Abstract
The aim of the present study was to investigate the effects of anabolic-androgenic steroids and resistance training (RT) on insulin sensitivity in ovariectomized rats. Adult female Wistar rats were divided into ten experimental groups (n = 5 animals per group): (1) sedentary (Sed-Intact); (2) sedentary ovariectomized (Sed-Ovx); (3) sedentary nandrolone (Sed-Intact-ND); (4) sedentary ovariectomized plus nandrolone (Sed-Ovx-ND); (5) trained (TR-Intact); (6) trained nandrolone (TR-Intact-ND); (7) trained ovariectomized (TR-Ovx); (8) trained ovariectomized plus nandrolone; (9) trained sham; and (10) trained ovariectomized plus sham. Four sessions of RT were used, during which the animals climbed a 1.1 m vertical ladder with weights attached to their tails. The sessions were performed once every 3 days, with between four and nine climbs and with eight to twelve dynamic movements per climb. To test the sensitivity of insulin in the pancreas, glucose and insulin tolerance tests were performed. For insulin sensitivity, there was a statistically significant interaction for the TR-Ovx group, which presented higher sensitivity than the Sed-Intact, Sed-Ovx, and TR-Intact groups. Sed-Intact-ND and TR-Intact-ND groups exhibited higher values of insulin sensitivity than the Sed-Intact group. Except for the TR-Intact group, sensitivity was greater in trained groups than in the Sed-Intact group. There was higher insulin sensitivity in the TR-Intact-ND group than in the Sed-Intact and Sed-Intact-ND groups (P < 0.05). In conclusion, ovariectomy and short-term RT alone induced no change on insulin action. Administration of nandrolone decanoate improved insulin action, mainly when it was associated with RT.
Introduction
Menopause has been associated with an increased risk of coronary artery disease, diabetes, skeletal muscle wasting (sarcopenia), bone mineral density loss (osteopenia), changes in body composition, lipid profile, fat deposition, and increased inflammatory markers.Citation1 Ovariectomy is an experimental model used to mimic menopause in animals, inducing an increase in food intake and body weight, insulin resistance, sarcopenia, and muscle force generation.Citation2–Citation4 It has been shown that these deleterious effects of ovariectomy can be partially reversed by exercise training and steroid hormone replacement.Citation2,Citation5,Citation6
Physical training, such as resistance, jumping, or swimming exercise, and wheel or treadmill running by rats has repeatedly been reported to be associated with enhanced insulin sensitivity, glucose transportation, increased bone density, improved immune system, and positive adaptations in cardiac muscle, skeletal muscle, and adipose tissue.Citation7–Citation12 However, these positive adaptations in ovariectomized rats have mainly been observed with long-term exercise training.
Anabolic-androgenic steroid (AAS) compounds are synthetic androgens commonly used by athletes to increase physical strength, endurance, and performanceCitation13 and to modify vascular function.Citation14 Although many studies have examined physiological, morphological, and psychological responses to AAS use in males, less is known about the incidence, patterns, and consequences of AAS use in females.Citation15–Citation17 AAS compounds may be used therapeutically in women, usually in small doses, to treat aplastic anemia and may also be used as antitumor agents in breast cancer and osteoporosis. In this regard, AASs such as nandrolone decanoate (ND) modulate transcription and translation of pancreatic islets cells and improve insulin action and glucose uptake.Citation15,Citation16 However, it is important to note that the World Anti-Doping AgencyCitation18 lists ND as a prohibited substance, with use of ND considered as doping.
Testosterone increases insulin mRNA levels in vitro as well as in vivo. Additionally, the stimulating effect of testosterone is also observed on insulin promoter activity, content, and release in male rats.Citation19 In this regard, nandrolone and other anabolic steroids have been used by athletes to build muscle mass and to enhance weight-lifting performance. A recent placebo-controlled study showed that supraphysiologic doses of testosterone resulted in an increase in muscle mass and strength in humans.Citation20
To the best of the authors’ knowledge, this is the first study to investigate the effects of ovarian hormone absence, short-term resistance training (RT), and AAS therapy on glucose tolerance and glucose-stimulated insulin response. The authors’ initial hypothesis was that resistance exercise associated with AAS would improve tissue response.
