Abstract
Background
Aerobic exercise can greatly assist in reducing collateral effects of metabolic syndrome (MetS). Moreover, aerobic exercise is associated with sympathetic activation and adaptive responses to sustain muscle engagement, changes in the release of Orexin A, a pleiotropic neuropeptide.
Aim
The aim of this study was to analyze the beneficial effects of aerobic exercise without dietary changes, in a cohort of MetS subjects, focusing on the role of sympathetic and orexinergic activity. Several blood parameters linked to MetS ROS production, heart rate, galvanic skin response, d-ROM test, and Orexin A serum levels were evaluated in ten males with MetS (BMI 30–34.9) before and after a period of 6 months of aerobic exercise compared to ten healthy subjects.
Methods
Ten male subjects (aged 54 ± 4.16) with MetS (MetS group) and ten healthy males (aged 49.7 ± 2.79, Healthy group) were told about the study protocol and possible risks, signed the informed consent, and voluntarily participated in the study. Several blood parameters were evaluated in the two tested groups and were re-evaluated in the MetS group after 6 months of training (MetS6M group). The training protocol consisted of more than 30 min/day of walking (average speed of 4.5 km/h) and 3 days/week of aerobic activities (jogging under heart rate control – 120–140 bpm for 45 min).
Results
The results showed that exercise induced a significant increase in GSR and plasma Orexin A but no significant increase in d-ROM values. Significant decreases in the serum ALT enzyme, triglycerides, and total cholesterol were found, while the HDL levels were significantly higher. Finally, a significant reduction of BMI and resting HR were reported.
Conclusion
The results of this study confirm that physical activity is associated with sympathetic activation, having a pivotal role against adverse effects linked to MetS. Moreover, this study demonstrates that, in patients with MetS, Orexin A is involved in hormonal adaptations to exercise.
Introduction
Metabolic syndrome (MetS) is a well-defined cluster of various metabolic abnormalities that come together in a single individual. Following the criteria of the International Diabetes Federation (2005), the clinical diagnosis of MetS is made when the increase of waist circumference (WC) is combined with any 2 of the following factors: lipid alteration (triglyceride levels ≥150 mg/dL; high-density lipoprotein cholesterol <40 mg/dL in men or <50 mg/dL in women), blood pressure modification (≥130 mm Hg systolic or ≥85 mm Hg diastolic or on hypertension treatment) or high glucose level (≥100 mg/dL, including diabetes) (International Diabetes Federation). Affected individuals are most often overweight or obese.Citation1–Citation3 One of the most severe complications related to MetS is non-alcoholic fatty liver disease (NAFLD).Citation4
Physical activity is an effective, practical and inexpensive way to improve health, to balance the internal homeostatic process, to control body weight and to reduce age-related cognitive decline.Citation5–Citation8 Particularly, aerobic exercise can greatly assist in reducing collateral effects of MetS, mainly by improving weight loss, reducing body fat, and increasing lean body mass.Citation9–Citation12 The data from randomized controlled trials have highlighted favorable changes in MetS criteria after aerobic training.Citation13
During the natural physiological process of all living beings, free radicals are generated. Oxidative stress is linked to the onset of several diseases, playing a central role in the promotion of chronic and degenerative disorders as well as aging.Citation14,Citation15 The terms “Reactive Oxygen Species (ROS)” and “Reactive Nitrogen Species (RNS)” have been frequently used to describe free radicals and other non-radical reactive derivatives. High levels of ROS can be lethal to cells, generating modifications in cellular lipids, DNA and proteins.Citation16 Regular physical activity helps to ease stress by decreasing pro-inflammatory mediators, even if a moderate increase in plasma ROS is considered healthy and fundamental in cellular adaptation to training. One of the unanswered questions is related to the paradox the extended muscle disuse can increase ROS production in skeletal muscle fibers as well as strenuous physical activity (contractile activity). In skeletal muscle fibers, ROS is a signaling molecule that can promote both anabolic responses (during particular conditions such as exercise training) and in other states of catabolic signaling.Citation17–Citation19
Moreover, the adaptive responses to sustain muscle engagement and sympathetic activation is associated with aerobic exercise. Furthermore, physical activity is related to changes in the release of Orexin A (hypocretin-1), a pleiotropic neuropeptide synthesized by neurons located in the perifornical region of the lateral hypothalamus area (LHA). Orexin A plays several roles in the regulation of various body systems. It has been found to be involved in several physiological processes, such as the regulation of food intake,Citation20,Citation21 arousal, metabolism, and cardiovascular and respiratory function.Citation22–Citation25 Moreover, it has been linked to an increase in blood pressure (BP), heart rate (HR) and metabolic rate.Citation26,Citation27
