Abstract
Long non-coding RNAs (lncRNAs) are a group of non-coding RNAs longer than 200 nucleotides, which are defined as transcripts. The lncRNAs are involved in regulating gene expression at epigenetic, transcriptional, and post-transcriptional levels. Recent studies have found that lncRNA is closely related to many diseases like neurological diseases, endocrine and metabolic disorders. Diabetic peripheral neuropathy (DPN) is one of the most common chronic complications of diabetes mellitus. In this review, we highlight the latest research related to lncRNAs in DPN.
Introduction
Long non-coding RNAs (lncRNAs) refer to non-coding RNAs with a length higher than 200 nucleotides. In mammalian, they account for the most substantial proportion of non-coding transcriptome.Citation1 Recent studies have shown that lncRNAs have essential regulatory functions and are involved in almost all biological processes and pathways.Citation2,Citation3 And some studies indicate lncRNAs may participate in the pathogenesis of diabetic peripheral neuropathy (DPN).Citation4,Citation5 In this review, the latest progress of lncRNAs in DPN will be demonstrated.
Pathophysiology of DPN
DPN is one of the most common severe and chronic complications affecting about 60–90% of patients with type 2 diabetes. In the early stage of diabetes, clinical symptoms and signs of peripheral neuropathy are not apparent. However, its nerve structure and function have been damaged, which means peripheral nerve showed segmental demyelination changes and often accompanied by axonal degeneration.Citation6 Nowadays, the pathogenesis of DPN is unclear, which is currently recognized as a multifactor. The main mechanisms of pathogenesis include metabolic toxicity, vascular injury, neurotrophin deficiency, oxidative stress, autoimmune, genetic susceptibility and other factors.Citation7–Citation11 Despite achieve control of blood sugar and blood pressure, supplemental neurotrophic factor, improve microcirculation and antioxidant therapy in clinical practice. However, most of the patients still inevitably suffer peripheral nerve injury. At present, DPN has become the leading cause of disability in type 2 diabetes mellitus.Citation12,Citation13
Function of LncRNA
Non-coding RNAs (ncRNAs) can be divided into short ncRNAs (<200 nt in length) and lncRNAs according to the base of size.Citation14,Citation15 LncRNAs range from 200 nt to more than 100 kb and often lack an apparent open reading frame.Citation16,Citation17 It has been confirmed lncRNAs regulate gene expression at epigenetic, transcriptional, and post-transcriptional levels.Citation2 LncRNAs participate in a diverse range of molecular and biological processes, and dysregulation of various human diseases. Many studies have demonstrated that lncRNAs disorders are involved in cancer, cardiovascular, neurodegenerative, and genetic diseases. Recent studies have posited that lncRNAs may also become a significant factor in DPN.Citation4,Citation5,Citation18 One clinical study found that there were 446 and 1327 differentially expressed lncRNAs between DM and DPN patients by the microarray analysis, respectively. Although there are more and more researches on lncRNAs, the specific biological role of most lncRNAs is still unknown. It may be possible to explain the specific function of lncRNAs by analyzing the action mechanism of mRNA co-expressed with lncRNAs. The KEGG pathway analysis of mRNAs co-expressed by differentially expressed lncRNAs between DM and DPN found that the most widely covered is the MAPK signaling pathway, which may be one of the specific mechanisms of lncRNAs participating in DPN.Citation4 At the same time, lncRNAs compete to occupy a large number of miRNAs in the cell, buffering and reducing their ability to interfere with the target mRNA like a sponge. Studying these ceRNA relationships has become a breakthrough for studying lncRNAs in DPN. (; ).
Table 1 Related LncRNAs in Diabetic Peripheral Neuropathy
Figure 1 Related lncRNAs in diabetic peripheral neuropathy.
Abbreviations: α-SMA, α-smooth muscle aorta; DPN, diabetic peripheral neuropathy; mRNA, messenger RNA; SGK3, serum and glucocorticoid regulated protein kinase 3; TNFAIP1, tumor necrosis factor, alpha-induced protein 1; TRPV1, transient receptor potential vanilloid type 1.