Methods
Animals
Fifty adult female Wistar rats (Rattus norvegicus var. albinus, Rodentia, Mammalia) from the breeding colony of the Methodist University of Piracicaba, Brazil, were used in this study. They were approximately 90 days old and had an initial weight of approximately 250 g, plus or minus 30 g. The animals were kept in collective cages (five rats per cage) and they received commercial rodent chow (Labina-Purina, Descalvado, São Paulo, Brazil) and water ad libitum. The room temperature was kept constant at 23°C, plus or minus 2°C, and the room had a cycle of 12 hours of light and 12 hours of dark, with lights on from 0600 to 1800 hours each day.
The Federal University of São Carlos Committee of Experimental Animals approved the research (protocol No. 048/2007). All animal procedures were conducted in accordance with the guide for care and use of laboratory animals from the National Research Council, 1996.
Experimental groups
The experimental design is presented in . Fifty rats were randomly distributed into the following ten experimental groups (n = 5 animals per group): (1) sedentary (Sed-Intact); (2) sedentary ovariectomized (Sed-Ovx); (3) sedentary nandrolone (Sed-Intact-ND); (4) sedentary ovariectomized plus nandrolone (Sed-OVX-ND); (5) trained (TR-Intact); (6) trained nandrolone (TR-Intact-ND); (7) trained ovariectomized (TR-Ovx); (8) trained ovariectomized plus nandrolone (TR-OVX-ND); (9) trained sham (TR-Intact-sham); and (10) trained ovariectomized plus sham (TR-OVX-sham).
Table 1 Experimental design
The Sed-Intact and Sed-Ovx animals were kept in their cages for 4 days without any type of exercise. The Sed-Ovx animals had their ovaries removed. Animals in the ND groups received an intramuscular injection of ND (Organon do Brasil, São Paulo, Brazil) into the hind limb every training day. Animals in the RT groups underwent four sessions of the proposed RT protocol. Sham groups received an intramuscular injection of the vehicle only (propylene glycol; Galena Química e Farmacêutica ltda, Campinas, SP, Brazil).
Ovariectomy
Rats were ovariectomized at 90 days of age, according to the technique described by Kaluo.Citation21 Ethyl ether was used as anesthetic in all animals undergoing ovariectomy. All animals were given 1 week of recovery after the surgical procedure.
RT exercise
The four sessions of RT included climbing exercise, and the sessions were performed once every 3 days. Initially, the rats were adapted to the RT protocol, which required the animals to climb a vertical ladder (1.1 × 0.18 m, 2 cm grid, 80° incline) with weights secured to their tails. The size of the ladder induced the animals to perform eight to twelve dynamic movements per climb. The load apparatus was secured to the tail by wrapping the proximal portion of the tail with a self-adhesive foam strip. A Velcro strap was wrapped around the foam strip and fastened. With the load apparatus secured to the tail, each rat was placed at the bottom of the ladder and familiarized with climbing. If necessary, a stimulus with tweezers was applied to the animal’s tail to initiate movement. At the top of the ladder, the rats reached a housing chamber (20 × 20 × 20 cm), where they were allowed to rest for 120 seconds. This procedure was repeated until the rats voluntarily climbed the ladder three consecutive times, without stimulus.
