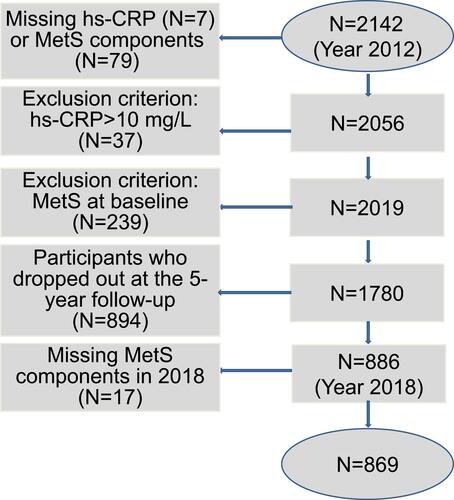
At both baseline and follow-up, we used the diagnostic criteria from the most updated Chinese Diabetes Society version (2017) to diagnose MetS.Citation36 Specifically, MetS was defined as having at least three of the following five components: (1) abdominal obesity: WC ≥ 90 cm in men or ≥ 80 cm in women; (2) hyperglycemia: fasting plasma glucose ≥ 6.1 mM and/or 2-h postprandial blood glucose ≥ 7.8 mM, or previously diagnosed as having type 2 diabetes and treated; (3) high blood pressure: systolic blood pressure (SBP)/diastolic blood pressure (DBP) ≥ 130/85 mmHg, or previously diagnosed as having hypertension and treated; (4) hypertriglyceridemia: triglyceride (TG) ≥ 1.7 mM, and (5) low high-density lipoprotein cholesterol (HDL-C) levels: HDL-C < 1.04 mM.Citation36 A total of 239 participants had MetS at baseline, and 79 had missing data regarding the MetS status (for one or more MetS components); thus, they were excluded from the current study.
Data Collection
Data on sociodemographic status, family, healthy lifestyle habits, and drug use were collected by structured questionnaires. The WC was measured according to the protocols recommended by the World Health Organization.Citation37 SBP and DBP were measured on the right arm using a calibrated mercury sphygmomanometer in a seated position. All subjects rested for at least 15 min, their SBP and DBP were measured three times in succession, and the average of three readings was taken.
Laboratory Measurement of hs-CRP and Other Biochemical Indicators
All blood specimens were collected after an overnight fasting for at least 10 h. First morning urine samples and fasting blood samples were collected, stored at 2–8 °C immediately after collection, and then transported to the Central Laboratory of the Third Affiliated Hospital of Southern Medical University within 3 h of collection.Citation38 Hs-CRP was tested using an enzymatic immunoassay turbidimetric method (reagent: Orion Corporation, Espoo, Finland; apparatus: Roche Cobas 6000, Penzberg, Germany). A total of 37 participants had extremely high hs-CRP levels (>10 mg/L), and 7 had missing hs-CRP values. After further excluding these participants, 1780 subjects were eligible for the current study at baseline. After the 5-year follow-up, 894 dropped out of the study, leaving a total of 886 for the current analysis. A total of 17 subjects had missing values for all components of MetS and thus were further excluded. Therefore, 869 subjects were selected for the current analysis. The detailed flowchart is shown in . Serum creatinine, urinary creatinine, serum uric acid (UA), fasting glucose, serum TG, low-density lipoprotein cholesterol, and HDL-C were measured by a colorimetric method. Urinary albumin was measured by an immune nephelometric method.Citation39
Statistical Analysis
Continuous variables with normal distributions were reported as the mean and standard deviation, while those with skewed distributions were reported as the median and interquartile range. Categorical data were presented as percentages. The baseline characteristics of the participants included in the current study and those excluded due to prevalent MetS were compared using the independent-samples Student’s t-test or analysis of variance for continuous variables, and the chi-squared test or Fisher’s exact test for categorical variables.