This study aimed to analyze the effects of physical activity, which could be considered a “natural drug”, against different health problems concerning metabolism. This study aimed to analyze the effects of aerobic physical activity, which could be considered a “natural drug”, against different health problems concerning metabolism. Particularly, this experimental model aimed to evaluate the beneficial effects of aerobic exercise in a cohort of MetS subjects, focusing on the role of sympathetic and orexinergic activity; the study was carried out maintaining the same diet regimen for all the study period. For this purpose, the anthropometric and biochemical parameters were analyzed to define the MetS group; moreover, to analyze the sympathetic and orexinergic system galvanic skin response, Orexin-A serum levels and heart rate measurements were tested in the same group. Indeed, as previously described, obesity, MetS, and also physical activity represent different stress conditions to active mechanisms of adaptation or re-adaptation, restoring altered homeostasis.Citation28–Citation30
As previously described, these parameters can be tested to evaluate the homeostasis alteration, allowing continuous measurements over a long period of time with minimal disturbance to the subject under examination. Moreover, skin conductance and heart rate variability could be used to measure the activity of the sympathetic nervous system.Citation31 Stressful events or emergency situations may cause dynamic changes in the autonomic nervous system, in particular the activity of the sympathetic nervous system increases.
Materials and Methods
Subjects
Ten male subjects (aged 54 ± 4.16) with MetS (MetS group) and ten healthy males (aged 49.7 ± 2.79, Healthy group) were told about the study protocol and possible risks, signed the informed consent, and voluntarily participated in the study. They were free to withdraw their participation at any time during the course of the study. The participants were enrolled in both groups if they were older than 40 years of age and had no familiarity for several conditions such as diabetes, cancer, heart disease or pathologies such as anemia, renal or liver disorders. Moreover, smokers, subjects who habitually consumed alcohol, and pregnant or lactating women were excluded. Following the scientific criteria of metabolic syndrome,Citation1,Citation2,Citation32 at the start of the experimental study, all participants included in the MetS group had the coexistence of at least three factors characterizing metabolic syndrome, in particular, they were obese (BMI > 30 Kg/m2) with waist circumference >102 cm (112.5 ± 1.2), triglyceride levels >150 mg/dl, blood pressure >130/85 mmHg,Citation33 and/or fasting glucose >100 mg/dL. Participants of both groups were all Caucasians, and were recruited in the same urban area and with similar social and economic status.
All procedures were performed in accordance with the Declaration of Helsinki and were approved by the Scientific Committee of the University of Foggia (Italy–FG_MetS_28/11/2018). Blood samples were collected from the antecubital vein into blood collection tubes containing sodium heparin for the measurement of reactive oxygen metabolites. The blood samples were centrifuged at 4°C immediately after collection, and the plasma was separated and stored at −80°C until analysis could be performed.
Experimental Model
Several blood parameters were evaluated in the MetS group and Healthy group, and are summarized in . Moreover, the same parameters were evaluated in the MetS group after 6 months of training (MetS6M group). The training protocol consists of more than 30 min/day of walking (each participant was free to choose their own walking speed) and 3 days/week of aerobic activities (jogging under hart rate control – 120–140 bpm for 45 min), following the criteria of Physical Activity Guidelines.Citation34 The training protocol was monitored evaluating the heart rate values with the Activio Sport System (Activio AB, Stockholm, Sweden). Moreover, heart rate, galvanic skin response, d-ROMs test, and Orexin A, were measured in the MetS6M group before and after 45 min of training in the last week of the training program.
Table 1 Tested Parameters in Both Groups
Blood Analysis
Blood was sampled after 12 h of fasting.
As previously reported, the following biochemical parameters were measured on a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, USA) using Roche reagents: aspartate aminotransaminase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), cholesterol (total, HDL and LDL) and triglycerides (non-fasting).Citation35
Serology tests (including analyses for hepatitis B and C viruses, and HIV virus) were negative for all patients.
