Abstract
Objective
Laparoscopic sleeve gastrectomy (LSG) is one of the most effective therapies to treat obesity. However, whether LSG affects thyroid function remains elusive. Due to a lack of longitudinal research, we explored changes in thyroid function in euthyroid patients with obesity before and after LSG.
Methods
In total, 109 participants (59 obese patients, 30 normal controls and 20 overweight subjects) were recruited from the Second Xiangya Hospital of Central South University (CSU). All patients underwent LSG, and metabolic indicators and free triiodothyronine (FT3), free thyroxine (FT4), and thyroid stimulating hormone (TSH) were evaluated at baseline, 6 and 12 months postoperatively.
Results
Compared to the normal control group, the concentrations of FT4 were decreased and TSH were increased in the obese group at baseline. Thyroid hormone levels in all participants were within the normal range during the 12 months after LSG. The concentrations of FT3 (4.83 ± 0.06 vs 5.03 ± 0.08, P = 0.023) and TSH (1.67 ± 0.11 vs 2.25 ± 0.18, P = 0.000) significantly decreased from baseline to 12 months postoperatively, while the concentrations of FT4 significantly increased (17.40 ± 0.52 vs 15.80 ± 0.32, P = 0.004). The decrease in fasting C-peptide (FCP) was related to the decline in FT3 and TSH during 12 months after LSG.
Conclusion
Obesity is closely related to thyroid function. LSG promoted a significant decrease in FT3 and TSH and a significant increase in FT4 in euthyroid patients with obesity after LSG. The decline in FCP may be involved in the decrease in FT3 and TSH after LSG.
Introduction
Obesity is an important public health concern worldwide, and the global prevalence of obesity in adults has been increasing over recent decades.Citation1 Moderate (5%) weight loss improves metabolic function in multiple organs simultaneously, and progressive weight loss causes dose-dependent alterations in key adipose tissue biological pathways.Citation2 Metabolic surgeries, including laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (RYGB), are effective interventions that can lead to marked weight loss and improved glucose controlCitation3 and have been considered one of the treatments for obesity.Citation4 It is worth mentioning that the LSG procedure has gained popularity in the past decade because it is easy to perform and is associated with a low incidence of serious complications.Citation5
The hypothalamic-pituitary-thyroid axis consists of the hypothalamus, the pituitary gland, and the adrenal glands, all of which can produce and secrete their own hormone cascade including thyrotropin-releasing hormone (TRH), thyroid stimulating hormone (TSH) and subsequent thyroid hormones. It is well established that thyroid hormone status correlates with body weight and energy expenditure.Citation6,Citation7 Hyperthyroidism promotes a hypermetabolic state characterized by increased resting energy expenditure, weight loss and gluconeogenesis, while hypothyroidism is associated with hypometabolism characterized by reduced resting energy expenditure, weight gain and reduced gluconeogenesis.Citation8 The relationship between thyroid hormones and obesity are closely.Citation9–Citation13 A low free triiodothyronine (FT3) level predicts weight gain in euthyroid persons,Citation9 and low free thyroxine (FT4) or slightly elevated serum TSH levels are associated with an increase in the occurrence of obesity.Citation10,Citation11 On the other hand, obesity has also been associated with significant alterations in thyroid function,Citation12,Citation13 as previous studies have shown that the concentrations of serum TSH are increased in obese patients.Citation12,Citation13
Previous studies have generated inconsistent results and involved mainly RYGB.Citation14–Citation17 The results showed that the concentrations of TSH decreasedCitation14,Citation15 or remained steadyCitation16,Citation17 after RYGB, while the concentrations of FT4 increased,Citation17 decreasedCitation15,Citation16 or remained steady.Citation14 In addition, the mechanism and clinical implications of this hormonal alteration remain unknown. Recently, the impact of LSG on thyroid function has caused great interest, but related evidence is limited. Therefore, our aim was to evaluate the effect of LSG on thyroid function in patients with obesity and normal thyroid function.