Three days after this familiarization, the first training session took place. The session consisted of four to eight ladder climbs while carrying progressively heavier loads. For the initial climb, the load carried was 75% of the animal’s body mass. After this, an additional 30 g weight was added, until a load was reached with which the rat could not climb the entire length of the ladder. Failure was determined when the animal could not progress up the ladder after three successive stimuli to the tail. The highest load successfully carried the entire length of the ladder was considered the rat’s maximal carrying capacity for that training session. The next training session consisted of four ladder climbs with 50%, 75%, 90%, and 100% of the rat’s previous maximal carrying capacity, determined in the previous session. During subsequent ladder climbs, an additional 30 g load was added until a new maximal carrying capacity was determined. The RT protocol was adapted from Hornberger and Faccar,Citation9 according to the needs of the present research.
AAS treatment
Nonphysiologic doses of ND (0.1 mg/kg) diluted in propylene glycolCitation22 were injected subcutaneously at the triceps surae on both posterior paws and in an alternating fashion. The drug was injected after every RT session. This dose is comparable with the dose reported as being frequently used for hormone replacement therapy.Citation22,Citation23
The control groups that did not receive AAS treatment (Sed-Intact, TR-Intact-sham, and TR-OVX-sham) received the vehicle propylene glycol.Citation24
Insulin sensitivity tests
Glucose tolerance test
Animals were anesthetized with sodium thiopental (40 mg/kg) (Abbott Laboratorios do Brasil, São Paulo, Brazil), and glucose solution (1 g/kg), was administered intraperitoneally after a 4-hour fast for the glucose tolerance test (GTT). Blood samples were collected before (0 minutes) and at 5, 10, 20, 30, 40, 60, and 90 minutes after glucose loading, and glycemia was determined using a glucometer (Advantage®; Boehringer Mannheim, Indianapolis, IN).
Insulin sensitivity was determined by calculating the area above the curve of the GTT. Lower values of area indicate higher insulin sensitivity, while higher values of area indicate lower insulin sensitivity. For animals in the training groups, the GTT was performed 24 hours after the last RT training session.
Insulin tolerance test
Twenty-four hours after the GTT, all animals were submitted to an insulin tolerance test (ITT). Briefly, animals were anesthetized with sodium thiopental (40 mg/kg) and a 1 U/kg (1 U/mL) dose of fast insulin (Biobrás, São Paulo, Brazil) was administered intraperitoneally. Blood samples were collected before (0 minutes) and at 2.5, 5, 10, 15, and 20 minutes after insulin loading, and glucose was determined by glucometer.
Tissue sensitivity was assayed by calculating the area above the curve of ITT. Lower values of area indicate higher tissue sensitivity, while higher values of area indicate lower tissue sensitivity.
Statistical analysis
All data are presented as mean plus or minus the standard deviation. The statistical analysis was performed initially using the Kolmogorov-Smirnov normality test and the homoscedasticity test (Bartlett criterion). All variables analyzed in the study presented normal distribution and homoscedasticity, which allowed the application of the analysis of variance test (variables: RT + ND + sham × different moments) and F test. Tukey’s post hoc test was applied in the event of a significant (P < 0.05) F ratio. The software package Statistica (version 6.1; StatSoft Inc, Tulsa, OK) was used, with a significance level of 0.05.
Results
Effects of ovariectomy and RT on insulin and tissue sensitivity
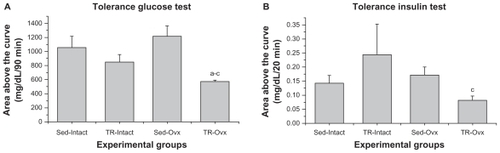
There was a statistically significant interaction among groups for insulin sensitivity. The TR-Ovx group presented a lower value for area above the curve, indicating higher insulin sensitivity than the Sed-Intact (45.2%), Sed-Ovx (52.4%), and TR-Intact (32.1%) groups (). For the tissue sensitivity, there was a statistically significant difference only between TR-Ovx and Sed-Ovx groups, indicated by the higher rate of glucose removed during the GTT in the TR-Ovx group ().