Blood Pressure Measurements
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured to the nearest one-millimeter of mercury while the participant was seated using a sphygmomanometer and stethoscope.
Resting Heart Rate (HR) Measurement
The HR measurement was carried out with a chest strap wired to a digital R-R recorder (BTL08 SD ECG); the QRS-signal wave-form R-R signal was sampled at the resolution of 1 ms. The HR (beats min-1) was calculated using the formula: HR = 60 R-R interval-1; the R-R interval was converted into seconds.
Galvanic Skin Response (GRS)
The GSR parameters were measured simultaneously using the SenseWear Pro Armband™ (version 3.0, BodyMedia, Inc. PA, USA), which was worn on the right arm over the triceps muscle at the midpoint between the acromion and olecranon processes, as recommended by the manufacturer. The SenseWear Pro Armband is a portable sensing device, 4.0 cm diameter and 1.9 cm high and 34 g in weight that has 4 sensors: • Accelerometer (3 axes); • Heat flow; • Skin temperature; • GSR. All physiological data were then processed by advanced algorithms to calculate and report total energy expenditure, metabolic physical activity, and sleep. However, in this study, the data about GSR parameters were collected at basal level (time 0, at rest before physical activity), after 45 min (at the end of training), and after 60 min. (15 min after training).
Plasma Orexin A Detection
Blood samples were withdrawn from a forearm vein, at 8:00 a.m. after an overnight fast, into Vacutainer tubes (BD, Franklin Lakes, NJ, USA) containing EDTA and 0.45 TIU/mL of aprotinin. After centrifugation at 3000 rpm for 12 min at 4°C, the plasma was separated and stored at −80°C until analysis. Enzyme-linked immunoassay kits (ELISA) were used to measure plasma Orexin A levels. Hypocretin Orexin A1 ELISA kits were purchased from Phoenix Pharmaceuticals. Before measurement, plasma Orexin A was extracted using Sep-Pak C18 columns (Waters, Milford, MA, USA), 10 mL of methanol and 20 mL of H2O were used to activate the columns. The following step consisted of adding 1–2 mL of the sample to the column and washing with 20 mL of water. Samples were eluted slowly with 80% acetonitrile and the resulting volume was reduced to 400 μL under nitrogen flow. An aliquot was used for exsiccation using the Speedvac (Savant Instruments, Holbrook, NY, USA). The dry residue was dissolved in water and used for the ELISA test. No cross-reactivity for hypocretin-1, hypocretin-2, and agouti-related protein amide had been previously reported. The kit used for the quantification has an intra-assay error <5% and an inter-assay error <14%. The detection concentration limit was 0.37 ng/mL.
The d-ROMs Test
The d-ROMs test was performed using the FRAS4 system according to the manufacturer’s instructions (Wismerll Co., Tokyo, Japan). For each participant, 20 µL plasma was tested, and tests were run in duplicate. The results obtained from the d-ROMs test were expressed in arbitrary units called “Carratelli units” (U.CARR). The results were presented and analyzed as mean values.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis of all parameters is summarized in and was performed using GraphPad Prism 5 software (GraphPad software, Inc, La Jolla, CA, USA) and by a researcher trained in biostatistics. Two-tailed Student’s t-test was used. A p-value of <0.05 was considered statistically significant. GSR, plasma Orexin A and d-ROMs values were subjected to a one-way analysis of variance ANOVA with Tukey HSD post hoc comparisons. The analysis was performed using SPSS software version 24 (IBM Japan, Tokyo, Japan), and the statistical level of significance was set at 0.05.
Results
The mean values of the blood parameters tested are summarized in , as well as the mean values of resting HR, BMI, and blood pressure.
The statistical analysis performed with Student’s t-test demonstrated significant differences between MetS and Healthy groups for all parameters: all values were statistically different. The results of Student’s t-test comparing the data of the MetS and Mets6M groups are interesting; after 6 months of training, all parameters were improved in the MetS6M group, even if 7 parameters had statistical differences. The blood values of triglycerides, fasting glucose, ALT, total cholesterol and HDL levels were statistically improved; moreover, the parameters of BMI and resting HR were significantly decreased.
