Abstract
Despite our cognizance that diabetes can enhance the chances of heart failure, causes multiorgan failure,and contributes to morbidity and mortality, it is rapidly increasing menace worldwide. Less attention has been paid to alert prediabetics through determining the comprehensive predictors of diabetic cardiomyopathy (DCM) and ameliorating DCM using novel approaches. DCM is recognized as asymptomatic progressing structural and functional remodeling in the heart of diabetics, in the absence of coronary atherosclerosis and hypertension. The three major stages of DCM are: (1) early stage, where cellular and metabolic changes occur without obvious systolic dysfunction; (2) middle stage, which is characterized by increased apoptosis, a slight increase in left ventricular size, and diastolic dysfunction and where ejection fraction (EF) is <50%; and (3) late stage, which is characterized by alteration in microvasculature compliance, an increase in left ventricular size, and a decrease in cardiac performance leading to heart failure. Recent investigations have revealed that DCM is multifactorial in nature and cellular, molecular, and metabolic perturbations predisposed and contributed to DCM. Differential expression of microRNA (miRNA), signaling molecules involved in glucose metabolism, hyperlipidemia, advanced glycogen end products, cardiac extracellular matrix remodeling, and alteration in survival and differentiation of resident cardiac stem cells are manifested in DCM. A sedentary lifestyle and high fat diet causes obesity and this leads to type 2 diabetes and DCM. However, exercise training improves insulin sensitivity, contractility of cardiomyocytes, and cardiac performance in type 2 diabetes. These findings provide new clues to diagnose and mitigate DCM. This review embodies developments in the field of DCM with the aim of elucidating the future perspectives of predictors and prevention of DCM.
Introduction
Diabetes mellitus (DM) is a metabolic disorder with multiple etiology and is one of the leading causes of morbidity and mortality worldwide.Citation1–Citation3 The prevalence of DM is increasing at an alarming rate and is predicted to occur in approximately 5% of the global population by 2025.Citation4,Citation5 There are two major types of DM: (a) type 1 diabetes (T1D) which is caused by deficiency or absence of insulin due to destruction of pancreatic beta cells and (b) type 2 diabetes (T2D) which is characterized by insulin insensitivity or intolerance. T1D is prevalent in the young (also called juvenile diabetes) and T2D is prevalent in adults.Citation6 However, the number of both T1D and T2D patients is expected to increase 3–4 fold by 2050 in the United States.Citation7 The progression from prediabetes to diabetes has also contributed to the rapid increase in the number of people diagnosed with diabetes. It is estimated that nearly 5%–10% of the global population per year either progress to diabetes or improve reverting to normal glucose levels. However, the size of the prediabetic population is increasing worldwide and it is estimated that nearly 470 million people will have prediabetes by 2030.Citation8
DM is associated with both microvascular (including retinopathy, nephropathy, and neuropathy) and macrovascular (including cardiovascular diseases) complications.Citation8–Citation12 Clinical studies suggest that the incidence of heart failure is 2–4 fold higher in diabetics when compared to nondiabetic patients.Citation13,Citation14 Diabetic cardiomyopathy (DCM) is described as the structural and functional changes in the myocardium that are associated with diabetes in the absence of ischemic heart diseases, hypertension, or other cardiac pathologies.Citation2,Citation3,Citation15–Citation19 Although it has been four decades since DCM was described, the pathogenesis and underlying mechanisms of the disease are not completely understood. Glucose has been considered as the main driving force for the development of DCM,Citation16 however, recent clinical trials (UK Prospective Diabetes Study 33[UKPDS33], the Action to Control Cardiovascular Risk in Diabetes [ACCORD], the Action in Diabetes and Vascular Disease [ADVANCE] and the Veterans’ Administration Diabetes [VADT])Citation20 have revealed no significant effect of intensive glycemic control on mortality and amelioration of cardiovascular events.Citation17 Hence, there is a dire need to understand the detailed mechanisms and factors associated with DCM. Additionally, novel approaches such as stem cell therapy, and micro-RNA (miRNA) may be a promising therapeutic target, for the treatment of DCM. This article embodies a brief overview of DCM, its predictors and preventative measures (at different stages of disease), and future perspectives of therapy.
