Abstract
Background
Diabetes mellitus (DM) and thyroid dysfunction (TD) are two closely associated disorders. The objective of the present study was to investigate the thyroid status and the relationships between thyroid hormones, diabetic complications and metabolic parameters in hospitalized patients with newly diagnosed type 2 DM (T2DM).
Methods
This was an observational cross-sectional study, conducting on 340 patients with newly diagnosed T2DM who were admitted to ward of endocrinology department and 120 matched individuals without diabetes. Anthropometric, clinical and biochemical data were collected. Spearman correlation coefficients were calculated to evaluate the correlations between thyroid hormones and other variables. Factors associated with diabetic nephropathy (DN) was analyzed with multivariate logistic regression.
Results
Levels of free triiodothyronine (FT3), free thyroxine (FT4) and thyroid stimulating hormone (TSH) were significantly lower in patients with T2DM as compared to control group without diabetes. The prevalence of TD was 21.2% in patients with diabetes, higher than that in controls (4.2%). The low T3 syndrome was the most frequent TD, shown in 14.7% of patients. The presence of diabetic complications DN, diabetic ketosis or ketoacidosis), metabolic and demographic factors, including age, glycemic control and insulin resistance were factors significantly associated with levels of thyroid hormones. FT3 level was inversely correlated with the level of urinary total protein (mg/24h) and the presence of DN. Multivariate analysis indicated low FT3 level as a strong independent risk factor (OR = 0.364, P = 0.001) for DN.
Conclusion
TD is not rarely seen in hospitalized patients with newly diagnosed T2DM. Diabetic complications and diabetes-related metabolic and demographic factors are related to thyroid hormone levels. Decreased FT3 is strongly correlated with the presence of DN.
Introduction
Studies have suggested a bidirectional influence of diabetes and thyroid disorders upon each other.Citation1,Citation2 Thyroid dysfunction (TD), which is usually defined as an abnormal thyroid function test result, is more common in patients with type 2 DM (T2DM) than in those without diabetes and can adversely influence metabolic control.Citation3,Citation4 The risk of T2DM increases in subjects with hypothyroidism and in those with lower free thyroxine (FT4) levels in the reference range.Citation5 Thyroid hormones can exert a direct influence on insulin secretion. Hypothyroidism, as a main form of TD in patients with diabetes, could lead to a decrease in insulin production. Hyperthyroidism results in an increase in beta-cell responsiveness to catecholamine or glucose due to increased beta-cell mass, and an increase in insulin clearance.Citation6 Also, both hypothyroidism and hyperthyroidism are able to influence the metabolism of insulin and thus induce insulin resistance.Citation7 On the other hand, diabetes can impair thyroid function by changing thyroid stimulating hormone (TSH) levels at the level of hypothalamus and by disturbing the conversion of thyroxine (T4) to triiodothyronine (T3) in peripheral tissues.Citation4 Long term coexistence of TD and T2DM can further increase the morbidity and mortality associated with diabetes.Citation4 Low levels of thyroid hormones, even in the normal range, were associated with diabetic complications including acute complications such as diabetic ketosis (DK) or diabetic ketoacidosis (DKA)Citation8 and chronic complications such as diabetic nephropathy (DN)Citation9 and diabetic retinopathy (DR).Citation10 Hypothyroidism was indicated to be related to increased risks of DR and chronic kidney disease.Citation11 The relationship between T2DM and TD is complex and has not been fully elucidated. Several studies have investigated the prevalence and risk factors of TD in patients with T2DM. But the population varied among these studies and researches in patients with newly diagnosed diabetes were relatively rare. Considering the addictive impact of diabetes progression on thyroid function, we suppose it necessary to focus on patients with newly diagnosed diabetes on this issue. Therefore, we designed this study, to investigate the prevalence of TD and the relationships between thyroid hormones, diabetic complications and metabolic parameters in hospitalized patients with newly diagnosed T2DM.