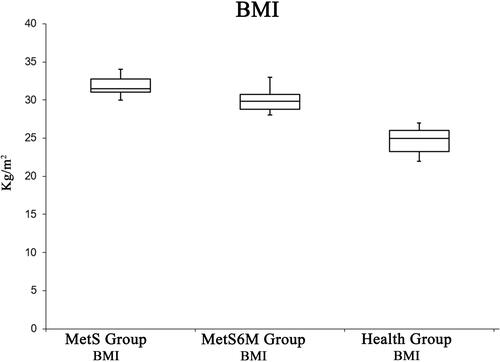
As shown in , resting HR improved after 6 months of aerobic training; the positive effects of physical activity are shown in the BMI values: the mean values of the MetS group are located in the obesity category (Class 1 obesity), while after a period of aerobic training the MetS group improved this parameter including the participants in the overweight category ().
Figure 1 Resting HR is statistically higher in the MetS Group with respect to the Healthy Group. Moreover, this parameter is significantly improved after 6 months aerobic training.
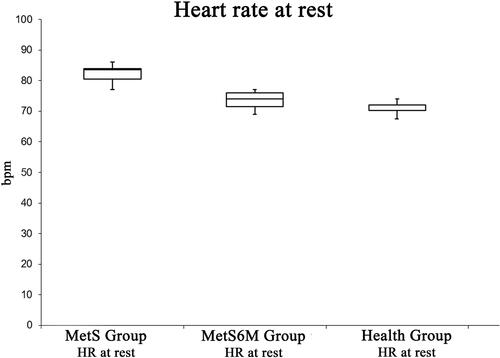
Figure 2 BMI is statistically higher in the MetS Group related to the Healthy Group. Moreover, this parameter is significantly improved after 6 months aerobic training.
In the last week of the training program, GSR, plasma Orexin A and d-ROMs were tested in the MetS6M group.
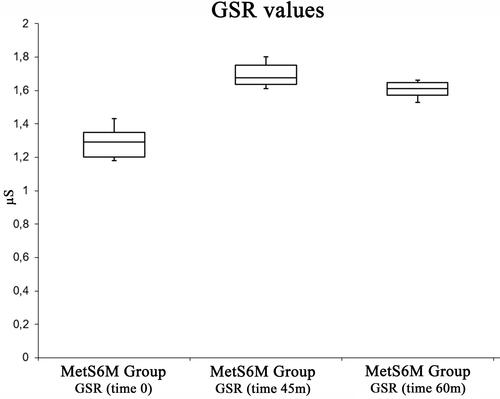
shows GSR changes. Physical activity caused an increase in GSR with the maximum level at 45 min. GSR (μS) increased from a basal value of 1.28±0.09 to 1.69±0.07 at 45 min, and it decreased to 1.6 ± 0.04 at 60 min. The ANOVA test showed significant effects [F(2, 27) =3.35, p <0.05, 1.8x10−12]. The post hoc test showed a difference between pre-exercise and post-exercise values at 0 min., 45 min., and 60 min.
Figure 3 Box plot analysis summarizing GSR variations among pre-exercise status, at the end of the exercise status.
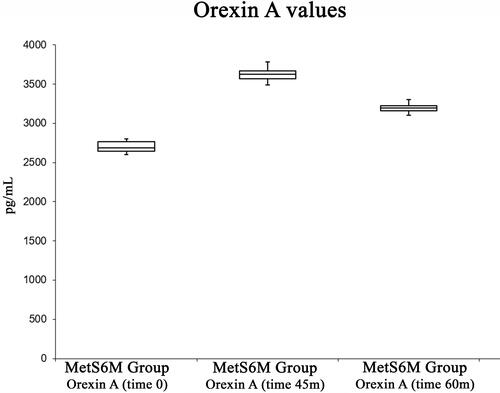
shows Orexin A changes. Physical activity caused an increase in Orexin A, from a basal level of 2700 ± 72.26 pg/mL to a maximum level (3626 ± 95.93 pg/mL) at 45 min. Orexin A decreased to a value of 3196 ± 60.22 at 60 min. The ANOVA test showed significant differences [F(2, 27) = 3.35, p <0.05, 3.83x10−20]. The post hoc test showed a difference between pre-exercise and post-exercise values at 0 min., 45 min., and 60 min.
Figure 4 Box plot analysis summarizing Orexin A variations among pre-exercise status, at the end of the exercise status and in the recovery periods.