DCM
Early studies have demonstrated that coronary artery disease (CAD) is the primary cause of cardiac death in diabetics.Citation19,Citation21 However, this notion was challenged by findings that there was a modest increase in atherosclerotic disease in diabetics when compared with age- and sex-matched nondiabetic controls,Citation22 and absence of narrowing of the lumen in the intramural vessels in diabetics.Citation23 These findings were enigmatic until 1972 when DCM was identified as heart failure without any clear symptom of hypertension, CAD, or valvular disease.Citation24 DCM was corroborated by examination of left ventricular function and coronary angiogram in uncomplicated adult diabetics with a family history,Citation25 where a significant reduction of stroke volume index and an elevation in end-diastolic pressure were demonstrated in diabetics when compared with age-matched controls. Although no difference in ejection fraction (EF) was recorded between diabetics and control, there was a significant increase in end-diastolic filling pressure to volume (indicator of end-diastolic wall stiffness) in diabetics.Citation25 Based on these findings, DCM is defined as a distinct entity characterized by the presence of abnormal myocardial performance or structure in the absence of epicardial CAD, hypertension, and significant valvular disease.Citation15,Citation26 Due to the multifactorial nature of diabetes, there are perturbations at both the cellular and molecular levels that predispose the heart to pathological structural and functional remodeling. These alterations may contribute to DCM; however, the detailed mechanism is not completely understood. Cardiomyopathy can be classified into two types: (1) primary cardiomyopathy where the cardiomyopathy primarily affects the function of the heart and (2) secondary cardiomyopathy where cardiac performance is affected due to a systemic syndrome.Citation27 Cardiomyopathy leads to heart failure which can be either systolic heart failure with reduced EF or diastolic heart failure with normal EF.Citation28 The definition of DCM has been extended to DCM with normal EF and DCM with reduced EF, and includes all associated diabetic diseases affecting central hemodynamics.Citation16
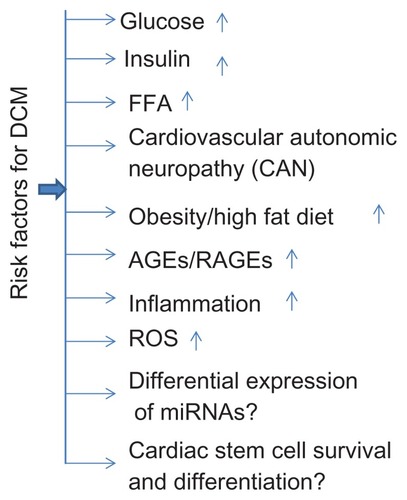
Risk factors for DCM
As diabetes is associated with DCM, elevated glucose level seems to be the major risk factor.Citation16 However, the risk factors that contribute to diabetes or heart failure are also associated with DCM. These risk factors include a high fat diet/obesity, cardiovascular autonomic neuropathy (CAN), inflammation and elevated levels of free fatty acid (FFA), advanced glycation end products (AGEs) and their receptors, and reactive oxygen species (ROS). Recently, differential expression of miRNAsCitation29,Citation30 and stem cell survival and differentiationCitation31 was associated with DCM ().
Mechanism underlying DCM
The disturbances in metabolism that lead to hyperlipidemia, insulin insensitivity causing hyperinsulinemia, and deficiency of insulin due to pancreatic beta cell death causing hyperglycemia, contribute to DCM.Citation3 The metabolic disturbances is mainly due to an elevation in nonesterified fatty acids, also called free fatty acid (FFA).Citation2,Citation3,Citation16 The heart has the potential to utilize both FFA and carbohydrate as a source of energy. However, the dominant source of energy is FFA, and this switches to carbohydrate with increased workload or starvation.Citation32 The switch from FFA to carbohydrate may be due to fetal gene reprogramming.Citation33 In the heart of diabetics, energy production by glucose utilization may be decreased and FFA utilization is increased and this causes depletion of glucose transporter (GLUT)-1 and -4.Citation34 The transgenic expression of GLUT-4 in diabetic mice restored cardiac metabolism and function, and this suggested that glucose metabolism was associated with DCM.Citation35 The elevated level of FFA is implicated in cellular insulin resistanceCitation3,Citation16 One mechanism by which FFA induces insulin resistance is through the protein kinase C (PKC) pathway. PKC (a serine/threonine kinase) phosphorylates inhibitor of kappa light polypeptide gene enhancers B- cells (IkKB) kinase, and this in turn phosphorylates insulin receptor substrate-1 (IRS-1). Phosphorylation of IRS-1 inhibits its ability to bind to the p85 regulatory subunit of phosphatidylinositol 3-kinase, impairing insulin signal transduction in skeletal muscle.Citation36 However, this signaling cascade is not demonstrated in the diabetic heart. Another mechanism of FFA-mediated insulin resistance is via peroxisome proliferator-activated receptor (PPAR)-γ. The activation of PPAR-γ is associated with increased FFA that in turn induces the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN). PTEN dephosphorylates phosphatidylinositol-3, 4, 5-triphosphate and this prevents activation of Akt (serine/threonine kinase)-1.Citation3,Citation37 Increased levels of insulin induces cardiac hypertrophy by inhibiting glycogen synthase kinase-3β, which inhibits nuclear transcription of the hypertrophic program through nuclear factor in activated lymphocytes.Citation38,Citation39 Elevated levels of insulin also upregulates Akt-1 and this induces mammalian target of rapamycin (mTOR) that in turn activates the p70 ribosomal subunit S6 kinase-1 and promotes protein synthesis contributing to cardiac hypertrophy.Citation40–Citation42
Peroxisome proliferator-activated receptor (PPAR-α) is also activated by FFA and its activation induces pyruvate dehydrogenase kinase-4 causing glucose oxidation and stimulating fatty acid uptake in the mitochondria. Along with an increase in long chain acyl carnitines, it promotes mitochondrial uncoupling of oxidative phospohorylation.Citation43 Mitochondrial uncoupling of oxidative phosphorylation results in decreased myocardial high energy reserves and contractile dysfunction.Citation19 The elevated level of FFA abrogates pyruvate dehydrogenase and this induces accumulation of glycolytic intermediates and ceramides, which may promote apoptosis.Citation44,Citation45 The lipotoxicity due to toxic metabolites from FFA opens K-ATP channelsCitation46 and this impairs the ability of cardiomyocytes to regulate calcium use, causing contractile dysfunction.Citation47–Citation49 The induction of apoptosis, hypertrophy, and contractile dysfunction leads to DCM ().
Figure 2 (A) Different pathways associated with increased free fatty acid mediated diabetic cardiomyopathy and (B) different pathways associated with hyperglycemia mediated diabetic cardiomyopathy.
Abbreviations: AGE, advanced glycation end product; AKT-1, serine/threonine kinase; DCM, diabetic cardiomyopathy; ECM, extracellular matrix; FFA, free fatty acid; GAPDH, glyceraldehyde phosphate dehydrogenase; GLUT, glucose transporter; GSK-3β, glycogen synthase kinase-3β; K-ATP, ATP sensitive potassium channel; miRNA, micro-RNA; MMP9, matrix metalloprotinease 9; mTOR, mammalian target of rapamycin; PARP, poly(ADP ribose) polymerase; PKC, protein kinase C; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; RyR, ryanodine receptor; SERCA2, sarco-endoplasmic reticulum-calcium ATPase 2; TNF-α, tumor necrosis factor-α.