According to the quartile distribution of serum hs-CRP in the baseline population, four groups were created, with the first group serving as a reference. The ranges of hs-CRP for the four groups were as follows: ≤0.39 mg/L, 0.39 mg/L to ≤0.79 mg/L, 0.79 mg/L to ≤1.73 mg/L, and 1.73 mg/L to ≤10 mg/L. The association between hs-CRP and incident MetS was analyzed using multivariate logistic regression models to compute the odds ratio (OR) and the corresponding 95% confidence interval (CI). Potential confounding factors were chosen based on both the biological plausibility and the statistical significance. Model 1 was adjusted for sex and age; model 2 was additionally adjusted for socioeconomic and lifestyle factors (education, exercise, smoking, and alcohol consumption). Model 3 was the final model including all of the variables in model 2 plus clinical biomarkers (UA, albumin-to-creatinine ratio [ACR], and estimated glomerular filtration rate [eGFR]). Since all three of these variables are biomarkers for kidney function and had high correlations, we included eGFR in the final model (Model 3). We did not include WC, glucose levels, blood pressure, or lipids in the final model because they were used to define the outcome of MetS. Statistical analyses were performed using the SPSS software version 20.0 (IBM Corp., Armonk, NY, USA). A two-sided P value of <0.05 was considered statistically significant.
Results
Baseline Characteristics
After a mean follow-up period of 5.4 years, 116 out of the 886 participants developed incident MetS in the current study. The baseline characteristics of the participants included in this study and those excluded due to prevalent disease are shown in . The average age of those who developed incident MetS was 52.0 ± 12.7 years old, and 68.7% were female. The average body mass index (BMI) was 22.8 ± 3.29 kg/m2. Compared to the recruited participants, those excluded from the study due to prevalent MetS were more likely to be men and older, and have higher levels of blood pressure (SBP and DBP), BMI, WC, total cholesterol (TC), TG, very-low-density lipoprotein (VLDL), hs-CRP, and FBG (P < 0.001) and lower levels of HDL. The proportion of participants with a high-school education or above in the non-MetS group was higher than that in the MetS group (P < 0.001). The LDL levels were similar between the two groups (P = 0.78).
Table 1 Baseline Characteristics of Participants Included in This Study and Those Excluded Participants with Prevalent Metabolic Syndrome (MetS) at Baseline
The distribution of baseline characteristics according to the quartiles of hs-CRP levels is shown in . Compared to quartile 1, the participants in quartile 4 were older, heavier, less educated, and more likely to be female, have a history of hypertension and coronary heart disease, and be nonsmokers. The levels of blood biomarkers, including SBP, TC, TG, LDL, VLDL, FBG, insulin, UA, and ACR, were higher and the level of HDL was lower in quartile 4 vs 1 ().
Table 2 Baseline Characteristics According to the Quartile Distribution of hs-CRP Levels
The incidence of MetS in the total study population as well as stratified by sex is shown in . The overall incidence of MetS was 13.3%, and it increased from 5.8% in quartile 1 to 10.8%, 16.6%, and 20.5% in quartiles 2–4, respectively (P < 0.001) (). When stratified by sex, the incidence in men was higher than that in women (16.1% vs 12.1%); however, a statistically significant trend was observed of the increment of MetS incidence across quartiles in women (P < 0.001) but not in men (P = 0.26) ().
Table 3 Logistic Regression Analysis of hs-CRP and Incident MetS in the Total Study Population and Stratified by Sex
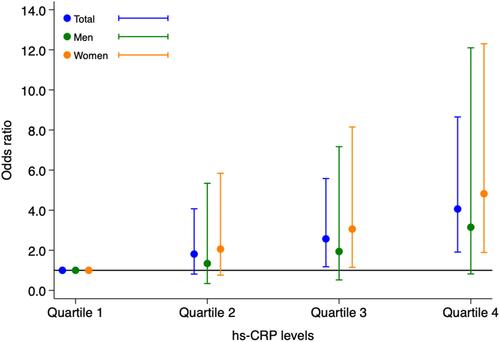
The multivariate logistic regression analyses showed consistent results (). In the total study population, a positive association between hs-CRP and MetS was observed in all three models. In the final model (model 3), the OR comparing the extreme quartiles of hs-CRP was 4.06 (95% CI: 1.91–8.65; P-trend < 0.001) ( and ). When stratified by sex, the positive association persisted in women (OR 4.82 [1.89–12.3]; P-trend< 0.001) but disappeared in men (OR 3.15 [0.82–12.1]; P-trend= 0.08) ( and ). The interaction between sex and the hs-CRP–MetS association was statistically significant (P-interaction = 0.039).