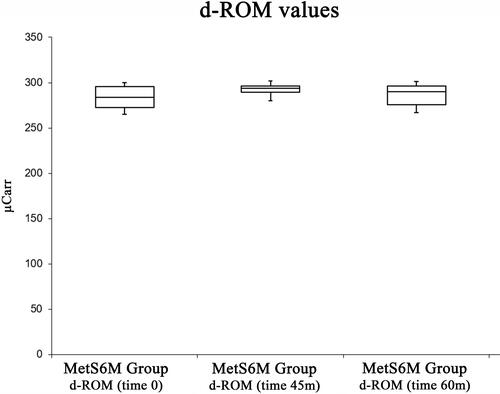
shows d-ROMs changes. Physical activity did not cause an increase in d-ROMs from a basal value of 284 ± 12.97 μCarr to a maximum level (292.4 ± 6.51 μCarr) at 45 min. d-ROMs decreased to a value of 285.7 ± 13.03 at 60 min. The ANOVA test did not show significant differences [F(2, 27) = 3.35, p =0.24].
Discussion and Conclusion
Characteristics of subjects with and without MetS have been extensively described: MetS is defined as the contemporary presence of several interconnected human diseases; physiological, biochemical, clinical, and metabolic factors can be combined, increasing the risk of atherosclerotic cardiovascular disease and diabetes; in particular cases, all these factors can cause the “exitus” of the subject.Citation36–Citation39 As shown in , the participants included in our study group had several parameters that confirmed the diagnoses of MetS: compared to the healthy group the values were significantly higher.
The guidelines on physical activity recommend a daily minimum of 30 min of moderate-intensity physical activity, in order to prevent organ damage and cardiovascular diseases. However, several experimental studies have demonstrated that 60 min of moderate-intense brisk walking could be very useful to improve several clinical parameters, especially in people living in developed countries.Citation40–Citation43 In our experimental model, we tested several blood parameters after 6 months of regular physical activity without dietary changes. It was very interesting that all blood parameters tested were improved with respect to the levels of the same parameters tested at time 0. The results of the present study show the positive effects of physical activities as “natural drugs” against metabolic syndrome. The results of this study confirm that physical activity is associated with sympathetic activation, as demonstrated by HR and GSR increases during exercise. The association between Orexin A plasma levels and sympathetic discharge is also described in this study. Physical activity induces an increase in plasmatic Orexin A,Citation22 as underlined by the results of the present experiment. Moreover, an adaptation of HR to physical activities is described in the results section. The present study suggests a correlation between plasmatic Orexin A and HR adaptation, as an expression of sympathetic activity.
As previously described, Orexin A affects both peripheral energy balance and the central nervous system, particularly when the subject is under stress; moreover, motivated behaviors (for example, food intake) and sleep-wakefulness can be coordinated by this neuropeptide.Citation7,Citation44 Orexin 1 receptor (OX1R) has been reported in various tissues such as the reproductive system, intestine, adrenal glands, pancreas, and kidney: binding its receptor, Orexin A has several pleiotropic functions.Citation15 Several vital body functions such as sleep/wake states, eating behavior, reward systems, energy homeostasis, cognition, and mood could be influenced by Orexin A levels. Likewise, it plays an important role in energy balance and obesity.Citation45–Citation47 Considering the important functions of the orexinergic system, it is well known that dysfunction in this system may be related to various pathological conditions.Citation7,Citation48 Moreover, to the best of our knowledge, this study is the first to demonstrate that, in patients with MetS, Orexin A is involved in hormonal adaptations to exercise, indicating that this peptide controls several adaptations (such as cardiovascular changes) to physical activity also in MetS.