Figure 1 (A) Insulin sensitivity evaluated in the glucose tolerance test and (B) glucose sensitivity evaluated in the insulin tolerance test for experimental groups of sedentary (Sed-Intact), trained (TR-Intact), sedentary ovariectomized (Sed-Ovx), and trained ovariectomized (TR-Ovx) rats (n = 5 animals per group).
Effects of ovariectomy and ND
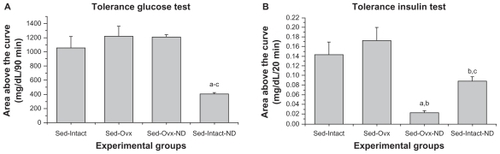
The Sed-Intact-ND group presented higher insulin sensitivity than the other sedentary groups: Sed-Intact, Sed-Ovx, and Sed-OVX-ND groups being 61.6%, 66.6%, and 66.4%, respectively (). Similarly, for the tissue sensitivity, the Sed-OVX-ND group presented improved values compared with the Sed-Intact, Sed-Ovx, and Sed-Intact-ND groups ().
Figure 2 (A) Insulin sensitivity evaluated in the glucose tolerance test and (B) glucose sensitivity evaluated in the insulin tolerance test for experimental groups of sedentary (Sed-Intact), sedentary ovariectomized (Sed-Ovx), sedentary ovariectomized plus nandrolone (Sed-OVX-ND), and sedentary nandrolone (Sed-Intact-ND) rats (n = 5 animals per group).
Effects of RT and ND
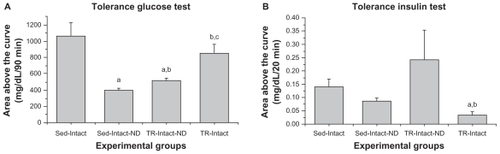
There was an interaction between the interventions (RT and ND) in insulin sensitivity. The Sed-Intact-ND and TR-Intact-ND groups exhibited higher values of insulin sensitivity than the Sed-Intact group, as shown in . Also, the TR-Intact group showed reduced insulin sensitivity compared with the Sed-Intact-ND and TR-Intact-ND groups (). The TR-Intact-ND group exhibited higher tissue sensitivity than the Sed-Intact and Sed-Intact-ND groups ().
Figure 3 (A) Insulin sensitivity evaluated in the glucose tolerance test and (B) glucose sensitivity evaluated in the insulin tolerance test for experimental groups of sedentary (Sed-Intact), sedentary nandrolone (Sed-Intact-ND), trained nandrolone (TR-Intact-ND), and trained (TR-Intact) rats (n = 5 animals per group).
Effects of ovariectomy, RT, and ND
Except for the TR-Intact group, the trained groups (TR-Intact-sham, TR-OVX-sham, TR-Ovx, and TR-OVX-ND) showed greater tissue sensitivity than the Sed-Intact group (). Moreover, the trained groups TR-Intact-sham, TR-OVX-sham, TR-Ovx, and TR-OVX-ND exhibited greater tissue sensitivity than the TR-Intact group ().
Figure 4 (A) Insulin sensitivity evaluated in the glucose tolerance test and (B) glucose sensitivity evaluated in the insulin tolerance test for experimental groups of sedentary (Sed-Intact), trained ovariectomized (TR-Ovx), trained sham (TR-Intact-sham), trained ovariectomized plus nandrolone (TR-OVX-ND), trained ovariectomized plus sham (TR-OVX-sham), and trained (TR-Intact) rats (n = 5 animals per group).
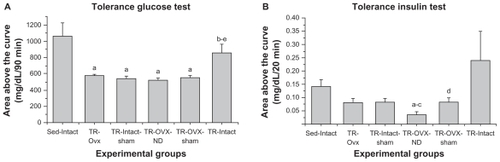
The TR-OVX-ND group showed greater tissue sensitivity than the Sed-Intact, TR-Ovx, and TR-Intact-sham groups. Furthermore, the TR-OVX-ND group exhibited higher tissue sensitivity than the TR-OVX-sham group ().