Materials and Methods
Study Population and Design
We studied patients with newly diagnosed T2DM who were admitted to ward of the Department of Endocrinology, The Second Affiliated Hospital of Guangzhou Medical University, from January 2014 to June 2019. The inclusion criteria were adults with newly diagnosed and treated T2DM based on the diagnostic criteria recommended by the Chinese Diabetes Society.Citation12 The exclusion criteria included: A. with known history of thyroid disease or thyroid surgery; B. severe primary liver and kidney dysfunctions; C. using drugs potentially altering thyroid hormone concentrations such as amiodarone, beta-blockers and corticosteroids. These patients were newly diagnosed with T2DM when seeking medical service due to diabetes-related symptoms, or undergoing routine physical examination in community hospitals. Patients were admitted due to high glycemic level (HbA1c ≥ 9%) or diabetic complications including acute complications (DK, DKA) and chronic complications (diabetic peripheral neuropathy (DPN), diabetic foot, etc.). Control group without diabetes was selected from a population undergoing an annual physical examination at the Health Examination Department, The Second Affiliated Hospital of Guangzhou Medical University, during the same period. Exclusion criteria were the same as the ones for patients with diabetes. Finally, a total of 340 patients with newly diagnosed T2DM and 120 subjects without T2DM were enrolled. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Guangzhou Medical University (Approval number 2021-hg-ks-10), following the Declaration of Helsinki. Written informed consent was waived due to the retrospective nature and low risk of the study. Personal identifiers were removed before data extraction.
Measurement and Data Collection
Demographic information including family history and habit of smoking was collected through the review of medical records. Body mass index (BMI) was calculated as weight (kg) divided by squared height (m). Blood pressure (BP) was detected twice in a sitting position after a 10-minute rest period and recorded as a mean of the two successive measurements. Hypertension was defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg, or with positive histories of hypertension. Venous blood samples were collected in the morning after an overnight fast for laboratory measurement in the second day of admission. Serum free T3 (FT3), FT4 and TSH were measured using electrochemiluminescence immunoassays. Normal ranges were provided by kit producers as follows: TSH 0.4–5.0 μIU/mL, FT3 2.63–5.70 pmol/L, and FT4 9.01–19.05 pmol/L. Euthyroid was considered if thyroid hormone levels fall within reference range and thyroid dysfunction was considered if thyroid hormones fall outside the reference range. The diagnostic categories for thyroid dysfunction were as follows: A. subclinical hypothyroidism (increased TSH values with normal FT4 levels); B. overt hypothyroidism (increased TSH values with decreased FT4 levels); C. subclinical hyperthyroidism (decreased TSH values with normal FT4 levels; D. overt hyperthyroidism (decreased TSH values with increased FT4 levels; E. low T3 syndrome (decreased FT3 values only, or decreased FT3, TSH, and/or FT4 levels). Routine biochemical parameters were measured by routine laboratory methods. The estimated glomerular filtration rate (eGFR) was calculated according to Modification of Diet in Renal Disease equation: eGFR (mL/min/1.73 m2) = 186 × (SCr/88.4)−1.154 × (age)−0.203 × (0.742 if female).Citation13 Urine samples of 24 hours were collected to measure urine albumin levels using a chemiluminescence assay. Spot urinary samples of patients were collected at 7:00–8:00 am. Urinary albumin concentration was measured by nephelometry immunoassay and urinary creatinine concentration was measured by velocity method. The average value of the urinary albumin-to-creatinine ratio (UACR) was calculated. Homeostatic model assessment of insulin resistance (HOMA-IR) and β cell function (HOMA-β) was calculated using well-established methods: HOMA-IR = 1.5 + fasting blood glucose (mmol/L) × fasting C-peptide (pmol/L)/2800, HOMA-β = 0.27 × fasting C-peptide (pmol/L)/(fasting blood glucose (mmol/L) – 3.5).Citation14 DN was defined as an increased UACR of ≥ 30 mg/g or albumin excretion rate (AER) ≥ 30 mg/24h in the absence of urinary tract infection or other renal abnormalities. DR was defined as either a non-proliferative or proliferative DR or previous laser photocoagulation therapy. DPN was identified on the basis of nerve conduction velocity tests together with neurological symptoms and signs (pain, burning, tingling, or numb sensation on the feet or hands). DK was diagnosed in patients with blood ketone body > 3 mmol/L or positive urine ketone body, blood glucose > 11 mmol/L, and bicarbonate (HCO3−) ≥ 15 mmol/L or arterial pH ≥ 7.3.Citation15 The diagnosis of DKA was made if serum HCO3− level was under 15 mmol/L and/or arterial pH level was under 7.3, and blood glucose was ranged from 16.7 to 33.3 mmol/L.Citation15 Carotid atherosclerosis was identified based on the ultrasonographic examinations of both common carotid arteries.