Materials and Methods
Participants
In this observational cohort study, 109 participants, including 59 obese patients (body mass index [BMI] ≥ 27.5 kg/m2), 30 normal controls (24.0 > BMI ≥ 18.5 kg/m2) and 20 overweight participants (27.5 > BMI ≥ 24.0 kg/m2), were recruited from the Second Xiangya Hospital of Central South University (CSU) (Changsha, Hunan, China). LSG was performed on all obese patients who met the surgical criteria.Citation4 The exclusion criteria for all the participants were a prior history of thyroid disease, abnormal thyroid hormone levels, a previous diagnosis of pituitary correlation disease such as pituitary tumor or hypopituitarism, therapy with drugs that might affect the serum thyroid hormone levels in the past 3 months, type 1 diabetes mellitus, secondary diabetes, inflammation, infectious diseases, or other autoimmune disorders, pregnancy, and malignant diseases. None of the normal controls or overweight participants had diabetes mellitus or other medical problems, nor were they taking any drugs. This study protocol was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Second Xiangya Hospital of CSU. All participants provided informed consent for the study programme.
Study Design and Laboratory Analysis
Obese subjects underwent assessments (after 10 h of fasting overnight) within 1 week before and 6 after and 12 months after surgery. Study physicians recorded the body height, weight and waist circumference before and 6 and 12 months after the therapy. BMI was calculated as the body weight (kg)/height (m)2. Fasting venous blood samples were tested for fasting plasma glucose (FPG), fasting C-peptide (FCP), haemoglobin A1c (HbA1c), FT3, FT4 and TSH.
Assays
HbA1c was measured using automated liquid chromatography (VARIANT II Hemoglobin Testing System; Bio-Rad Laboratories, Hercules, CA). FCP was measured by the chemiluminescence method (ADVIA Centaur; Siemens, Munich, Germany). FT3, FT4 and TSH were determined using a direct chemiluminescent immunoassay (Siemens Healthcare Diagnostics Inc., Berlin, Germany). The intra-assay coefficients of variation for FT3, FT4 and TSH were 2.6, 3.8, and 6.5%, respectively.
Surgical Procedure
All LSG surgeries were performed in the Department of Metabolic Surgery at the Second Xiangya Hospital of CSU.
Statistical Analysis
For the statistical analysis, SPSS version 20.0 (IBM Corporation, Chicago, IL) and GraphPad Prism 5 (GraphPad Software, San Diego, CA) were used. Data with Gaussian distribution are presented as the mean ± SEM, and data with skewed distribution are presented as the median (interquartile range), as determined using the Shapiro–Wilk test. To compare the changes from baseline to the postsurgical time points within groups, repeated measures ANOVA with the Bonferroni post hoc test was performed. The relationship between the changes in thyroid function and other parameters was estimated by Pearson correlations. A multiple stepwise regression analysis was performed to identify the clinical factors associated with changes in thyroid function. Two-tailed P < 0.05 was considered statistically significant.
Results
The Concentrations of FT4 Were Decreased While Those of TSH Were Increased in Obese Patients
As shown in , a total of 109 participants were recruited for this study: 59 obese patients (23 females), with a mean age of 30.7 ± 1.3 years, and age- and sex-matched normal controls (n=30) and overweight participants (n=20). BMI, waist circumference, hip circumference, FPG, FCP and HbA1c were significantly higher in the obese group than in the normal control group and in the overweight group (all P = 0.000). Thyroid hormone levels in all the participants were within the normal range at baseline. Compared to the normal control group, the concentrations of FT4 were decreased (15.08 ± 0.32 vs 17.71 ± 0.35, P = 0.000) and those of TSH were increased (2.25 ± 0.18 vs 1.72 ± 0.13, P = 0.033) in the obese group, while the concentrations of FT3, FT4 and TSH were not different in the overweight group (P > 0.05). Compared to the overweight group, the concentrations of FT4 were also decreased (15.08 ± 0.32 vs 17.39 ± 0.43, P = 0.006) in the obese group.
Table 1 Clinical Characteristics of Patients with Obesity at Baseline and 6 and 12 Months After LSG
At baseline, as shown in , Pearson’s analysis showed that BMI, waist circumference, hip circumference, FCP and HbA1c had significant negative relationships with the concentrations of FT4 (all P < 0.05), while BMI, waist circumference and hip circumference were significantly positively related to the concentrations of TSH (all P < 0.05). As shown in , the multiple stepwise regression analysis indicated that BMI correlated with the concentrations of FT4 (β = −0.373, P = 0.022), while waist circumference was related to TSH (β = 0.191, P = 0.047).