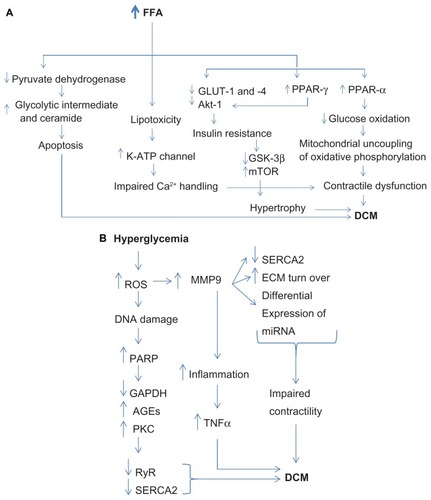
Hyperglycemia also triggers ROSCitation50–Citation52 by inducing glucose oxidation and generating mitochondrial superoxide.Citation52–Citation55 ROS activates matrix metalloproteinases 9 (MMP9) which degrades extracellular matrix, increases matrix turnover, attenuates sarco-endoplasmic reticulum-calcium ATPase 2 (SERCA2), and alters the expression of several miRNAs that leads to contractile dysfunction and ultimately DCM.Citation31,Citation55,Citation56 In diabetes, induction of MMP9 also increases inflammation by inducing pro-inflammatory tumor necrosis factor (TNF)-α and mitigating the anti-inflammatory interleukin (IL)-10 cytokine (unpublished data, 2013) which exacerbates DCM.Citation55–Citation60 Differential expression of several miRNAs also induces TNF-α, inhibits IL-10, and regulates inflammation.Citation61–Citation63 Several miRNAs (such as miR-155 and miR-223) are anti-inflammatory and cardioprotective.Citation64,Citation65 Diabetes-mediated generation of superoxide also causes DNA damage that triggers the reparative enzyme poly (ADP ribose) polymerase (PARP).Citation50 The induction of PARP attenuates glyceraldehyde phosphate dehydrogenase and this diverts glucose from the glycolytic pathway into alternative pathways such as advanced glycation end products (AGEs) and the PKC pathway which downregulates the calcium regulating receptor and enzyme ryanodine receptor and SERCA2 respectively, impairing the contractility of cardiomyocytes, and inducing the ventricular stiffness that leads to DCM ().Citation66–Citation72
Diabetes cardiovascular autonomic neuropathy is manifested in both T1D and T2D, and is diagnosed with abnormal variation in diurnal and nocturnal blood pressure, resting heart rate disorder, exercise intolerance, and prolongation of QT interval in ECG.Citation72 The attenuations of beta-1 and -2 adrenergic receptors (which are part of sympathetic tone) is also associated with DCM.Citation74–Citation77 The alterations in myocardial autonomic neurotransmitters cause toxic effects on catecholamines and apoptosisCitation19 which contributes to DCM. The microenvironment (oxidative stress, myocardial stiffness, differential expression of miRNAs) of the myocardium is changed in the heart of diabetics, and this is implicated in defective cardiac progenitor cell growth and differentiation,Citation16,Citation31 which contributes to DCM.
Pathophysiology and remodeling in DCM
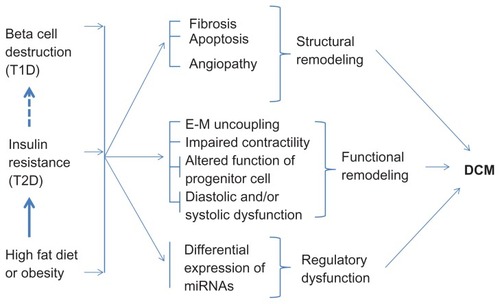
The high fat diet/obesity is associated with insulin resistance, T1D and T2D. In T2D, high blood glucose levels trigger pancreatic beta cells to release insulin; however, due to insulin insensitivity, glucose levels remain high. These hyperglycemic signals continuously activate beta cells to release insulin leading to hyperinsulinemia. The beta cells, due to their continued workload, die and thus, in the long-term, T2D leads to T1D (). Diabetes is associated with structural (fibrosis,Citation55,Citation78 apoptosis,Citation55,Citation79,Citation80 angiopathyCitation81–Citation83), functional (endothelium–myocytes uncoupling,Citation55 impaired contractility of cardiomyocytes,Citation75 decreased survival and differentiation of cardiac stem cells,Citation31 diastolic and systolic dysfunctionCitation55,Citation75,Citation84,Citation85), and regulatory (alteration in the levels of miRNAsCitation29,Citation30,Citation86 and signaling molecules involved in glucose metabolismCitation2,Citation3,Citation16,Citation87) remodeling that leads to DCM ().
Figure 3 Effect of high fat diet, type 1 diabetes, and type 2 diabetes on cardiac remodeling leading to diabetic cardiomyopathy.