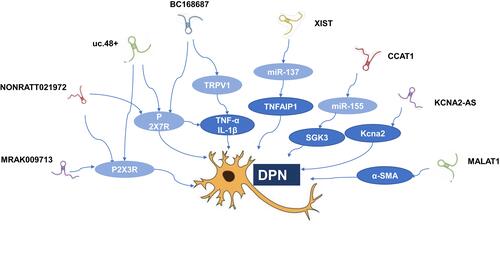
LncRNA BC168687
Diabetic neuropathic pain (DNP) is the most common clinical symptom of DPN. DNP affects patients’ quality of life and causes great pain. There are reports in the literature that nociceptive TRP (Transient receptor potential) channels are involved in inflammatory pain, neuropathic pain, visceral pain, and pathological pain.Citation19 Several of these studies have shown that activation of transient receptor potential vanilloid type 1 (TRPV1) in neurons plays an important role in peripheral neuralgia caused by diabetes.Citation20–Citation22 A research showed that in the streptozotocin-induced diabetic rat model, lncRNA BC168687 siRNA increased the thermal withdrawal latency (TWL) and mechanical withdrawal threshold (MWT) of diabetic rats and alleviated DPN by regulating transient receptor potential vanilloid type 1 (TRPV1) and cytokines (TNF-α and IL-1β).Citation23 The production of TNF-α and IL-1β and other inflammatory factors is closely related to the activation of ERK (extracellular regulated protein kinases) and p38 pathways.Citation24 This study indicates that lncRNA BC168687 siRNA can up-regulate the expression of p-ERK and p-p38 in DRG of DNP rats. ERK and p38 signaling pathways may be involved in the process of lncRNA BC168687 siRNA to relieve TRPV1-mediated DNP.Besides, another study found that lncRNA BC168687 siRNA could decrease the expression of P2X7 in dorsal root ganglion (DRG) neurons and the concentrations of NO in serum in DPN rats, thus attenuating DPN.Citation25
LncRNA uc.48+
One study about lncRNA array analysis in rats revealed that in the SCG of diabetic sympathetic neuropathy, the expression of the lncRNA uc.48+ was significantly increased. At the same time, one study showed that both in the DRG and the DM patients’ serum samples, the expression of lncRNA uc.48+ were increased. Moreover, the experiments showed that the MWT and TWL values of DM rats treated with siRNA of lncRNA uc.48+ were increased compared to those of DM rats, while compared with DM rats.Citation26 In order to further study whether the target of lncRNA uc.48 + exists in the pathophysiology of DPN, the data showed that In a subpopulation of small-diameter primary afferent neurons, the P2X3 receptor, as a suitable targeting marker for analgesics, had the highest expression and played an important role in the pain transmission of peripheral neurons.Citation27–Citation29 After the completion of the experiment, the expression of P2X3 protein and mRNAs in DRG of DM rats was increased, and they was significantly reduced after treatment with lncRNA uc.48 + siRNA. These changes are in common with the IOD ratio of p-ERK1/2, ERK1/2 and the expression of TNF-α. The results suggested that uc.48 + siRNA treatment could reduce the activation and phosphorylation of ERK1/2 in DRG of DM rats, and then reduce the hyperalgesia mediated by P2X3 receptor.
Diabetic autonomic neuropathy belongs to a subtype of DPN, which includes the sympathetic ganglionic dysfunction. P2X7 receptors in the superior cervical ganglia (SCG) are involved in the pathological changes of cardiac dysfunction.Citation30 It has been found lncRNAuc.48+ and P2X7 receptor in superior cervical ganglia (SCG) participates in the pathological changes which happen when Cardiac dysfunction. Cardiovascular complications of DM come from the damage of blood vessels and autonomic nerve fibers that govern the heart, which control heart rate and hemodynamics. Clinically, abnormal changes in HR and blood pressure are related to diabetic sympathetic neuropathy.Citation31 The lncRNAuc.48+ small interference RNA (siRNA) improved the cardiac autonomic dysfunction and reduced P2X7 upregulation by up-regulating ratio of p-ERK1/2 to ERK1/2 in SCG of type 2 diabetic rats.Citation32
LncRNA NONRATT021972
One research found that the concentration of LncRNA NONRATT021972 was significantly higher in the blood of patients with type 2 diabetes, and the level of LncRNA NONRATT021972 was positively correlated with neuropathic pain scores. The animal experiment showed that LncRNA NONRATT021972 siRNA could alleviate inflammation by reducing TNF-α.Citation33