Discussion
The purpose of the present study was to analyze the influence of ovariectomy, RT, and AASs on insulin and tissue sensitivity. The authors’ initial hypothesis was partially confirmed, in that ovariectomy and AASs changed tissue responsivity. Additionally, AASs improved insulin sensitivity, mainly when associated with RT, short-term RT alone caused minor effects on glucose homeostasis, and AASs appeared to modulate insulin action.
Studies on the effects of exogenous AASs on tissue and insulin sensitivity have shown conflicting results. For example, AAS administration has been reported to increase the transcription and translation of insulin from pancreatic islet cells and to improve insulin action, modulating glucose levels.Citation15,Citation16 Marin et alCitation25 reported that anabolic steroids alter insulin sensitivity, and therefore a single supraphysiologic dose of ND (250 mg) improved insulin sensitivity, while a higher dose (500 mg) showed no effects on insulin sensitivity.
Regarding the effects of RT in intact animals without the use of exogenous ND, there was no increase in insulin and tissue sensitivity, which might have been related with the short-term exercise training (). This can be partly explained by a study by Hernandez et alCitation26 in which animals showed a temporal pattern for changes in rate of glucose uptake after resistance exercise: glucose uptake initially decreased after a period of 3 hours and then markedly increased 6 hours later, remaining elevated 12 hours post exercise in male intact rats. Furthermore, chronic exercise training has been reported to promote an increase in insulin receptors associated with increased activity of the PI3-kinase intracellular pathway and the transportation of GLUT4 protein to the plasma membrane, which enhances insulin signaling in tissue.Citation27,Citation28 On the other hand, Christ et alCitation29 showed that adaptations occurred during training could lead to improved insulin-stimulated muscle glucose uptake without affecting insulin receptor signaling through the PI3-kinase pathway.
Regarding the effects of ND and RT, the present study revealed that, independently of the training status (sedentary or trained), the animals that received ND showed higher sensitivity response, with lower values for area above the curve. This response could be associated with the capacity of AAS administration in modifying influx of calcium related to insulin secretion.Citation30 In addition, Hernandez et alCitation26 showed that arterial plasma insulin concentrations are not different between trained and sedentary groups when measured during an isotope infusion.
Ovarian hormones, especially estrogen, participate in the regulation of the pancreatic secretion of insulin, insulin sensitivity, and carbohydrate metabolism. The absence of estrogen hormone is associated with several metabolic disorders such as insulin resistance and altered glucose metabolism.Citation31–Citation34 The results from the present study revealed no alteration in insulin tissue sensitivity in ovariectomized rats. These results might have been associated with short-term ovariectomy, since a period of 14–21 days seems to be necessary for postoperative hormonal re-adaptation, as for the manifestation of the deleterious effects of estrogen absence.Citation26 Furthermore, studies with long-term ovariectomy showed negative effects of it on the morphology of pancreatic islets, insulin secretion, and glucose oxidation.Citation35–Citation37 Ovariectomized rats submitted to short-term resistance exercise exhibited an improvement in insulin sensitivity, with lower effects on peripheral sensitivity.
In this study, administration of ND improved insulin action and, as a consequence, increased glucose uptake. Hobbs et alCitation38 showed that acute higher doses of ND in humans enhanced glucose availability with no change in glucose metabolism. The chronic effects of ND require further clinical tests in order to guarantee its use as a therapeutic drug. Thus, the results from the present study should be considered as preliminary. As well as this, some limitations of the present study should be considered. For example, the absence of serial measurements after the first and last RT sessions and 2–4 weeks after the last RT session, which would clarify if these effects were derived from short-term RT or simply a session of resistance exercise. Additionally, the administration of ether stimulates catecholamine release, which may result in a moderate rise in blood glucose concentrations and a wide range of other metabolic changes.Citation39
Conclusion
In conclusion, the presented study data provide new insights into some aspects of the regulation of insulin and tissue sensitivity. Ovariectomy and short-term RT induced minor change in insulin tissue sensitivity. Acute administration of ND improved insulin action. When associated with short-term RT, ND also improved insulin and tissue sensitivity.