There are three major stages of DCM: early stage, middle stage, and late stage. The early stage is asymptomatic, where the heart becomes hypertrophic and has diastolic dysfunction with normal EF.Citation88 However, at the molecular level, increased levels of FFA, altered calcium homeostasis, and depletion of GLUT-1 and -4 are evident.Citation2,Citation16,Citation34,Citation89 The middle stage is recognized by increased left ventricle (LV) size, wall thickness, and mass, which is accompanied by diastolic dysfunction and a slight decrease in systolic function (EF < 50%). It is also accompanied by insulin resistance, AGE formation, increased levels of renin-angiotensin-aldosterone system (RAAS) and tumor growth factor-β1, reduced levels of insulin growth factor-1, apoptosis, necrosis, fibrosis, and mild CAN.Citation2,Citation16,Citation17 The progression from middle stage to late stage disease is associated with additional severities including microvascular changes, CAD, and CAN, which impairs both systolic and diastolic functions ().Citation2,Citation16,Citation17
Table 1 The phenotype and functional impairment in different stages of diabetic cardiomyopathy
Predictors and prevention of DCM
The alarming increase in the number of diabetic patients with cardiomyopathy warrants the implementation of diagnostic strategies for DCM to identify the disease at its early stages. Currently, there is no well recognized method for early diagnosis of DCM. DCM induces changes in the heart structure (myocardial hypertrophy, fibrosis, and fat droplet deposition) and early changes in cardiac function are evident by the abnormal diastolic function that progresses to systolic dysfunction in the later stage of disease. These changes in patients with DCM can be diagnosed using the following methods:
Echocardiography and Doppler imaging: In the early stage of DCM and in the majority (75%) of asymptomatic diabetic patients, diastolic dysfunction characterized with heart failure with normal EF is present.Citation88 Diastolic dysfunction, mitral inflow patterns, mitral E/E′, transmitral E/A, cardiac stiffness, and dilatation of the LV can be assessed by echocardiography.Citation16,Citation90–Citation92 Therefore, echocardiography and Doppler imaging can be utilized to evaluate structural and functional remodeling in the heart of diabetics. However, numerous factors such as myocardial fibrosis, hypertrophy, and contractile asynchrony changes in calcium cycling are involved in altering the normal LV diastolic function.Citation90 These changes are not confined to DCM but are also present in other cardiac diseases,Citation15,Citation91 and therefore, other approaches are required for diagnosing DCM.
Magnetic resonance imaging (MRI): MRI is a highly sensitive tool for detecting LV wall motion abnormalities, geometry, and cardiac hypertrophy.Citation91,Citation93–Citation95 MRI is considered to be a favorable tool for the accurate measurement of LV mass, volume, and function.Citation96–Citation99. Additionally, it provides information on arrhythmia and cardiomyopathy.Citation100
Serological biomarkers:
High levels of glucose and hemoglobin A1c are indicators of diabetes.
Increased levels of N-terminal pro-brain natriuretic peptide and brain natriuretic peptide are markers of heart failure.
Troponin present in the plasma, is an indicator of necrosis.
Elevated level of MMPs (especially MMP9) and decreased levels of tissue inhibitor of metalloproteinases (TIMPs) are indicators of fibrosis.Citation16
Levels of the enzyme beta O-GlcNAc (o-linked N-acetylglucosamine) can also be used as a predictor of DCM as it is increased in hypertrophy and cardiovascular diseases.Citation101–Citation103
Heart catheterization and coronary angiography: Different stages of DCM can be diagnosed by left heart catheterization that assesses LV end diastolic pressure and right heart catheterization that measures mean pulmonary wedge pressure which is often associated with increases in mean pulmonary pressure.Citation16 Coronary angiography determines stenosis in the coronary artery which is often present in late stages of DCM.Citation15
MiRNA as a potential biomarker for DCM
MiRNA are small (~22 nucleotide), conserved, non-coding RNA molecules that modulate gene expression either through mRNA degradation or translational repression.Citation104 They are emerging as promising therapeutic targets for cardiovascular disease and diabetes.Citation29,Citation30 The levels of miRNAs are altered in the hearts of diabetics.Citation105,Citation106 Recently, circulating miRNAs were reported as biomarkers for cardiovascular disease.Citation107–Citation116 Therefore, the differential expression of specific circulating miRNAs can be used to diagnose different stages of DCM ().
Table 2 Predictors of and preventative measures for diabetic cardiomyopathy
Prevention of DCM
Although tight regulation of glucose levels is thought to ameliorate DCM, recent clinical trials (UKPDS 33, ACCORD, ADVANCE, and VADT)Citation21 have failed to support this.Citation17 Based on the different stages of DCM, different preventative measures should be taken.
Early stage DCM: Changes in lifestyle and diet are the measurable factors that prevent DCM progression and may even cure the disease. A low fat and glucose diet, and physical exercise can mitigate early DCM.
Middle stage DCM: In addition to exercise and diet control, treatment with metformin for T2D, insulin for T1D, pioglitazone for ameliorating diastolic dysfunction, and beta-blockers for reducing blood pressure may be required.
Late stage DCM: In addition to the above mentioned preventative measures for middle stage DCM, angioplasty is required to mitigate micro- and macro-angiopathy and for coronary stenosis ().