Figure 2 Associations between hs-CRP and MetS in the general population (total) as well as in men and women, separately. The odds ratio and 95% confidence interval of hs-CRP associated with MetS were based on Model 3 from .
Abbreviations: hs-CRP, high-sensitivity C-reactive protein; MetS, metabolic syndrome.
To test the robustness of the sensitivity analysis results, we further categorized hs-CRP into a binary variable using the sex-specific median value as the cut-off value and evaluated its association with MetS. Similar to the main analysis, higher levels of hs-CRP were significantly associated with incident MetS (OR 2.30 [1.42–3.72]). When stratified by sex, the positive association was only observed in women (OR 2.47 [1.39–4.37]) but not in men (OR 2.05 [0.85–4.93]) ().
Table 4 Logistic Regression Analysis of Binary hs-CRP and Incident MetS in the Total Study Population and Stratified by Sex
Discussion
In this Chinese population-based cohort study, we found a positive association between hs-CRP levels and the risk of developing MetS. We also observed a significant sex interaction, where the positive association was only observed in women but not in men. To the best of our knowledge, this is the first prospective study among a Chinese population to examine the association between hs-CRP and incident MetS in the general population as well as the potential sex heterogeneity.
The positive association between hs-CRP and MetS observed in the current study was largely consistent with the positive correlations that have been reported from cross-sectional studies.Citation7–Citation15 Among the few prospective cohort studies conducted in Finland,Citation22,Citation23 Mexico,Citation24 and Korea,Citation25 the results have not been entirely consistent. Among the three prospective studies conducted in men only,Citation22,Citation24,Citation25 two studies observed a positive association between CRP and MetS,Citation22,Citation24 while the other study found no association.Citation25 The observed difference may be due to the difference in sampling methods, genetic makeups, diagnostic criteria for MetS, measurements for hs-CRP, and model adjustment.Citation22,Citation24,Citation25 For example, the Mexican study lacked information on the population sources and sampling methods, and the age range (35–64 years) did not represent the general population;Citation24 in addition, the diagnostic criteria did not include WC in that study. The study conducted in Finland had a relatively low follow-up rate (26.4%), and the association between hs-CRP and MetS disappeared in the final model after including factors associated with insulin resistance.Citation22 Furthermore, another study from Finland included a small sample size of 103 women with a narrow age range (60–70 years).Citation23 Among the other two prospective studies that included women, a consistent positive association was observed between CRP and MetS,Citation20,Citation23 which corroborated with the results found in the current study. The sex heterogeneity also has been observed in various cross-sectional studies.Citation26–Citation29 Of note, a study in Japan has identified a lower cut-off point for the identification of MetS in women compared to men (0.25 mg/L vs 0.45 mg/L).Citation26
Inflammation has been hypothesized to be pivotal in several components of MetS, such as obesityCitation40–Citation43 and insulin resistance,Citation42,Citation43 which could explain the observed association between hs-CRP and MetS. Although the underlying mechanism behind the observed sex heterogeneity is not clear yet, two possible explanations are proposed. First of all, compared to men, women have a higher visceral fat level,Citation44 which has been shown to be associated with MetS risk.Citation45 Second, the sex hormone estrogen may exhibit proinflammatory roles;Citation27,Citation46 therefore, the increment of hs-CRP may have a more pronounced impact in leading to MetS in women compared to men. In addition, the observed sex difference has important clinical and public health implications. This finding suggests that prediction and modulation of the MetS risk by targeting hs-CRP should be focused in women. Future prospective studies and controlled trials are warranted to validate our findings and to evaluate the sex-specific prediction and intervention of MetS via targeting hs-CRP.