Furthermore, other experiment showed that lncRNA NONRATT021972 siRNA decreased DNP mediated through the P2X3 receptor in DRG of T2DM rat model. The TWL and MWT in T2DM rats were lower compared to control rats. When treated with LncRNA NONRATT021972 siRNA, TWL and MWT in T2DM rats were higher compared with those in T2DM rats. At the same time, compared to T2DM rats, lncRNA NONRATT021972 siRNA-treated T2DM rats had significantly lower expression levels of the P2X3 protein and mRNA. Thus, LncRNA NONRATT021972 siRNA treatment may suppress the up-regulated expression and activation of the P2X3 receptor in T2DM rats, and reduce the hyperalgesia potentiated by the pro-inflammatory cytokine TNF-α.Citation34
Some studies found that LncRNA NONRATT021972 siRNA alleviated activation of p38 MAPK signalling pathway and expression of the P2X7 receptor in PC12 cells. Further experiments showed that there was crosstalk between LncRNA NONRATT021972 and the p38 MAPK signalling pathway. Inhibition of p38 MAPK signalling decreased the expression of the P2X7 receptor-induced by LncRNA NONRATT021972. In the same way, in the DRG neurons of type 2 DM rats models, LncRNA NONRATT021972 siRNA treatment might decrease the expression levels of P2X7 mRNA and protein. Moreover, LncRNA NONRATT021972 siRNA treatment reduced the release of inflammatory factors TNF-α, thereby, this study explores the effects of lncRNA NONRATT021972 siRNA on DNP mediated by the P2X7 receptor in the rat DRG.Citation35,Citation36
Other Possible LncRNAs
Growing data has clearly shown that nearly 40% of lncRNAs is exclusively present in the nervous system. The genome-wide expression patterns of lncRNAs and genes were investigated in the spinal dorsal horn of DNP mice. Gene chip analysis identified 1096 differentially expressed mRNAs and 1481 differentially expressed lncRNAs in DNP mice. Finally, 289 adjacent and 57 overlapping lncRNA-mRNA pairs were found, including AK081017-Usp15 and ENSMUST00000150952-Mbp, which may be related to the pathogenesis of DNP.Citation5
Emerging evidence suggested that lncRNAs play a crucial role in the development of neuropathic pain. Neuropathic pain is a chronic and potentially disabling pain caused by abnormalities in the somatosensory nervous system, spinal cord injury or various chronic conditions such as postherpetic neuralgia, autoimmune diseases, cancers and metabolic disorders, and diabetes mellitus become its most common cause. Therefore, DNP and neuropathic pain still have some similarities in the pathogenesis. Some studies suggested that specific regulation of lncRNAs, such as KCNA2-AS, XIST, CCAT1, MRAK009713 or their downstream targets might be involved in providing the development of neuropathic pain. Hence, these dysregulated lncRNAs were speculated to be involved in the occurrence, development and process of DNP.Citation37–Citation41
Diabetic gastroparesis is the manifestation of diabetic autonomic nerve damage in the digestive system. In a rat model of diabetic gastroparesis, it was found that expression of MALAT1 was up-regulated. Furthermore, the research revealed that the appearance of MALAT1 was increased in the samples from diabetic patients with diabetic gastroparesis symptoms, and the inhibition of MALAT1 might increase the α-SMA expression, inhibit the potential of cell migration and induce cell apoptosis in human gastric smooth muscle cells. Finally, it indicated that MALAT1 played an crucial role in the pathogenesis of diabetic gastroparesis.Citation42
Conclusions
Taken together, lncRNAs are essential regulators of gene expression. In addition to the articles mentioned, more lncRNAs will be found to be involved in the pathogenesis of DPN. At the same time, they have recently been characterized as important modulators of neuronal functions. As shown above, some specific lncRNAs participate in the pathophysiological processes of DPN. This review provides a better understanding of the emerging roles of lncRNAs in DPN.
With further research in basic and clinical fields, this may contribute to new strategies for the development of novel targeted drugs and biomarkers to treat this disease.
Acknowledgments
This study was supported by grants from the Fund of Shanghai Municipal Health Commission (201940362), Jinshan Science and Technology Commission (2019-03-02), and Outstanding Young Talents training plan of Jinshan District Health Committee (JSYQ201903).
Disclosure
The authors have no competing interests to declare.
References
- Derrien T, Guigo R, Johnson R. The long non-coding RNAs: a new player in the “dark matter”. Front Genet. 2011;2:107.