Statistical Analysis
Numeric values were presented as mean (standard deviation) or median (interquartile range). Categorical values were presented as number (%). Bivariate comparisons for continuous variables were performed using Student’s t-test for parametric variables and Mann–Whitney U-test for non-parametric variables. One-way analysis of variance (ANOVA) were used for comparisons of multiple continuous variables. Chi-square test was used for comparisons of categorical variables. For evaluation of correlation between FT3, FT4, FT3/FT4 ratio or TSH and other variables, Spearman correlation coefficient was calculated. Factors associated with DN was analyzed with multivariate logistic regression. A two-sided P value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, New York, USA).
Results
Baseline Characteristics
Detailed baseline demographic and clinical characteristics of included subjects were presented in . The two groups were age- and sex-matched. The median (interquartile range) age of the patients with T2DM was 55.5 (18.0) years old, ranging from 18 to 90 years. Male patients constituted the majority (n = 221, 65.0%). The median (interquartile range) BMI of the patients was 24.0 (5.1) kg/m2. About 30% of the study population had a family history of diabetes. Social history of smoking was reported by 28.5% of the patients. Median (interquartile range) HbA1c level was 12.0 (3.5) %. Levels of FT3, FT4 and TSH were significantly lower in patients with T2DM (FT3 3.54 (0.98) pmol/L, FT4 14.33 (3.47) pmol/L, TSH 1.31 (1.17) μIU/mL) as compared to controls (FT3 4.95 (0.84) pmol/L, FT4 16.90 (3.60) pmol/L, TSH 2.08 (1.63) μIU/mL) (P < 0.001). TD was found in 72 (21.2%) patients with T2DM and 5 (4.3%) in control group (P < 0.001). To avoid the impact of acute condition on thyroid function, we exclude the patients with DK or DKA. Levels of thyroid hormones (FT3 3.73 (0.81) pmol/L, FT4 14.38 (3.19) pmol/L, TSH 1.27 (1.13) μIU/mL) remained to be lower and the prevalence of TD (n = 31, 13.0%) remained to be higher in patients with diabetes than in controls (All P < 0.001).
Table 1 Demographic and Clinical Characteristics of Subjects
Analyses of Associated Factors of Thyroid Function
Among the categories of thyroid disorders, low T3 syndrome (n = 50, 14.7%) was the most common form, followed by subclinical hyperthyroidism (n = 14, 4.1%), hypothyroidism (n = 6, 1.8%) and subclinical hypothyroidism (n = 2, 0.6%). Distribution of thyroid status and the corresponding thyroid hormone levels were shown in . Higher prevalence of TD was found in patients over 60 years old (n = 32, 27.4%) than in younger patients (n = 40, 17.2%) (P = 0.044). We found a lower level of FT3 (3.08 (1.05) pmol/L) in patients with DK or DKA than patients without DK or DKA (3.73 (0.81) pmol/L) (P < 0.001). Moreover, the level of FT3 further decreased with the deterioration of DK (2.41 (1.10) pmol/L) (P < 0.001). The level of FT4 (12.54 (3.64) pmol/L) was also significantly lower in patients with DKA (P = 0.004). But no significant difference was shown between patients with DK (14.06 (4.38) pmol/L) and patients without DK (14.38 (3.19) pmol/L) in FT4 level (Supplementary Table S1). Lower level of FT3 (2.99 (1.42) pmol/L) was also found in patients with DN (P < 0.001), accompanied with lower levels of FT4 and TSH, compared with patients with normoalbuminuria. The levels of FT3 and FT4 were lower in patients over 60 years old (FT3 3.40 (1.04) pmol/L, FT4 14.08 (2.80) pmol/L) than in patients with younger age (FT3 3.66 (1.02) pmol/L, FT4 14.55 (3.53) pmol/L) (P = 0.001, P = 0.016), respectively). Spearman correlation analysis revealed negative factors of FT3 level including DK or DKA, DN, age and HbA1c. Positive correlated factors of FT3 level included BMI, eGFR, diastolic BP (DBP), high-density lipoprotein cholesterol (HDL-C), fasting C-peptide, 2-h C-peptide, HOMA-IR and HOMA-β. Age and carotid atherosclerosis were indicated to be negative correlated factors of FT4. DK or DKA, DN and HbA1c remained to be negative correlated factors of FT3/FT4 ratio. Except for BMI, no other metabolic or demographic parameter was found to be strongly associated with TSH level ().