In addition, significant decreases in the serum ALT enzyme, triglycerides, fasting glucose, and total cholesterol were found, while the HDL levels were significantly higher. It is well described that aerobic activity induces positive effects on HDL plasma levels, depending on the entry level.Citation49 Moreover, the low plasma levels of HDL were associated with high levels of triglycerides. In agreement with Couillard et al, the results of the present study confirmed that aerobic activity may be related to the reduction of triglyceride levels combined with improved HDL levels.Citation50
Physical activity is not considered the only way to fight MetS: the best approach to produce lifestyle modification treatment involves a multidisciplinary team composed of physicians and non-physician health professionals. To date, professionals with a master degree in exercise physiology should be collaborating with other specialists such as biologists, dieticians, and behavioral psychologists. Moreover, lifestyle modification is a complex route that should lead subjects to modify their habits both related to diet and physical activities. In this way, an ambitious goal could be achieved, reducing all risk factors linked with metabolic syndrome.Citation43,Citation51-Citation53
Finally, the results of the present study show that physical activity did not cause an increase in d-ROMs values in the study group. Previous studies have demonstrated that physical exercise increases the metabolic demands of skeletal muscles leading to greater oxygen uptake and blood flow to the muscles and other organsCitation54,Citation55 thus leading to a higher ROS generation.Citation35,Citation47,Citation48 According to the “mitochondrial hypothesis of aging”, mitochondria are the main source of ROS generation, especially during exercise, and their generating rate is related to mitochondrial oxygen consumption.Citation56 Nevertheless, the presented results highlight that these modifications described in athlete groups are not presented in our study group, suggesting that the status of MetS negatively influenced the beneficial effects of physical activity. In agreement with our results, several studies demonstrate that exercise leads to oxidative stress only when it is exhaustive due to an increase in lipid peroxidation products and, consequently, oxidative stress and damage of muscle mitochondrial membranes.Citation57–Citation62
The main limitation of the present study is linked to the small number (10 for both groups) of participants. Moreover, the choice of comparing the positive effects of physical activity in the same group at different times (MetS vs MetS6M), on the one hand, represents an advantage allowing comparison in a homogeneous group; on the other hand, it undoubtedly removed the MetS group from the final evaluation. Furthermore, the exercise effects on the control group were not evaluated. Finally, the results concerning oxidative stress and sympathetic activity should be confirmed increasing the number of tested markers. Moreover, an added limitation of the present study is that there is not a pre-post 6 months’ exercise comparison in the healthy group.
For these reasons, further experiments will have to be performed to verify these hypotheses; moreover, it could be interesting to explore the role of Orexin A on the peripheral adaptations to physical activity and oxidative stress levels: these studies could confirm the multitasking role of this neuropeptide. Indeed, physical activity is associated with sympathetic activation, having a pivotal role against adverse effects linked to MetS. Moreover, this study demonstrates that, in patients with MetS, Orexin A is involved in hormonal adaptations to exercise. In addition, the exercise induced a significant increase in GSR, and plasma Orexin A, but no significant increase of d-ROM values, and an amelioration in anthropometric and biochemical parameters such as glycemic and lipid profile. For these reasons, physical activity, in particular aerobic exercise, may be considered a valid strategy to improve health status without changes in diet in metabolic syndrome.
Disclosure
The authors report no conflicts of interest in this work.
References
- Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438.
- Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:1–21. doi:10.1155/2014/943162
- Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. doi:10.1242/dmm.001180
- Byrne CD, Patel J, Scorletti E, Targher G. Tests for diagnosing and monitoring non-alcoholic fatty liver disease in adults. BMJ. 2018;362. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85050204997&doi=10.1136%2Fbmj.k2734&partnerID=40&md5=aeb23fda673a2fb5ff83173b00797b39. Accessed June 24, 2020.
- Obici S, Magrisso IJ, Ghazarian AS, et al. Moderate voluntary exercise attenuates the metabolic syndrome in melanocortin-4 receptor-deficient rats showing central dopaminergic dysregulation. Mol Metab. 2015;4(10):692–705. doi:10.1016/j.molmet.2015.07.003