Acknowledgments
The Methodist University of Piracicaba supported this research. The authors would like to thank EB Carvalho, MI Montebelo, V Guzzoni, T Prando, and G Vasconcelos for reviewing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Leite RD Prestes J Pereira GB Shiguemoto GE Perez SE Menopause: highlighting the effects of resistance training Int J Sports Med 2010 31 11 761 767 21058218
- Landau IT Zucker I Estrogenic regulation of body weight in the female rat Horm Behav 1976 7 1 29 39 1278845
- Moran AL Nelson SA Landisch RM Warren GL Lowe DA Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice J Appl Physiol 2007 102 4 1387 1393 17218423
- Prestes J de Cássia Marqueti R Shiguemoto GE Effects of ovariectomy and resistance training on MMP-2 activity in skeletal muscle Appl Physiol Nutr Metab 2009 34 4 700 706 19767806
- Laudenslager ML Wilkinson CW Carlisle HJ Hammel HT Energy balance in ovariectomized rats with and without estrogen replacement Am J Physiol 1980 238 5 R400 R405 7377378
- Bongbélé J Gutierrez A Cardin S Lavoie JM Effect of physical training on insulin response to intravenous glucose in male peripubertal rats J Appl Physiol 1992 73 4 1227 1231 1447063
- Hansen PA McCarthy TJ Pasia EN Spina RJ Gulve EA Effects of ovariectomy and exercise training on muscle GLUT-4 content and glucose metabolism in rats J Appl Physiol 1996 80 5 1605 1611 8727546
- Allen DL Harrison BC Maass A Bell ML Byrnes WC Leinwand LA Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse J Appl Physiol 2001 90 5 1900 1908 11299284
- Hornberger TAJr Farrar RP Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat Can J Appl Physiol 2004 29 1 16 31 15001801
- Shiguemoto GE Rossi EA Baldissera V Gouveia CH de Valdez Vargas GM de Andrade Perez SE Isoflavone-supplemented soy yoghurt associated with resistive physical exercise increase bone mineral density of ovariectomized rats Maturitas 2007 57 3 261 270 17368767
- Prestes J de Ferreira CK Dias R Lymphocyte and cytokines after short periods of exercise Int J Sports Med 2008 29 12 1010 1014 18600609
- Leite RD Prestes J Bernardes CF Effects of ovariectomy and resistance training on lipid content in skeletal muscle, liver, and heart; fat depots; and lipid profile Appl Physiol Nutr Metab 2009 34 6 1079 1086 20029517
- Wilson JD Androgen abuse by athletes Endocr Rev 1988 9 2 181 199 3042375
- Bruder-Nascimento T Cordellini S Vascular adaptive responses to physical exercise and to stress are affected differently by nandrolone administration Braz J Med Biol Res 2011 44 4 337 344 21445526
- Landon J Wynn V Samols E The effect of anabolic steroids on blood sugar and plasma insulin levels in man Metabolism 1963 12 924 935 14068083
- Green IC El Seifi S Perrin D Howell SL Cell replication in the islets of langerhans of adult rats: effects of pregnancy, ovariectomy and treatment with steroid hormones J Endocrinol 1981 88 2 219 224 7009774
- Tricker R Casaburi R Storer TW The effects of supraphysiological doses of testosterone on angry behavior in healthy eugonadal men: a clinical research center study J Clin Endocrinol Metab 1996 81 10 3754 3758 8855834
- World Anti-Doping Agency (WADA) The 2010 prohibited list Available from: http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/WADA_Prohibited_List_2010_EN.pdf. [September 19, 2009] Accessed August 29, 2011