The current study used the stratified random sampling method to recruit a representative population of Chinese adults. We chose hs-CRP instead of CRP as a more sensitive marker for inflammation and excluded subjects with hs-CRP levels greater than 10 mg/L to avoid those with acute inflammation. The diagnostic criteria of MetS were based on the most updated Chinese Diabetes Society version (2017) and are similar to the present Adult Treatment Panel III criteria.Citation47 However, some limitations merit consideration. First of all, the participants included in the current study were all ethnic Han Chinese from Zhuhai City; thus, they may not be representative of other ethnic groups. In addition, the hs-CRP levels were measured only once, and some measurement error is evitable. Residual confounding may also exist as some of the confounding factors may not have been collected in the current study. Furthermore, although this is the first Chinese prospective study, many cross-sectional studies have been conducted on this topic and the implication of our results is not new. Future studies with larger sample sizes, longer follow-up times, and repeated measurements of hs-CRP are warranted to validate our findings so that detailed subgroup analyses can be performed.
Conclusions
In conclusion, in this prospective cohort study among a Chinese population, we found a positive association between hs-CRP and incident MetS, which was only observed in women and not in men. The results may facilitate the sex-specific prediction and treatment of MetS by targeting hs-CRP. However, future studies are warranted to validate our findings and to evaluate related interventions.
Abbreviations
hs-CRP, high-sensitivity C-reactive protein; MetS, Metabolic syndrome; OR, Odds ratio; WC, waist circumference; BMI, body mass index; UA, uric acid; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; UA, uric acid; ACR, urinary albumin-to-creatinine ratio.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Southern Medical University and was conducted in accordance with the Declaration of Helsinki. All subjects provided their written informed consent at recruitment.
Consent for Publication
All data published here are under the consent for publication.
Data Sharing Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting, or revising the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi:10.1007/s11906-018-0812-z
- Zafar U, Khaliq S, Ahmad HU, Manzoor S, Lone KP. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones (Athens). 2018;17(3):299–313. doi:10.1007/s42000-018-0051-3
- Lu J, Wang L, Li M, et al. Metabolic syndrome among adults in China: the 2010 China noncommunicable disease surveillance. J Clin Endocrinol Metab. 2017;102(2):507–515. doi:10.1210/jc.2016-2477
- Povel CM, Beulens JW, van der Schouw YT, et al. Metabolic syndrome model definitions predicting Type 2 diabetes and cardiovascular disease. Diabetes Care. 2013;36(2):362–368. doi:10.2337/dc11-2546
- de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54(6):945–955. doi:10.1373/clinchem.2007.100156
- Luan -Y-Y, Yao Y-M. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol. 2018;9:1302. doi:10.3389/fimmu.2018.01302
- Song Y, Yang SK, Kim J, Lee DC. Association between C-reactive protein and metabolic syndrome in Korean adults. Korean J Fam Med. 2019;40(2):116–123. doi:10.4082/kjfm.17.0075
- Frohlich M, Imhof A, Berg G, et al. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23(12):1835–1839. doi:10.2337/diacare.23.12.1835
- Suhett LG, Hermsdorff HHM, Rocha NP, et al. Increased C-reactive protein in Brazilian children: association with cardiometabolic risk and metabolic syndrome components (PASE study). Cardiol Res Pract. 2019;2019:3904568. doi:10.1155/2019/3904568