Table 2 Correlation Between Thyroid Function and the Clinical Data of All Subjects at Baseline
Table 3 Multiple Stepwise Regression Analysis of Thyroid Function and the Clinical Data of All Subjects at Baseline
The Concentrations of FT3 and TSH Decreased While Those of FT4 Increased in Obese Patients at 12 Months After LSG
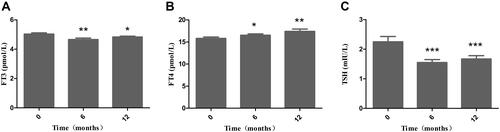
As shown in , BMI, waist circumference, hip circumference, FPG, FCP and HbA1c were all significantly decreased in the obese patients from baseline to 12 months after LSG (all P = 0.000). Thyroid hormone levels in all the obese patients were within the normal range at baseline and 12 months after LSG. As shown in , the concentrations of FT3 were decreased significantly at 6 months and 12 months after LSG compared to those at baseline (4.66 ± 0.10, 4.83 ± 0.06 vs 5.03 ± 0.08; P = 0.001, P = 0.023, respectively). As shown in , the concentrations of FT4 were increased significantly at 6 months and 12 months after LSG compared to those at baseline (16.54 ± 0.28, 17.40 ± 0.52 vs 15.80 ± 0.32; P = 0.034, P = 0.004, respectively). As shown in , the concentrations of TSH were decreased significantly at 6 months and 12 months after LSG compared to those at baseline (1.55 ± 0.10, 1.67 ± 0.11 vs 2.25 ± 0.18; P = 0.000, P = 0.000, respectively).
Figure 1 Alterations (± SEM) in the levels of FT3 (A) FT4 (B) and TSH (C) in obese patients during the 12 months after LSG. *P < 0.05, **P < 0.01, ***P < 0.001 compared with those at baseline.
The Decrease in FCP Was Related to the Decline in FT3 and TSH During the 12 Months After LSG
As shown in , Pearson’s analysis showed that the declines in FPG and HbA1c had significant negative relationships with the decrease in FT3 during the 12 months after LSG (P = 0.018, P = 0.005, respectively), while the decrease in FCP had a positive relationship with the decrease in FT3 (P = 0.000). The decline in hip circumference had a significant negative relationship with the increase in FT4 during the 12 months after LSG (P = 0.18), and the decline in FCP had a significant positive relationship with the decrease in TSH (P = 0.041). As shown in , the multiple stepwise regression analysis indicated that the decrease in FCP was related to the decline in FT3 (β = 0.365, P = 0.006) and TSH (β = 0.272, P = 0.045) during the 12 months after LSG.
Table 4 Correlation Between Changes in Thyroid Function and the Clinical Data of Obese Subjects During the 12 Months After LSG
Table 5 Multiple Stepwise Regression Analysis of Thyroid Function and Clinical Data During the 12 Months After LSG
Discussion
In a population of patients with obesity and normal thyroid function, we found that the concentrations of FT4 were decreased while those of TSH were increased compared to normal controls. Moreover, the concentrations of FT3 and TSH decreased while those of FT4 increased in obese patients at 12 months after LSG. The decrease in FCP was related to the decline in FT3 and TSH during the 12 months after LSG. Previous studies have reported mainly RYGB.Citation14–Citation17 Since all the subjects underwent LSG, our research adds evidence of the effect of LSG on thyroid function in obese patients.
In our study, the concentrations of FT4 were decreased and those of TSH were increased in obese individuals compared to normal controls (). Moreover, the concentrations of FT4 were also decreased in the obese group compared to the overweight group (). Correlation analysis suggested that indicators of obesity, including BMI and waist and hip circumferences, were all closely related to FT4 and TSH ( and ). The mechanism and the clinical implications of TSH elevation in obesity remain unknown. As a cause of elevated TSH concentrations in obesity, the adipocyte-derived hormone leptin has been demonstrated to alter the hypothalamic pituitary axis compensatory activation.Citation18,Citation19 TSH receptors are less expressed on the white adipose tissue of obese individuals.Citation20 Moreover, TSH receptors in brown adipose tissue play a direct role in the regulation of thermogenesis.Citation21 This reduction in TSH receptor expression may induce the downregulation of thyroid hormone receptors and thyroid hormone action, thereby further increasing TSH concentrations and constituting a condition of peripheral thyroid hormone resistance.Citation20 Although thyroid function in obese patients is different from that in normal controls, it has been suggested that reference values for TSH may be inadequate to define hypothyroidism in persons with morbid obesity.Citation22