Antidiabetic drugs for treatment of DCM
Metformin (one of the most commonly prescribed drugs) improves peripheral sensitivity to insulin, promotes hyperglycemic control, and acts as an anti-inflammatory agent.Citation92,Citation117,Citation118 Glucagon-like peptide (GLP)-1 is an incretin hormone that stimulates postprandial insulin secretion and improves insulin sensitivity. Individuals treated with GLP-1 also have improved left ventricular ejection fraction.Citation92
Other classes of drugs for treatment of DCM
The trials with statins and RAAS inhibitors also show positive results for mitigating DCM.Citation91,Citation92,Citation119–Citation125 Statins inhibit cholesterol biosynthesis, and have anti-inflammatory and anti-oxidative stress functions. They also improve LV function and reduce fibrosis that ameliorates DCM.Citation92,Citation119–Citation125 RAAS inhibitors are also cardioprotective. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are commonly used to block the RAAS. Results based on clinical and experimental studies suggest that RAAS inhibitors not only reduce blood pressure but also help to improve insulin sensitivity and enhance blood glucose uptake. Diastolic dysfunction is ameliorated by RAAS inhibitor treatment.Citation91 The use of beta-blockers in diabetic patients is not common considering different parameters such as blood glucose, insulin resistance, and dyslipidemia; however, recent meta-analyses suggest that the use of these beta-blockers improves glycemic levels and insulin resistance when compared with other antidiabetic drugs.Citation91
Conclusions and future directions
Several hypotheses have been proposed to describe how DCM develops,Citation3,Citation16 however, DCM is still a valid challenge in medical science as the number of diabetics in the population is rapidly increasing. Although recent trials have revealed that tight glucose regulation is not as effective as first thought, control of hyperglycemia is essential to mitigate DCM. It is clear that DCM is regulated not only by high glucose levels; there are several other factors and mechanisms (–) that contribute to DCM. The role of high blood pressure, hyperlipidemia, and oxidative stress also contributes to, and exacerbates, DCM. Early diagnosis is essential for preventing and reverting DCM. For early diagnosis, serological markers, echocardiography, and MRI are important. Recent advances in the areas of miRNA and stem cell therapy provide a new dimension to explore DCM and its therapy. The levels of specific miRNA during early, middle, and late stage DCM can be used as a biomarker for different stages of DCM. The use of miRNA mimics and antagomiR (if a miRNA is attenuated and up regulated, respectively) can be exploited to mitigate DCM. Similarly, stem cells can be used for regenerating pancreatic beta cells and myocardium to improve glucose metabolism and cardiac function, respectively.
Acknowledgement
This work is supported in part by the American Heart Association grant 11BGIA 7690055 and National Institute of Health grants HL-113281 (P.K.M.), HL-108621 (SCT) and HL-74185 (SCT).
Disclosure
The authors report no conflicts of interest in this work.
References
- Ashcroft FM Rorsman P Diabetes mellitus and the beta cell: the last ten years Cell 2012 148 6 1160 1171 22424227
- Goyal BR Mehta AA Diabetic cardiomyopathy: pathophysiological mechanisms and cardiac dysfunction Hum Exp Toxicol Epub November 22, 2012
- Poornima IG Parikh P Shannon RP Diabetic cardiomyopathy: the search for a unifying hypothesis Circ Res 2006 98 5 596 605 16543510
- King H Aubert RE Herman WH Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections Diabetes Care 1998 21 9 1414 1431 9727886
- Wild S Roglic G Green A Sicree R King H Global prevalence of diabetes: estimates for the year 2000 and projections for 2030 Diabetes Care 2004 27 5 1047 1053 15111519
- Mishra PK Singh SR Joshua IG Tyagi SC Stem cells as a therapeutic target for diabetes Front Biosci 2010 15 461 477 20036830
- Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States 2011 Department of Health and Human Services Atlanta, GA, USA 2011 Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed March 01, 2012
- Tabak AG Herder C Rathmann W Brunner EJ Kivimaki M Prediabetes: a high-risk state for diabetes development Lancet 2012 379 9833 2279 2290 22683128
- Arora MK Singh UK Molecular mechanisms in the pathogenesis of diabetic nephropathy: An update Vascul Pharmacol Epub January 11, 2013
- Campos C Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae Postgrad Med 2012 124 6 90 97 23322142
- Nguyen DV Shaw LC Grant MB Inflammation in the pathogenesis of microvascular complications in diabetes Front Endocrinol (Lausanne) 2012 3 170 23267348
- Rahman S Rahman T Ismail AA Rashid AR Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis Diabetes Obes Metab 2007 9 6 767 780 17924861
- Mathew V Gersh BJ Williams BA Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial Circulation 2004 109 4 476 480 14732749
- Pignone M Alberts MJ Colwell JA Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation Diabetes Care 2010 33 6 1395 1402 20508233
- Aneja A Tang WH Bansilal S Garcia MJ Farkouh ME Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options Am J Med 2008 121 9 748 757 18724960
- Maisch B Alter P Pankuweit S Diabetic cardiomyopathy – fact or fiction? Herz 2011 36 2 102 115 21424347
- Miki T Yuda S Kouzu H Miura T Diabetic cardiomyopathy: pathophysiology and clinical features Heart Fail Rev Epub March 28, 2012