- Mirhafez SR, Ebrahimi M, Saberi Karimian M, et al. Serum high-sensitivity C-reactive protein as a biomarker in patients with metabolic syndrome: evidence-based study with 7284 subjects. Eur J Clin Nutr. 2016;70(11):1298–1304. doi:10.1038/ejcn.2016.111
- Mazidi M, Toth PP, Banach M. C-reactive protein is associated with prevalence of the metabolic syndrome, hypertension, and diabetes mellitus in US adults. Angiology. 2018;69(5):438–442. doi:10.1177/0003319717729288
- Mahajan A, Jaiswal A, Tabassum R, et al. Elevated levels of C-reactive protein as a risk factor for metabolic syndrome in Indians. Atherosclerosis. 2012;220(1):275–281. doi:10.1016/j.atherosclerosis.2011.10.031
- Hillman AJ, Lohsoonthorn V, Hanvivatvong O, Jiamjarasrangsi W, Lertmaharit S, Williams MA. Association of high sensitivity C-reactive protein concentrations and metabolic syndrome among Thai adults. Asian Biomed. 2010;4(3):385–393. doi:10.2478/abm-2010-0047
- Ridker P, Buring J, Cook N, Rifai N. C-Reactive protein, the metabolic syndrome, and risk of incident cardiovascular events an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi:10.1161/01.CIR.0000055014.62083.05
- Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB Sr., Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham offspring study. Circulation. 2004;110(4):380–385. doi:10.1161/01.CIR.0000136581.59584.0E
- Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of Type 2 diabetes. A systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175.
- Kanmani S, Kwon M, Shin M-K, Kim MK. Association of C-reactive protein with risk of developing Type 2 diabetes mellitus, and role of obesity and hypertension: a large population-based Korean cohort study. Sci Rep. 2019;9(1):4573. doi:10.1038/s41598-019-40987-8
- Pan A, Wang Y, Yuan J-M, Koh W-P. High-sensitive C-reactive protein and risk of incident type 2 diabetes: a case-control study nested within the Singapore Chinese health study. BMC Endocr Disord. 2017;17(1):8. doi:10.1186/s12902-017-0159-5
- Morimoto H, Sakata K, Oishi M, et al. Effect of high-sensitivity C-reactive protein on the development of diabetes as demonstrated by pooled logistic-regression analysis of annual health-screening information from male Japanese workers. Diabetes Metab. 2013;39(1):27–33. doi:10.1016/j.diabet.2012.03.004
- Chiu F-H, Chuang CH, Li W-C, et al. The association of leptin and C-reactive protein with the cardiovascular risk factors and metabolic syndrome score in Taiwanese adults. Cardiovasc Diabetol. 2012;11:40. doi:10.1186/1475-2840-11-40
- Torres JL, Ridker PM. Clinical use of high sensitivity C-reactive protein for the prediction of adverse cardiovascular events. Curr Opin Cardiol. 2003;18(6):471–478. doi:10.1097/00001573-200311000-00008
- Laaksonen DE, Niskanen L, Nyyssonen K, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47(8):1403–1410. doi:10.1007/s00125-004-1472-x
- Hassinen M, Lakka TA, Komulainen P, Gylling H, Nissinen A, Rauramaa R. C-reactive protein and metabolic syndrome in elderly women: a 12-year follow-up study. Diabetes Care. 2006;29(4):931–932. doi:10.2337/diacare.29.04.06.dc05-2508
- Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25(11):2016–2021. doi:10.2337/diacare.25.11.2016
- Yoon K, Ryu S, Lee J, Park JD. Higher and increased concentration of hs-CRP within normal range can predict the incidence of metabolic syndrome in healthy men. Diabetes Metab Syndr. 2018;12(6):977–983. doi:10.1016/j.dsx.2018.06.008
- Oda E, Kawai R. Tentative cut point of high-sensitivity C-reactive protein for a component of metabolic syndrome in Japanese. Circ J. 2009;73(4):755–759. doi:10.1253/circj.CJ-08-0848