Altered thyroid function and the effect of metabolic surgery on postoperative thyroid function evolution are still not fully understood. Our data show that the concentrations of FT3 and TSH decreased while those of FT4 increased in obese patients at 12 months after LSG ( and ). Most,Citation15,Citation23,Citation24 but not all,Citation16,Citation25 studies evaluating the evolution of TSH after metabolic surgery have also found a decrease in circulating TSH concentrations after intervention. Similarly, the changes in FT3 and FT4 were also inconsistent after metabolic surgery. Most studies evaluating the effect of metabolic surgery on thyroid hormones showed a significant decrease in FT3.Citation14,Citation25,Citation26 Thyronine is mainly produced in peripheral tissues by outer-ring deiodination of thyroxine by the action of iodothyronine deiodinases.Citation27 The gene for type I iodothyronine 5ʹ-deiodinase (D1) is upregulated and its activated is stimulated in adipose tissue of obese humans.Citation28 Evidence shows the decline in FT3 concentration after metabolic surgery is related to the decrease in D1 activity,Citation29 which could also explain the reduced level of FT3 instead of increase when TSH was decreased after metabolic surgery in our research. On the other hand, the impact of bariatric surgery on FT4 levels is more controversial, with different studies reporting decreasing,Citation26 stable,Citation14,Citation23,Citation30 or increasingCitation17,Citation25 levels after metabolic surgery. In the above studies, we found that the operations performed on obese patients covered RYGB,Citation14,Citation26 SGCitation23 or gastric banding,Citation25 and one study even included two or more surgical procedures.Citation17,Citation30 Furthermore, the differences between the above studies could be due not only to the types of metabolic surgery performed but also to the characteristics of the control group and the studied patients or to the different statistical powers of the studies. Our study could supplement the effects of LSG on thyroid function in euthyroid patients with obesity.
Moreover, our correlation analysis suggested that the decline in FCP was related to the decrease in FT3 and TSH ( and ), which has never been found before. The level of C-peptide could reflect the level of endogenous insulin.Citation31 A positive correlation between the concentrations of insulin and TSH has been observed in obese patients.Citation32 Additionally, increased FCP could predict the presence of insulin resistance,Citation33 and LSG can relieve insulin resistance.Citation34 Evidence shows that thyroid function may be influenced by insulin resistance in euthyroid obese subjects.Citation35 Combined with our data, these results suggest that the decrease in FT3 and TSH in thyroid hormones after LSG may be related to the change in FCP or insulin resistance.
Although an association between metabolic surgery and changes in thyroid hormones has been found, the underlying reasons responsible for this association are not yet well understood and may be explained by several mechanisms. First, the change in thyroid hormones is probably mediated by weight loss, while appears to be not an intrinsic effect of LSG. Decreased circulating TSH has been found to be independently associated with massive body weight loss after surgery.Citation24 Weight loss is accompanied by a significant reduction in total energy expenditure due to a decrease in both resting and non-resting energy expenditure, and losses in both fat mass and fat-free mass induced by metabolic surgery may influence the thyroid function.Citation36 It was worth noting that the thyroid function of obese patients had been changed at 12 months after lifestyle treatment to weight loss,Citation37 while not changed at 3 months.Citation38 Second, as the key component of obesity, adipose tissue can secrete a mass of leptin, which has been proposed to have a stimulatory impact on thyroid activity and thereby increase TSH and FT3 secretion.Citation39 As leptin levels declined after metabolic surgery,Citation40 weight loss induced by surgery could cause a decline in the concentration of TSH and FT3. Third, there may also be an added impact of metabolic surgery in addition to weight reduction. This standpoint was supported by evidence that a reduction in TSH concentrations did not correlate with the percentage of excess weight loss in some studies.Citation14,Citation23 This phenomenon is similar to other effects of bariatric surgery, such as T2DM remission, which is connected not only to weight loss but also to the surgery itself through other mechanisms, including the mediation of gastrointestinal hormones. With regard to this aspect, ghrelin modulates pituitary TSH cells and decreases the level of serum TSH, consequently changing thyroid morphology and function by reducing the T4 hormone level in the serum.Citation41 Therefore, a reduction in ghrelin succeeding LSGCitation42 would contribute to a decrease in TSH. Finally, organochlorine compounds released into the serum during lipid mobilization subsequent to weight loss play a role in T3 and FT3 reduction.Citation43
In summary, our study showed that obesity was closely related to thyroid function. LSG promoted a significant decrease in FT3 and TSH and a significant increase in FT4 in euthyroid patients with obesity after the procedure. The decline in FCP was related to the decrease in FT3 and TSH.