- Sharma V McNeill JH Diabetic cardiomyopathy: where are we 40 years later? Can J Cardiol 2006 22 4 305 308 16568154
- Tarquini R Lazzeri C Pala L Rotella CM Gensini GF The diabetic cardiomyopathy Acta Diabetol 2011 48 3 173 181 20198391
- Bloomgarden ZT Glycemic control in diabetes: A tale of three studies Diabetes care 2008 31 1913 1919 18753670
- Kessler II Mortality experience of diabetic patients. A twenty-six-year follow-up study Am J Med 1971 51 6 715 724 5129542
- Vihert AM Zhdanov VS Matova EE Atherosclerosis of the aorta and coronary vessels of the heart in cases of various diseases J Atheroscler Res 1969 9 2 179 192 5770400
- Ledet T Histological and histochemical changes in the coronary arteries of old diabetic patients Diabetologia 1968 4 5 268 272 4243356
- Rubler S Dlugash J Yuceoglu YZ Kumral T Branwood AW Grishman A New type of cardiomyopathy associated with diabetic glomerulosclerosis Am J Cardiol 1972 30 6 595 602 4263660
- Regan TJ Lyons MM Ahmed SS Evidence for cardiomyopathy in familial diabetes mellitus J Clin Invest 1977 60 4 884 899 893679
- Fang ZY Prins JB Marwick TH Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications Endocr Rev 2004 25 4 543 567 15294881
- Elliott P Andersson B Arbustini E Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases Eur Heart J 2008 29 2 270 276 17916581
- Paulus WJ Tschope C Sanderson JE How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology Eur Heart J 2007 28 20 2539 2550 17428822
- Mishra PK Tyagi N Kumar M Tyagi SC MicroRNAs as a therapeutic target for cardiovascular diseases J Cell Mol Med 2009 13 4 778 789 19320780
- Tyagi AC Sen U Mishra PK Synergy of microRNA and stem cell: a novel therapeutic approach for diabetes mellitus and cardiovascular diseases Curr Diabetes Rev 2011 7 6 367 376 21864292
- Mishra PK Chavali V Metreveli N Tyagi SC Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix Can J Physiol Pharmacol 2012 90 3 353 360 22394373
- Goodwin GW Taylor CS Taegtmeyer H Regulation of energy metabolism of the heart during acute increase in heart work J Biol Chem 1998 273 45 29530 29539 9792661
- Depre C Shipley GL Chen W Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy Nat Med 1998 4 11 1269 1275 9809550
- Rodrigues B Cam MC McNeill JH Metabolic disturbances in diabetic cardiomyopathy Mol Cell Biochem 1998 180 1–2 53 57 9546630
- Belke DD Larsen TS Gibbs EM Severson DL Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice Am J Physiol Endocrinol Metab 2000 279 5 E1104 E1113 11052966
- Kim JK Kim YJ Fillmore JJ Prevention of fat-induced insulin resistance by salicylate J Clin Invest 2001 108 3 437 446 11489937
- Schwartzbauer G Robbins J The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival J Biol Chem 2001 276 38 35786 35793 11448956
- Morisco C Condorelli G Trimarco V Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes Circ Res 2005 96 2 180 188 15591229
- O’Neill BT Abel ED Akt1 in the cardiovascular system: friend or foe? J Clin Invest 2005 115 8 2059 2064 16075047
- Khamzina L Veilleux A Bergeron S Marette A Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance Endocrinology 2005 146 3 1473 1481 15604215
- Manning BD Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis J Cell Biol 2004 167 3 399 403 15533996
- Shah OJ Wang Z Hunter T Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies Curr Biol 2004 14 18 1650 1656 15380067
- Boudina S Abel ED Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes Physiology (Bethesda) 2006 21 250 258 16868314
- Eckel J Reinauer H Insulin action on glucose transport in isolated cardiac myocytes: signalling pathways and diabetes-induced alterations Biochem Soc Trans 1990 18 6 1125 1127 1965168
- Liedtke AJ DeMaison L Eggleston AM Cohen LM Nellis SH Changes in substrate metabolism and effects of excess fatty acids in reperfused myocardium Circ Res 1988 62 3 535 542 3342476
- Liu GX Hanley PJ Ray J Daut J Long-chain acyl-coenzyme A esters and fatty acids directly link metabolism to K(ATP) channels in the heart Circ Res 2001 88 9 918 924 11349001
- Abe T Ohga Y Tabayashi N Left ventricular diastolic dysfunction in type 2 diabetes mellitus model rats Am J Physiol Heart Circ Physiol 2002 282 1 H138 H148 11748057
- Malhotra A Sanghi V Regulation of contractile proteins in diabetic heart Cardiovasc Res 1997 34 1 34 40 9217870
- Takeda N Nakamura I Hatanaka T Ohkubo T Nagano M Myocardial mechanical and myosin isoenzyme alterations in streptozotocin-diabetic rats Jpn Heart J 1988 29 4 455 463 2846906
- Du X Matsumura T Edelstein D Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells J Clin Invest 2003 112 7 1049 1057 14523042
- Nishikawa T Edelstein D Brownlee M The missing link: a single unifying mechanism for diabetic complications Kidney Int Suppl 2000 77 S26 S30 10997687
- Nishikawa T Edelstein D Du XL Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage Nature 2000 404 6779 787 790 10783895
- Cai L Kang YJ Oxidative stress and diabetic cardiomyopathy: a brief review Cardiovasc Toxicol 2001 1 3 181 193 12213971
- Farhangkhoee H Khan ZA Mukherjee S Heme oxygenase in diabetes-induced oxidative stress in the heart J Mol Cell Cardiol 2003 35 12 1439 1448 14654370
- Mishra PK Tyagi N Sen U Joshua IG Tyagi SC Synergism in hyperhomocysteinemia and diabetes: role of PPAR gamma and tempol Cardiovasc Diabetol 2010 9 49 20828387
- Mishra PK Metreveli N Tyagi SC MMP-9 gene ablation and TIMP-4 mitigate PAR-1-mediated cardiomyocyte dysfunction: a plausible role of dicer and miRNA Cell Biochem Biophys 2010 57 2–3 67 76 20422465