- Lai MM, Li CI, Kardia SL, et al. Sex difference in the association of metabolic syndrome with high sensitivity C-reactive protein in a Taiwanese population. BMC Public Health. 2010;10:429. doi:10.1186/1471-2458-10-429
- Garcia VP, Rocha HN, Sales AR, Rocha NG, da Nobrega AC. Sex differences in high sensitivity C-reactive protein in subjects with risk factors of metabolic syndrome. Arq Bras Cardiol. 2016;106(3):182–187. doi:10.5935/abc.20160027
- Wen J, Liang Y, Wang F, et al. Association of C-reactive protein and metabolic syndrome in a rural Chinese population. Clin Biochem. 2009;42(10–11):976–983. doi:10.1016/j.clinbiochem.2009.03.026
- Zhang L, Chen S, Deng A, et al. Association between lipid ratios and insulin resistance in a Chinese population. PLoS One. 2015;10(1):e0116110. doi:10.1371/journal.pone.0116110
- Li Y, Zhou C, Shao X, et al. Hypertriglyceridemic waist phenotype and chronic kidney disease in a Chinese population aged 40 years and older. PLoS One. 2014;9(3):e92322. doi:10.1371/journal.pone.0092322
- Li Y, Chen Y, Liu X, et al. Metabolic syndrome and chronic kidney disease in a Southern Chinese population. Nephrology. 2014;19(6):325–331. doi:10.1111/nep.2014.19.issue-6
- Chen S, Liu H, Liu X, et al. Central obesity, C-reactive protein and chronic kidney disease: a community-based cross-sectional study in southern China. Kidney Blood Press Res. 2013;37(4–5):392–401. doi:10.1159/000355718
- Chen S, Chen Y, Liu X, et al. Insulin resistance and metabolic syndrome in normal-weight individuals. Endocrine. 2014;46(3):496–504. doi:10.1007/s12020-013-0079-8
- Chen S, Chen Y, Liu X, et al. Association of insulin resistance with chronic kidney disease in non-diabetic subjects with normal weight. PLoS One. 2013;8(9):e74058. doi:10.1371/journal.pone.0074058
- Society. CD. Guideline for prevention and treatment of Type 2 diabetes in China; 2017. Available from: http://www.xayxfw.com/uploadfiles/2018/03/201803160832403240.pdf. Accessed October 24, 2019.
- Ma W-Y, Yang C-Y, Shih S-R, et al. Measurement of waist circumference: midabdominal or iliac crest? Diabetes Care. 2013;36(6):1660–1666. doi:10.2337/dc12-1452
- Sun M, Zhang L, Chen S, Liu X, Shao X, Zou H. Association of C-reactive protein and metabolic disorder in a Chinese population. Int J Environ Res Public Health. 2015;12(7):8228–8242. doi:10.3390/ijerph120708228
- Liu X, Liu Y, Chen Y, et al. Body mass index (BMI) is associated with microalbuminuria in Chinese hypertensive patients. Int J Environ Res Public Health. 2015;12(2):1998–2008. doi:10.3390/ijerph120201998
- Ley SH, Harris SB, Connelly PW, et al. Adipokines and incident type 2 diabetes in an aboriginal Canadian [corrected] population: the sandy lake health and diabetes project. Diabetes Care. 2008;31(7):1410–1415. doi:10.2337/dc08-0036
- Thorand B, Löwel H, Schneider A, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the monica augsburg cohort study, 1984-1998. Arch Intern Med. 2003;163(1):93–99. doi:10.1001/archinte.163.1.93
- Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of Type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–1805. doi:10.2337/diabetes.52.7.1799
- Festa A, D’Agostino R, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of Type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51(4):1131–1137. doi:10.2337/diabetes.51.4.1131
- Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108(3–5):272–280. doi:10.1016/j.jsbmb.2007.09.001
- You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89(11):5517–5522. doi:10.1210/jc.2004-0480
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. doi:10.1210/er.2007-0001
- Grundy SM, Brewer HB Jr., Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi:10.1161/01.CIR.0000111245.75752.C6