- Melissari MT, Grote P. Roles for long non-coding RNAs in physiology and disease. Pflugers Arch. 2016;468(6):945–958. doi:10.1007/s00424-016-1804-y
- Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription. 2014;5(4):e944014. doi:10.4161/21541272.2014.944014
- Luo L, Ji LD, Cai JJ, et al. Microarray analysis of long noncoding RNAs in female diabetic peripheral neuropathy patients. Cell Physiol Biochem. 2018;46(3):1209–1217. doi:10.1159/000489071
- Du H, Liu Z, Tan X, Ma Y, Gong Q. Identification of the genome-wide expression patterns of long non-coding RNAs and mRNAs in mice with streptozotocin-induced diabetic neuropathic pain. Neuroscience. 2019;15(402):90–103. doi:10.1016/j.neuroscience.2018.12.040
- Said G, Baudoin D, Toyooka K. Sensory loss, pains, motor deficit and axonal regeneration in length-dependent diabetic polyneuropathy. J Neurol. 2008;255(11):1693–1702. doi:10.1007/s00415-008-0999-z
- El Boghdady NA, Badr GA. Evaluation of oxidative stress markers and vascular risk factors in patients with diabetic peripheral neuropathy. Cell Biochem Funct. 2012;30(4):328–334. doi:10.1002/cbf.2808
- Tavakkoly-Bazzaz J, Amoli MM, Pravica V, et al. VEGF gene polymorphism association with diabetic neuropathy. Mol Biol Rep. 2008;255(11):1693–1702. doi:10.1007/s11033-010-0013-6
- Feldman EL, Nave KA, Jensen TS, Bennett DL. New Horizons in Diabetic Neuropathy: mechanisms, Bioenergetics, and Pain. Neuron. 2017;93(6):1296–1313. doi:10.1016/j.neuron.2017.02.005
- Tesfaye S, Chaturvedi N, Eaton SEM, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi:10.1056/NEJMoa032782
- Politi C, Ciccacci C, D’Amato C, et al. Recent advances in exploring the genetic susceptibility to diabetic neuropathy. Diabetes Res Clin Pract. 2016;120:198–208. doi:10.1016/j.diabres.2016.08.006
- Tesfaye S. Advances in the management of diabetic peripheral neuropathy. Curr Opin Support Palliat Care. 2009;3(2):136–143. doi:10.1097/SPC.0b013e32832b7df5
- Boulton A, Tesfaye S. Diabetic Neuropathy - ODL Oxford Diabetes Library. Oxford University Press; 2010.
- Modarresi F, Faghihi MA, Patel NS, Sahagan BG, Wahlestedt C, Lopez-Toledano MA. Knockdown of BACE1-AS nonprotein-coding transcript modulates beta-amyloid-related hippocampal neurogenesis. Int J Alzheimers Dis. 2011;2011:929042.
- Magistri M, Faghihi MA, St Laurent G, Wahlestedt C. Regulation of chromatin structure by long non-coding RNAs: focus on natural antisense transcripts. Trends Genet. 2012;28(8):389–396. doi:10.1016/j.tig.2012.03.013
- Knauss JL, Sun T. Regulatory mechanisms of long non-coding RNAs in vertebrate central nervous system development and function. Neuroscience. 2013;235:200–214. doi:10.1016/j.neuroscience.2013.01.022
- Khorkova O, Hsiao J, Wahlestedt C. Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev. 2015;87:15–24. doi:10.1016/j.addr.2015.05.012
- Li G, Sheng X, Xu Y, et al. Co-expression changes of lncRNAs and mRNAs in the cervical sympathetic ganglia in diabetic cardiac autonomic neuropathic rats. J Neurosci Res. 2017;95(8):1690–1699. doi:10.1002/jnr.24000
- Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM. Roles of transient receptor potential channels in pain. Brain Res Rev. 2009;60(1):2–23. doi:10.1016/j.brainresrev.2008.12.018
- Brito R, Sheth S, Mukherjea D, Rybak L, Ramkumar V. TRPV1: a potential drug target for treating various diseases. Cells. 2014;3(2):517–545. doi:10.3390/cells3020517
- Marche P, Dubois S, Abraham P, et al. Neurovascular microcirculatory vasodilation mediated by C-fibers and Transient receptor potential vanilloid-type-1 channels (TRPV 1) is impaired in type 1 diabetes. Sci Rep. 2017;7(1):44322. doi:10.1038/srep44322
- Aaron M, Andrew S, Durga M. Nociceptive TRP channels: sensory detectors and transducers in multiple pain pathologies. Pharmaceuticals. 2016;9(4):72.