- Barac A Wang H Shara NM Markers of inflammation, metabolic risk factors, and incident heart failure in American Indians: the Strong Heart Study J Clin Hypertens (Greenwich) 2012 14 1 13 19 22235819
- Bradham WS Moe G Wendt KA TNF-alpha and myocardial matrix metalloproteinases in heart failure: relationship to LV remodeling Am J Physiol Heart Circ Physiol 2002 282 4 H1288 H1295 11893563
- Bradham WS Bozkurt B Gunasinghe H Mann D Spinale FG Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective Cardiovasc Res 2002 53 4 822 830 11922892
- Calle MC Fernandez ML Inflammation and type 2 diabetes Diabetes Metab 2012 38 3 183 191 22252015
- Moschos SA Williams AE Perry MM Birrell MA Belvisi MG Lindsay MA Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids BMC Genomics 2007 8 240 17640343
- Perry MM Moschos SA Williams AE Shepherd NJ Larner-Svensson HM Lindsay MA Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells J Immunol 2008 180 8 5689 5698 18390754
- Roy S Sen CK MiRNA in innate immune responses: novel players in wound inflammation Physiol Genomics 2011 43 10 557 565 21139022
- Schaefer JS Montufar-Solis D Vigneswaran N Klein JR Selective upregulation of microRNA expression in peripheral blood leukocytes in IL-10−/− mice precedes expression in the colon J Immunol 2011 187 11 5834 5841 22043014
- van de Vrie M Heymans S Schroen B MicroRNA involvement in immune activation during heart failure Cardiovasc Drugs Ther 2011 25 2 161 170 21503626
- Bidasee KR Nallani K Yu Y Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels Diabetes 2003 52 7 1825 1836 12829653
- Candido R Forbes JM Thomas MC A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes Circ Res 2003 92 7 785 792 12623881
- Cooper ME Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease Am J Hypertens 2004 17 12 Pt 2 31S 38S 15607433
- Guo M Wu MH Korompai F Yuan SY Upregulation of PKC genes and isozymes in cardiovascular tissues during early stages of experimental diabetes Physiol Genomics 2003 12 2 139 146 12441406
- Herrmann KL McCulloch AD Omens JH Glycated collagen cross-linking alters cardiac mechanics in volume-overload hypertrophy Am J Physiol Heart Circ Physiol 2003 284 4 H1277 H1284 12595292
- Koya D King GL Protein kinase C activation and the development of diabetic complications Diabetes 1998 47 6 859 866 9604860
- Wakasaki H Koya D Schoen FJ Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy Proc Natl Acad Sci U S A 1997 94 17 9320 9325 9256480
- Karayannis G Giamouzis G Cokkinos DV Skoularigis J Triposkiadis F Diabetic cardiovascular autonomic neuropathy: clinical implications Expert Rev Cardiovasc Ther 2012 10 6 747 765 22894631
- Lambert GW Straznicky NE Lambert EA Dixon JB Schlaich MP Sympathetic nervous activation in obesity and the metabolic syndrome – causes, consequences and therapeutic implications Pharmacol Ther 2010 126 2 159 172 20171982
- Mishra PK Givvimani S Metreveli N Tyagi SC Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy Biochem Biophys Res Commun 2010 401 2 175 181 20836991
- Mishra PK Awe O Metreveli N Qipshidze N Joshua IG Tyagi SC Exercise mitigates homocysteine – beta2-adrenergic receptor interactions to ameliorate contractile dysfunction in diabetes Int J Physiol Pathophysiol Pharmacol 2011 3 2 97 106 21760968
- Wang G Zhu X Xie W Rad as a novel regulator of excitation-contraction coupling and beta-adrenergic signaling in heart Circ Res 2010 106 2 317 327 19926875
- Fowlkes V Clark J Fix C Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts Life Sci Epub January 16, 2013
- Delucchi F Berni R Frati C Resveratrol treatment reduces cardiac progenitor cell dysfunction and prevents morpho-functional ventricular remodeling in type-1 diabetic rats PLoS One 2012 7 6 e39836 22768138
- Zou MH Xie Z Regulation of interplay between autophagy and apoptosis in the diabetic heart: New role of AMPK Autophagy Epub February 4, 2013
- Marciano C Galderisi M Gargiulo P Effects of type 2 diabetes mellitus on coronary microvascular function and myocardial perfusion in patients without obstructive coronary artery disease Eur J Nucl Med Mol Imaging 2012 39 7 1199 1206 22526959
- Papa G Degano C Iurato MP Licciardello C Maiorana R Finocchiaro C Macrovascular complication phenotypes in type 2 diabetic patients Cardiovasc Diabetol Epub January 18, 2013
- Wang CC Reusch JE Diabetes and cardiovascular disease: changing the focus from glycemic control to improving long-term survival Am J Cardiol 2012 110 Suppl 9 58B 68B
- Ernande L Thibault H Bergerot C Systolic myocardial dysfunction in patients with type 2 diabetes mellitus: identification at MR imaging with cine displacement encoding with stimulated echoes Radiology 2012 265 2 402 409 22929334
- Zhang H Zhang Y Li Z Left ventricular radial systolic dysfunction in diabetic patients assessed by myocardial acceleration derived from velocity vector imaging J Ultrasound Med 2012 31 8 1179 1186 22837281
- Asrih M Steffens S Emerging role of epigenetics and miRNA in diabetic cardiomyopathy Cardiovasc Pathol 2013 22 2 117 125 22951386
- Boudina S Abel ED Diabetic cardiomyopathy, causes and effects Rev Endocr Metab Disord 2010 11 1 31 39 20180026
- Boyer JK Thanigaraj S Schechtman KB Perez JE Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus Am J Cardiol 2004 93 7 870 875 15050491
- Taegtmeyer H Cardiac metabolism as a target for the treatment of heart failure Circulation 2004 110 8 894 896 15326079
- Cosson S Kevorkian JP Left ventricular diastolic dysfunction: an early sign of diabetic cardiomyopathy? Diabetes Metab 2003 29 5 455 466 14631322