Abbreviations
BMI, body mass index; CSU, Central South University; D1, type I iodothyronine 5ʹ-deiodinase; FCP, fasting C-peptide; FPG, fasting plasma glucose; FT3, free triiodothyronine; FT4, free thyroxine; HbA1c, haemoglobin A1c; LSG, laparoscopic sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; TRH, thyrotropin-releasing hormone; TSH, thyroid stimulating hormone.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- NCD-RisC NRFC. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;10026(387):1377–1396.
- Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. doi:10.1016/j.cmet.2016.02.005
- Salminen P, Helmio M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–254. doi:10.1001/jama.2017.20313
- Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–877. doi:10.2337/dc16-0236
- Khorgami Z, Shoar S, Andalib A, et al. Trends in utilization of bariatric surgery, 2010–2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017;13(5):774–778. doi:10.1016/j.soard.2017.01.031
- Fox CS, Pencina MJ, D’Agostino RB, et al. Relations of thyroid function to body weight cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168(6):587–592. doi:10.1001/archinte.168.6.587
- Iwen KA, Schroder E, Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. 2013;2(2):83–92. doi:10.1159/000351249
- Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. doi:10.1152/physrev.00030.2013
- Ortega E, Pannacciulli N, Bogardus C, Krakoff J. Plasma concentrations of free triiodothyronine predict weight change in euthyroid persons. Am J Clin Nutr. 2007;85(2):440–445. doi:10.1093/ajcn/85.2.440
- Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019–4024. doi:10.1210/jc.2004-2225
- Amouzegar A, Kazemian E, Abdi H, et al. Association between thyroid function and development of different obesity phenotypes in euthyroid adults: a nine-year follow-up. Thyroid. 2018;28(4):458–464. doi:10.1089/thy.2017.0454
- Rotondi M, Leporati P, La Manna A, et al. Raised serum TSH levels in patients with morbid obesity: is it enough to diagnose subclinical hypothyroidism? Eur J Endocrinol. 2009;160(3):403–408. doi:10.1530/EJE-08-0734
- Michalaki MA, Vagenakis AG, Leonardou AS, et al. Thyroid function in humans with morbid obesity. Thyroid. 2006;16(1):73–78. doi:10.1089/thy.2006.16.73
- Moulin DMC, Mancini MC, de Melo ME, et al. Prevalence of subclinical hypothyroidism in a morbidly obese population and improvement after weight loss induced by Roux-en-Y gastric bypass. Obes Surg. 2005;15(9):1287–1291. doi:10.1381/096089205774512537
- Liu F, Di J, Yu H, et al. Effect of Roux-en-Y gastric bypass on thyroid function in euthyroid patients with obesity and type 2 diabetes. Surg Obes Relat Dis. 2017;13(10):1701–1707. doi:10.1016/j.soard.2017.06.001
- Zhang H, Liu W, Han X, et al. Effect of laparoscopic Roux-en-Y gastric bypass surgery on thyroid hormone levels in Chinese patients, could it be a risk for thyroid nodules? Obes Surg. 2017;27(10):2619–2627. doi:10.1007/s11695-017-2684-8
- MacCuish A, Razvi S, Syed AA. Effect of weight loss after gastric bypass surgery on thyroid function in euthyroid people with morbid obesity. Clin Obes. 2012;2(1–2):25–28. doi:10.1111/j.1758-8111.2012.00036.x
- Ghizzoni L, Mastorakos G, Ziveri M, et al. Interactions of leptin and thyrotropin 24-hour secretory profiles in short normal children. J Clin Endocrinol Metab. 2001;86(5):2065–2072. doi:10.1210/jcem.86.5.7452
- Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86(7):3284–3291. doi:10.1210/jcem.86.7.7644
- Nannipieri M, Cecchetti F, Anselmino M, et al. Expression of thyrotropin and thyroid hormone receptors in adipose tissue of patients with morbid obesity and/or type 2 diabetes: effects of weight loss. Int J Obes (Lond). 2009;33(9):1001–1006. doi:10.1038/ijo.2009.140
- Endo T, Kobayashi T. Thyroid-stimulating hormone receptor in brown adipose tissue is involved in the regulation of thermogenesis. Am J Physiol Endocrinol Metab. 2008;2(295):E514–E518.