Table 2 Distribution of Thyroid Status
Table 3 Correlation Between Thyroid Function and Demographic and Metabolic Parameters
Analyses of Associated Factors of DN
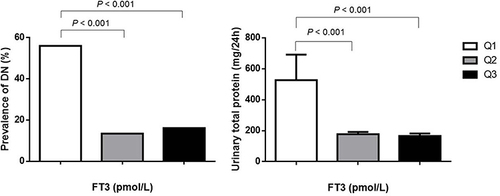
Comparisons between patients with DN and patients with normoalbuminuria was shown in Supplementary Table S2. FT3 level (2.99 (1.42) vs 3.64 (0.81), P < 0.001) and FT3/FT4 ratio (0.22 (0.06) vs 0.26 (0.05), P < 0.001) was significantly lower in patients with DN. Furthermore, there was a decline of FT3 level with progressing albuminuria. FT3 level was inversely correlated with the level of urinary total protein (mg/24h) and the presence of DN (, ). After adjusting various confounding factors, multivariate analysis indicated low FT3 level as a strong independent risk factor (OR = 0.364, P = 0.001) for DN (). Considering the possible impact of acute conditions on renal function, further multivariate analysis was performed by excluding the patients with DK or DKA. Decreased FT3 remained to be strongly correlated with the presence of DN (OR = 0.228, P < 0.001) (Supplementary Table S3).
Table 4 Multivariate Analysis of Associated Factors of Diabetic Nephropathy in Patients with Newly Diagnosed T2DM
Figure 1 Prevalence of DN and levels of urinary total protein in different FT3 quartiles. Quartile 1 (Q1, n = 109), 1.54 to < 3.26 pmol/L; Quartile 2 (Q2, n = 119), 3.26 to < 3.88 pmol/L; Quartile 3 (Q3, n = 112), 3.88 to ≤ 7.18 pmol/L.
Discussion
In this study, lower levels of thyroid hormones and a higher prevalence of TD were found in patients with diabetes as compared to the control group, which is in accordance with previous studies.Citation16,Citation17 The prevalence of TD varied in patients with DM in different regions, ranging from 4% to over 20%.Citation2 The differences can be explained by the large population diversity, the varied degree of iodine intake, different diagnostic criteria of TD and different sensitivities of laboratory assays.Citation11 Subclinical hypothyroidism or hypothyroidism was reported to be the most common form of TD in several studies.Citation18–21 No significant differences were found in the prevalence or incidence of TD by diabetes type in community-based study or study recruiting outpatients.Citation22–24 Compared with T2DM, the association between type 1 DM (T1DM) and autoimmune thyroid diseases was stronger.Citation22,Citation23 Studies recruiting T1DM patients with DKA showed higher prevalence of low T3 syndrome and hypothyroidism.Citation8,Citation22–24 In this study, hospitalized patients with newly diagnosed T2DM were included and a relatively high prevalence of TD was found, among which low T3 syndrome constituted the majority.
Low T3 syndrome, also known as euthyroid sick syndrome (ESS) or nonthyroidal illness syndrome (NTIS), was initially described in the 1970s. It represents a state of alterations in thyroid hormone economy, which usually present in critically ill patients.Citation25 Low T3 syndrome is characterized by decreased serum T3 and T4 concentrations, increased serum reverse T3 (rT3) concentrations and unaltered or inappropriately low serum TSH.Citation26 Complicated mechanisms were involved in its pathogenesis, including downregulation of TRH and TSH production, changes in thyroid hormone metabolism and inhibitory effect of cytokines on the thyroid gland.Citation27 The presence of low T3 syndrome is a predictor of poor prognosis of acute or chronic illnesses. The high prevalence of low T3 syndrome in this study was comparable to the one in patients with advanced kidney disease reported by Peters et al.Citation28 Actually, low T3 syndrome is often overseen and therefore underdiagnosed in clinical practice. Since it was considered as a reflection of underlying diseases rather than an abnormality of thyroid status, it was not included for discussion in many studies investigating thyroid dysfunction. Nevertheless, low FT3 and FT4 levels were found to be associated with various alterations except their anticipated relationships with acute conditions.