- Morimoto S Fernandez-Mejia C Romero-Navarro G Morales-Peza N Díaz-Sánchez V Testosterone effect on insulin content, messenger ribonucleic acid levels, promoter activity, and secretion in the rat Endocrinology 2001 142 4 1442 1447 11250923
- Bhasin S Storer TW Berman N The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men N Engl J Med 1996 335 1 1 7 8637535
- Kalu DN The ovariectomized rat model of postmenopausal bone loss Bone Miner 1991 15 3 175 191 1773131
- Kindlundh AM Lindblom J Bergström L Nyberg F The anabolic-androgenic steroid nandrolone induces alterations in the density of serotonergic 5HT1B and 5HT2 receptors in the male rat brain Neuroscience 2003 119 1 113 120 12763073
- Lindblom J Kindlundh AM Nyberg F Bergström L Wikberg JE Anabolic androgenic steroid nandrolone decanoate reduces hypothalamic pro-opiomelanocortin mRNA levels Brain Res 2003 986 1–2 139 147 12965238
- Farrell SF McGinnis MY Effects of pubertal anabolic-androgenic steroid (AAS) administration on reproductive and aggressive behaviors in male rats Behav Neurosci 2003 117 5 904 911 14570541
- Mårin P Krotkiewski M Björntorp P Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissue Eur J Med 1992 1 6 329 336 1341460
- Hernandez JM Fedele MJ Farrell PA Time course evaluation of protein synthesis and glucose uptake after acute resistance exercise in rats J Appl Physiol 2000 88 3 1142 1149 10710414
- Beeson M Sajan MP Dizon M Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise Diabetes 2003 52 8 1926 1934 12882907
- Krisan AD Collins DE Crain AM Resistance training enhances components of the insulin signaling cascade in normal and high-fat-fed rodent skeletal muscle J Appl Physiol 2004 96 5 1691 1700 14707149
- Christ CY Hunt D Hancock J Garcia-Macedo R Mandarino LJ Ivy JL Exercise training improves muscle insulin resistance but not insulin receptor signaling in obese Zucker rats J Appl Physiol 2002 92 2 736 744 11796688
- Benten WP Lieberherr M Sekeris CE Wunderlich F Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells FEBS Lett 1997 407 2 211 214 9166901
- Liu ML Xu X Rang WQ Li YJ Song HP Influence of ovariectomy and 17beta-estradiol treatment on insulin sensitivity, lipid metabolism and post-ischemic cardiac function Int J Cardiol 2004 97 3 485 493 15561337
- Bouwens L Rooman I Regulation of pancreatic beta-cell mass Physiol Rev 2005 85 4 1255 1270 16183912
- Sitnick M Foley AM Brown M Spangenburg EE Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass J Appl Physiol 2005 100 1 286 293 16150841
- Song D Arikawa E Galipeau DM Chronic estrogen treatment modifies insulin-induced insulin resistance and hypertension in ovariectomized rats Am J Hypertens 2005 18 9 Pt 1 1189 1194 16182108
- Holloszy JO Coyle EF Adaptations of skeletal muscle to endurance exercise and their metabolic consequences J Appl Physiol 1984 56 4 831 838 6373687
- James DE Kraegen EW Chisholm DJ Effects of exercise training on in vivo insulin action in individual tissues of the rat J Clin Invest 1985 76 2 657 666 3897288
- Puah JA Bailey CJ Effect of ovarian hormones on glucose metabolism in mouse soleus muscle Endocrinology 1985 117 4 1336 1340 3896756
- Hobbs CJ Jones RE Plymate SR Nandrolone, a 19-nortestosterone, enhances insulin-independent glucose uptake in normal men J Clin Endocrinol Metab 1996 81 4 1582 1585 8636371
- Flecknell P Laboratory Animal Anaesthesia 3rd ed London Academic Press 1996