- Valdes S, Maldonado-Araque C, Lago-Sampedro A, et al. Reference values for TSH may be inadequate to define hypothyroidism in persons with morbid obesity: [email protected] study. Obesity (Silver Spring). 2017;25(4):788–793. doi:10.1002/oby.21796
- Abu-Ghanem Y, Inbar R, Tyomkin V, et al. Effect of sleeve gastrectomy on thyroid hormone levels. Obes Surg. 2015;25(3):452–456. doi:10.1007/s11695-014-1415-7
- Neves JS, Castro OS, Souteiro P, et al. Effect of weight loss after bariatric surgery on thyroid-stimulating hormone levels in patients with morbid obesity and normal thyroid function. Obes Surg. 2018;28(1):97–103. doi:10.1007/s11695-017-2792-5
- Dall’Asta C, Paganelli M, Morabito A, et al. Weight loss through gastric banding: effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity (Silver Spring). 2010;18(4):854–857. doi:10.1038/oby.2009.320
- Di J, Zhang H, Yu H, et al. Effect of Roux-en-Y gastric bypass on the remission of type 2 diabetes: a 3-year study in Chinese patients with a BMI <30 kg/m2. Surg Obes Relat Dis. 2016;7(12):1357–1363.
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;1(23):38–89.
- Ortega FJ, Jilkova ZM, Moreno-Navarrete JM, et al. Type I iodothyronine 5ʹ-deiodinase mRNA and activity is increased in adipose tissue of obese subjects. Int J Obes (Lond). 2012;36(2):320–324. doi:10.1038/ijo.2011.101
- Dall’Asta C, Paganelli M, Morabito A, et al. Weight loss through gastric banding: effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity (Silver Spring). 2010;18(4):854–857.
- Chikunguwo S, Brethauer S, Nirujogi V, et al. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg Obes Relat Dis. 2007;3(6):631–636. doi:10.1016/j.soard.2007.07.011
- Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabetic Med. 2013;30(7):803–817. doi:10.1111/dme.12159
- Galofre JC, Pujante P, Abreu C, et al. Relationship between thyroid-stimulating hormone and insulin in euthyroid obese men. Ann Nutr Metab. 2008;53(3–4):188–194. doi:10.1159/000172981
- Yanai H, Hirowatari Y. Fasting serum C-peptide levels (>1.6ng/mL) can predict the presence of insulin resistance in Japanese patients with type 2 diabetes. Diabetes Metab. 2017;1(43):97–98.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi:10.1056/NEJMoa1200225
- Ambrosi B, Masserini B, Iorio L, et al. Relationship of thyroid function with body mass index and insulin-resistance in euthyroid obese subjects. J Endocrinol Invest. 2010;33(9):640–643. doi:10.1007/BF03346663
- Iwen KA, Oelkrug R, Brabant G. Effects of thyroid hormones on thermogenesis and energy partitioning. J Mol Endocrinol. 2018;3(60):R157–R170.
- Agnihothri RV, Courville AB, Linderman JD, et al. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid. 2014;24(1):19–26. doi:10.1089/thy.2013.0055
- Kouidrat Y, Diouf M, Desailloud R, Louhou R. Effects of a diet plus exercise program on thyroid function in patients with obesity. Metabol Open. 2019;2:100008. doi:10.1016/j.metop.2019.100008
- Kok P, Roelfsema F, Langendonk JG, et al. High circulating thyrotropin levels in obese women are reduced after body weight loss induced by caloric restriction. J Clin Endocrinol Metab. 2005;90(8):4659–4663. doi:10.1210/jc.2005-0920
- Jouan Y, Blasco H, Bongrani A, et al. Preoperative chemerin level is predictive of inflammatory status 1 year after bariatric surgery. Obes Surg. 2020;30(10):3852–3861. doi:10.1007/s11695-020-04584-3
- Sosic-Jurjevic B, Stevanovic D, Milosevic V, Sekulic M, Starcevic V. Central ghrelin affects pituitary-thyroid axis: histomorphological and hormonal study in rats. Neuroendocrinology. 2009;89(3):327–336. doi:10.1159/000188603
- Tsoli M, Chronaiou A, Kehagias I, Kalfarentzos F, Alexandrides TK. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surg Obes Relat Dis. 2013;9(5):667–677. doi:10.1016/j.soard.2012.12.006
- Pelletier C, Doucet E, Imbeault P, Tremblay A. Associations between weight loss-induced changes in plasma organochlorine concentrations, serum T(3) concentration, and resting metabolic rate. Toxicol Sci. 2002;67(1):46–51. doi:10.1093/toxsci/67.1.46