- Maya L Villarreal FJ Diagnostic approaches for diabetic cardiomyopathy and myocardial fibrosis J Mol Cell Cardiol 2010 48 3 524 529 19595694
- Murarka S Movahed MR Diabetic cardiomyopathy J Card Fail 2010 16 12 971 979 21111987
- Achenbach S Ropers D Regenfus M Noninvasive coronary angiography by magnetic resonance imaging, electron-beam computed tomography, and multislice computed tomography Am J Cardiol 2001 88 2A 70E 73E
- Charoenpanichkit C Morgan TM Hamilton CA Left ventricular hypertrophy influences cardiac prognosis in patients undergoing dobutamine cardiac stress testing Circ Cardiovasc Imaging 2010 3 4 392 397 20442370
- Gebker R Mirelis JG Jahnke C Influence of left ventricular hypertrophy and geometry on diagnostic accuracy of wall motion and perfusion magnetic resonance during dobutamine stress Circ Cardiovasc Imaging 2010 3 5 507 514 20576810
- Alter P Rupp H Rominger MB B-type natriuretic peptide and wall stress in dilated human heart Mol Cell Biochem 2008 314 1–2 179 191 18461428
- Alter P Rupp H Maisch B Assessment and relevance of ventricular wall stress in heart failure Eur Heart J 2008 29 18 2316 18621773
- Alter P Rupp H Rominger MB A new method to assess ventricular wall stress in patients with heart failure and its relation to heart rate variability Int J Cardiol 2010 139 3 301 303 18952305
- Gottlieb I Macedo R Bluemke DA Lima JA Magnetic resonance imaging in the evaluation of non-ischemic cardiomyopathies: current applications and future perspectives Heart Fail Rev 2006 11 4 313 323 17131077
- Macedo R Schmidt A Rochitte CE Lima JA Bluemke DA MRI to assess arrhythmia and cardiomyopathies J Magn Reson Imaging 2006 24 6 1197 1206 17083108
- Jones SP A bittersweet modification: O-GlcNAc and cardiac dysfunction Circ Res 2005 96 9 925 926 15890978
- Lunde IG Aronsen JM Kvaloy H Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure Physiol Genomics 2012 44 2 162 172 22128088
- Zachara NE The roles of O-linked beta-N-acetylglucosamine in cardiovascular physiology and disease Am J Physiol Heart Circ Physiol 2012 302 10 H1905 H1918 22287582
- Bartel DP MicroRNAs: target recognition and regulatory functions Cell 2009 136 2 215 233 19167326
- Chavali V Tyagi SC Mishra PK MicroRNA-133a regulates DNA methylation in diabetic cardiomyocytes Biochem Biophys Res Commun 2012 425 3 668 672 22842467
- Feng B Chen S George B Feng Q Chakrabarti S miR133a regulates cardiomyocyte hypertrophy in diabetes Diabetes Metab Res Rev 2010 26 1 40 49 20013939
- Ajit SK Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules Sensors (Basel) 2012 12 3 3359 3369 22737013
- Dimmeler S Zeiher AM Circulating microRNAs: novel biomarkers for cardiovascular diseases? Eur Heart J 2010 31 22 2705 2707 20605798
- Fichtlscherer S de RS Fox H Circulating microRNAs in patients with coronary artery disease Circ Res 2010 107 5 677 684 20595655
- Fichtlscherer S Zeiher AM Dimmeler S Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol 2011 31 11 2383 2390 22011751
- Li C Pei F Zhu X Duan DD Zeng C Circulating microRNAs as novel and sensitive biomarkers of acute myocardial Infarction Clin Biochem 2012 45 10–11 727 732 22713968
- McManus DD Ambros V Circulating MicroRNAs in cardiovascular disease Circulation 2011 124 18 1908 1910 22042926
- Scholer N Langer C Kuchenbauer F Circulating microRNAs as biomarkers – True Blood? Genome Med 2011 3 11 72 22112937
- Tijsen AJ Pinto YM Creemers EE Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases Am J Physiol Heart Circ Physiol 2012 303 9 H1085 H1095 22942181
- van Empel VP De Windt LJ da Costa Martins PA Circulating miRNAs: reflecting or affecting cardiovascular disease? Curr Hypertens Rep 2012 14 6 498 509 22996205
- Weiland M Gao XH Zhou L Mi QS Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases RNA Biol 2012 9 6 850 859 22699556
- Da SD Ausina P Alencar EM Coelho WS Zancan P Sola-Penna M Metformin reverses hexokinase and phosphofructokinase downregulation and intracellular distribution in the heart of diabetic mice IUBMB Life 2012 64 9 766 774 22730258
- Molavi B Rassouli N Bagwe S Rasouli N A review of thiazolidinediones and metformin in the treatment of type 2 diabetes with focus on cardiovascular complications Vasc Health Risk Manag 2007 3 6 967 973 18200815
- Bellia A Rizza S Galli A Early vascular and metabolic effects of rosuvastatin compared with simvastatin in patients with type 2 diabetes Atherosclerosis 2010 210 1 199 201 20018286
- Lin CL Cheng H Tung CW Simvastatin reverses high glucose-induced apoptosis of mesangial cells via modulation of Wnt signaling pathway Am J Nephrol 2008 28 2 290 297 18004065
- Ma T Tien L Fang CL Liou YS Jong GP Statins and new-onset diabetes: a retrospective longitudinal cohort study Clin Ther 2012 34 9 1977 1983 22939164
- Paolisso G Sgambato S De RS Simvastatin reduces plasma lipid levels and improves insulin action in elderly, non-insulin dependent diabetics Eur J Clin Pharmacol 1991 40 1 27 31 2060542
- Tawfik HE El-Remessy AB Matragoon S Ma G Caldwell RB Caldwell RW Simvastatin improves diabetes-induced coronary endothelial dysfunction J Pharmacol Exp Ther 2006 319 1 386 395 16849625
- Wei P Grimm PR Settles DC Balwanz CR Padanilam BJ Sansom SC Simvastatin reverses podocyte injury but not mesangial expansion in early stage type 2 diabetes mellitus Ren Fail 2009 31 6 503 513 19839828
- Yao XM Ye SD Zai Z Simvastatin protects diabetic rats against kidney injury through the suppression of renal matrix metalloproteinase-9 expression J Endocrinol Invest 2010 33 5 292 296 19820293