- Sessa F, Messina G, Valenzano A, et al. Sports training and adaptive changes. Sport Sci Health. 2018;14(3):705–708. doi:10.1007/s11332-018-0464-z
- Chieffi S, Messina G, Villano I, et al. Neuroprotective effects of physical activity: evidence from human and animal studies. Front Neurol. 2017;8. doi:10.3389/fneur.2017.00188
- Wiklund P. The role of physical activity and exercise in obesity and weight management: time for critical appraisal. J Sport Health Sci. 2016;5(2):151–154. doi:10.1016/j.jshs.2016.04.001
- Normandin E, Chmelo E, Lyles MF, Marsh AP, Nicklas BJ. Effect of resistance training and caloric restriction on the metabolic syndrome. Med Sci Sports Exerc. 2017;49(3):413–419. doi:10.1249/MSS.0000000000001122
- Donini LM, Cuzzolaro M, Gnessi L, et al. Obesity treatment: results after 4 years of a nutritional and psycho-physical rehabilitation program in an outpatient setting. Eat Weight Disord. 2014;19(2):249–260. doi:10.1007/s40519-014-0107-6
- Van Der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The effects of physical exercise on fatty liver disease. Gene Expr. 2018;18(2):89–101. doi:10.3727/105221617X15124844266408
- Sessa F, Anna V, Messina G, et al. Heart rate variability as predictive factor for sudden cardiac death. Aging (Albany NY). 2018;10(2):166–177. doi:10.18632/aging.101386
- Messina G, Vicidomini C, Viggiano A, et al. Enhanced parasympathetic activity of sportive women is paradoxically associated to enhanced resting energy expenditure. Auton Neurosci Basic Clin. 2012;169(2):102–106. doi:10.1016/j.autneu.2012.05.003
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi:10.1152/physrev.00018.2001
- Phull A-R, Nasir B, Ul Haq I, Kim SJ. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact. 2018;281:121–136. doi:10.1016/j.cbi.2017.12.024
- Simioni C, Zauli G, Martelli AM, et al. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9(24):17181–17198. doi:10.18632/oncotarget.24729
- Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016;2016:1–18. doi:10.1155/2016/3565127
- Messina A, Monda M, Valenzano A, et al. Functional changes induced by orexin a and adiponectin on the sympathetic/parasympathetic balance. Front Physiol. 2018;9. doi:10.3389/fphys.2018.00259
- Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95(1):1–9. doi:10.1113/expphysiol.2009.050526
- Monda V, Salerno M, Fiorenzo M, et al. Role of sex hormones in the control of vegetative and metabolic functions of middle-aged women. Front Physiol. 2017;8(OCT). doi:10.3389/fphys.2017.00773
- Messina A, Monda V, Avola R, et al. Role of the orexin system on arousal, attention, feeding behaviour and sleep disorders. Acta Medica Mediterr. 2017;33(4):645–649.
- Messina G, Di Bernardo G, Viggiano A, et al. Exercise increases the level of plasma orexin A in humans. J Basic Clin Physiol Pharmacol. 2016;27(6):611–616. doi:10.1515/jbcpp-2015-0133
- Marra ML, Valenzano A, Ruberto M, et al. The effects of overweight and obesity on cognitive functions and psychological well-being. Acta Medica Mediterr. 2017;33:1225–1231.
- Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017:1–8. doi:10.1155/2017/3831972
- Monda V, Salerno M, Sessa F, et al. Functional changes of orexinergic reaction to psychoactive substances. Mol Neurobiol. 2018;55(8):1–7.
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol Regul Integr Comp Physiol. 1999;277(6):R1780–R1785. doi:10.1152/ajpregu.1999.277.6.R1780
- Hart EC, Head GA, Carter JR, et al. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol. 2017;312(5):H1031–H1051. doi:10.1152/ajpheart.00703.2016
- Holvoet P. Stress in obesity and associated metabolic and cardiovascular disorders. Scientifica (Cairo). 2012;2012:1–19. doi:10.6064/2012/205027
- Chaput J-P, Doucet É, Tremblay A. Obesity: a disease or a biological adaptation? An update. Obes Rev. 2012;13(8):681–691. doi:10.1111/j.1467-789X.2012.00992.x
- Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040. doi:10.1098/rsfs.2014.0040
- Mackersie CL, Calderon-Moultrie N. Autonomic nervous system reactivity during speech repetition tasks: heart rate variability and skin conductance. Ear Hear. 2016;37:118S–125S. doi:10.1097/AUD.0000000000000305
- Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and alzheimer’s diseases. Neurobiol Dis. 2014;72:3–12. doi:10.1016/j.nbd.2014.08.025
- Things you need to know about blood pressure and hypertension. Can J Cardiol. 2006;22(7):601–602.
- 2018 Physical Activity Guidelines Advisory Committee. 2018 physical activity guidelines advisory committee scientific report. US Dep Heal Hum Serv. 2018.
- Valenzano A, Polito R, Trimigno V, et al. Effects of very low calorie ketogenic diet on the orexinergic system, visceral adipose tissue, and ROS production. Antioxidants. 2019;8(12):643. doi:10.3390/antiox8120643
- Chieffi S, Messina G, Villano I, et al. Exercise influence on hippocampal function: possible involvement of orexin-a. Front Physiol. 2017;8. doi:10.3389/fphys.2017.00085
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006.