- Liu C, Li C, Deng Z, Du E, Xu C. Long non-coding RNA BC168687 is involved in TRPV1-mediated diabetic neuropathic pain in rats. Neuroscience. 2018;15(374):214–222. doi:10.1016/j.neuroscience.2018.01.049
- Hensellek S, Brell P, Schaible HG, Bräuer R, Banchet GSV. The cytokine TNF-α increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36(3):381–391. doi:10.1016/j.mcn.2007.07.010
- Liu C, Tao J, Wu H, et al. Effects of LncRNA BC168687 siRNA on diabetic neuropathic pain mediated by P2X(7) Receptor on SGCs in DRG of rats. Biomed Res Int. 2017;7831251. doi:10.1155/2017/7831251
- Wang S, Xu H, Zou L, et al. LncRNA uc.48+ is involved in diabetic neuropathic pain mediated by the P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2016;12(1):139–148. doi:10.1007/s11302-015-9488-x
- Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15(15):1717–1735. doi:10.2174/138161209788186335
- Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99(1):16–34.
- Liang S, Xu C, Li G, Gao Y. P2X receptors and modulation of pain transmission: focus on effects of drugs and compounds used in traditional Chinese medicine. Neurochem Int. 2010;57(7):705–712. doi:10.1016/j.neuint.2010.09.004
- Kong F, Liu S, Xu C, et al. Electrophysiological studies of upregulated P2X7 receptors in rat superior cervical ganglia after myocardial ischemic injury. Neurochem Int. 2013;63(3):230–237. doi:10.1016/j.neuint.2013.06.003
- Verrotti A, Prezioso G, Scattoni R, Chiarelli F. Autonomic neuropathy in diabetes mellitus. Front Endocrinol. 2014;5:205.
- Wu B, Zhang C, Zou L, et al. LncRNA uc.48+ siRNA improved diabetic sympathetic neuropathy in type 2 diabetic rats mediated by P2X7 receptor in SCG. Auton Neurosci. 2016;197:14–18. doi:10.1016/j.autneu.2016.04.001
- Yu W, Zhao GQ, Cao RJ, Zhu ZH, Li K. LncRNA NONRATT021972 was associated with neuropathic pain scoring in patients with type 2 diabetes. Behav Neurol. 2017;2017:2941297. doi:10.1155/2017/2941297
- Peng H, Zou L, Xie J, et al. lncRNA NONRATT021972 siRNA decreases diabetic neuropathic pain mediated by the p2x(3) receptor in dorsal root ganglia. Mol Neurobiol. 2017;54(1):511–523. doi:10.1007/s12035-015-9632-1
- Xu H, He L, Liu C, et al. LncRNA NONRATT021972 siRNA attenuates P2X7 receptor expression and inflammatory cytokine production induced by combined high glucose and free fatty acids in PC12 cells. Purinergic Signal. 2016;12(2):259–268. doi:10.1007/s11302-016-9500-0
- Liu S, Zou L, Xie J, et al. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol Brain. 2016;23(9):44. doi:10.1186/s13041-016-0226-2
- Li Z, Li X, Chen X, et al. Emerging roles of long non-coding RNAs in neuropathic pain. Cell Prolif. 2019;52(1):e12528. doi:10.1111/cpr.12528
- Dou L, Lin H, Wang K, et al. Long non-coding RNA CCAT1 modulates neuropathic pain progression through sponging miR-155. Oncotarget. 2017;8(52):89949–89957. doi:10.18632/oncotarget.21192
- Dou L, Lin H, Wang K, et al. Long non-coding RNA CCAT1 modulates neuropathic pain progression through sponging miR-155. Oncotarget. 2017;8(52):89949. doi:10.18632/oncotarget.21192
- Zhao X, Tang Z, Zhang H, et al. A long non-coding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci. 2013;16(8):1024–1031. doi:10.1038/nn.3438
- Li G, Jiang H, Zheng C, et al. Long non-coding RNA MRAK009713 is a novel regulator of neuropathic pain in rats. Pain. 2017;158(10):2042–2052. doi:10.1097/j.pain.0000000000001013
- Zhao Y, Li S, Xia N, Shi Y, Zhao CM. Effects of XIST/miR-137 axis on neuropathic pain by targeting TNFAIP1 in a rat model. J Cell Physiol. 2018;233(5):4307–4316. doi:10.1002/jcp.26254