- O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. doi:10.1111/obr.12229
- Ritchie SA, Connell JMC. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–326. doi:10.1016/j.numecd.2006.07.005
- Thompson PD, Buchner D, Piña IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (subcommittee on exercise, rehabilitation, and prevention) and the Council on Nutrition, Physical. Circulation. 2003;107(24):3109–3116. doi:10.1161/01.CIR.0000075572.40158.77
- Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents. A scientific statement from the American Heart Association atherosclerosis, hypertension, and obesity in the young committee of the Council on Cardiovascular Disease in the Young. Circulation. 2009;119(4):628–647. doi:10.1161/CIRCULATIONAHA.108.191394
- Messina A, Monda V, Sessa F, et al. Sympathetic, metabolic adaptations, and oxidative stress in autism spectrum disorders: how far from physiology? Front Physiol. 2018;9(MAR). doi:10.3389/fphys.2018.00261
- Monda V, Villano I, Messina A, et al. Aerobic exercise and orexin A: role of sympathetic activity and redox system. J Biol Regul Homeost Agents. 2019;33(2):587–592.
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61(2):162–176. doi:10.1124/pr.109.001321
- Perez-Leighton CE, Billington CJ, Kotz CM. Orexin modulation of adipose tissue. Biochim Biophys Acta Mol Basis Dis. 2014;1842(3):440–445. doi:10.1016/j.bbadis.2013.06.007
- Polito R, Nigro E, Messina A, et al. Adiponectin and orexin-A as a potential immunity link between Adipose tissue and central nervous system. Front Physiol. 2018;9. doi:10.3389/fphys.2018.00982
- Salerno M, Villano I, Nicolosi D, et al. Modafinil and orexin system: interactions and medico-legal considerations. Front Biosci. 2019;24:564–575.
- Sperandeo R, Maldonato MN, Messina A, et al. Orexin system: network multi-tasking. Acta Medica Mediterr. 2018;34(2):349–356.
- Williams PT, Stefanick ML, Vranizan KM, Wood PD. The effects of weight loss by exercise or by dieting on plasma high-density lipoprotein (HDL) levels in men with low, intermediate, and normal-to-high HDL at baseline. Metabolism. 1994;43(7):917–924. doi:10.1016/0026-0495(94)90277-1
- Couillard C, Després J-P, Lamarche B, et al. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides. Arterioscler Thromb Vasc Biol. 2007.
- Dongiovanni P, Lanti C, Riso P, Valenti L. Nutritional therapy for nonalcoholic fatty liver disease. J Nutr Biochem. 2016;29:1–11. doi:10.1016/j.jnutbio.2015.08.024
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
- Monda M, Messina G, Scognamiglio I, et al. Short-term diet and moderate exercise in young overweight men modulate cardiocyte and hepatocarcinoma survival by oxidative stress. Oxid Med Cell Longev. 2014;2014.
- Sallam N, Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid Med Cell Longev. 2016;2016:1–32. doi:10.1155/2016/7239639
- Li G, He H. Hormesis, allostatic buffering capacity and physiological mechanism of physical activity: a new theoretic framework. Med Hypotheses. 2009;72(5):527–532. doi:10.1016/j.mehy.2008.12.037
- Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Integr Comp Physiol. 2004;286(3):R505–R511. doi:10.1152/ajpregu.00208.2003
- Radák Z, Naito H, Kaneko T, et al. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445(2):273–278. doi:10.1007/s00424-002-0918-6
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55(1):373–399. doi:10.1146/annurev.arplant.55.031903.141701
- Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal. 2011;15(9):2477–2486. doi:10.1089/ars.2011.3976
- Cazzola R, Russo-Volpe S, Cervato G, Cestaro B. Biochemical assessments of oxidative stress, erythrocyte membrane fluidity and antioxidant status in professional soccer players and sedentary controls. Eur J Clin Invest. 2003;33(10):924–930. doi:10.1046/j.1365-2362.2003.01227.x
- Rizzo AM, Corsetto PA, Montorfano G, et al. Effects of long-term space flight on erythrocytes and oxidative stress of rodents. PLoS One. 2016;7(3):e32361. doi:10.1371/journal.pone.0032361
- Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Med. 2006;36(4):327–358. doi:10.2165/00007256-200636040-00004