As predicted, DK or DKA was found to be closely related to low T3 syndrome in the present study. Previous studies in DKA mainly focused on T1DM, especially on pediatric patients with T1DM, and showed similar results. The high prevalence of low T3 syndrome, which is comparable to previous studies in T1DM, may be explained by the relatively high proportions of patients with DK or DKA (29.7%) in this study. TD including low T3 syndrome and hypothyroidism was more common in patients with DKA.Citation8,Citation29–31 The presence of low T3 syndrome was associated with poor glycemic controlCitation3,Citation21 and free thyroid hormones were correlated with the severity of DKA,Citation29 which was in accordance with our findings. The decreased thyroid hormones usually could increase to normal soon after correction of DKA.Citation8,Citation30
The relationship between thyroid hormones and DN is becoming a concern these years. A study in euthyroid subjects with T2DM showed that low levels of thyroid hormones (FT3 and FT4) were associated with DN.Citation9 The prevalence of kidney disorders in patients with T2DM increased with decreasing FT3 level.Citation32 DN was a risk factor of TD in patients with T2DM.Citation18,Citation19 High levels of TSH and low levels of FT3 were observed in T2DM patients with DN.Citation33 Moreover, high levels of TSH and/or low levels of FT3 were associated with more severe proteinuria, renal insufficiency and glomerular lesions in patients with DN.Citation32,Citation34 We also observed that FT3 and FT4 were positively associated with eGFR levels. Patients with DN demonstrated lower FT3 level and FT3/FT4 ratio. The presence of DN was significantly associated with decreased FT3, even within the normal range.Citation35 Low FT3 level was an independent risk factor for incidence and progression of DN in patients with T2DM.Citation36 A prior study in adult euthyroid patients with T1DM showed that higher FT3 level was related to lower prevalence of microangiopathy and better metabolic control,Citation37 which further supported our findings. The exact mechanisms are not fully elucidated. Thyroid hormones play important roles in the growth, development and physiology of kidneys, and also, in maintaining vascular and endothelial functions.Citation38,Citation39 It was found that T3 increases phosphatidylinositol 3-kinase (PI3K), reduces expression of transforming growth factor β1 (TGF-β1), improves structurally damaged kidneys, and reduces albuminuria.Citation40 TD including subclinical clinical hypothyroidism and low T3 syndrome is involved in the impairment of vascular function and damage of endothelial dilatation function, which may be associated with the pathogenesis of DN.Citation41 Also, alterations in thyroid status, especially lower FT3 levels and/or elevated TSH levels, are associated with worse endothelial function in patients with advanced chronic kidney disease or end-stage renal disease.Citation42
Other diabetic microvascular complications, such as DR and DPN, were also found to be correlated with thyroid disorders in diabetes.Citation43 Subclinical hypothyroidism was the most common reported type of TD to be involved in previous studies. Cross-sectional studies showed that subclinical hypothyroidism was highly prevalent in patients with DR and DPN, and was closely related to their severity.Citation44–46 Low FT3 within the normal range was also indicated to be independently associated with DRCitation10 and DPNCitation47 in euthyroid patients with T2DM in recently researches. But notably, the relationships between thyroid hormone levels and diabetic microvascular complications such as DR were found to be weak or negative in some researchesCitation48 and there is a lack of large-scale prospective clinical research on the impact of thyroid on the occurrence and course of diabetic chronic complications.Citation49 In the present study, we did not find positive relationships. But since fundus examinations were performed only for patients with corresponding symptoms, the incidence of other diabetic microvascular complications might be underestimated.
Compared with diabetic microvascular complications and acute complications, the evidences of the association between thyroid hormone and diabetic macrovascular complications are more limited and inconsistent. High TSH levels were shown to be related to higher incidence or risk of cardiovascular diseases, especially in patients with obesity.Citation50 Total thyroid hormones were not indicated to be independent risk factors of cardiovascular events in patients with T2DM in a cross-sectional study.Citation51 Another retrospective study in euthyroid patients with T2DM reported that low but clinically normal free thyroid hormones was associated with elevated risk of diabetic macrovascular complications.Citation52 We also found that the prevalence of carotid atherosclerosis tended to elevate along with the decrease of FT3 and FT4 levels (Supplementary Figure S1). But systemic measurements of macrovascular complications were not performed in the present study. Further investigations are needed to clarify these relationships.
Levels of thyroid hormones were also suggested to be associated with some metabolic and demographic parameters. Insulin resistance was reported to play a critical role in the connection between TD and T2DM.Citation6 A study in individuals without diabetes demonstrated that low T3 levels were significantly associated with decreased HOMA-IR, which indicated an association of thyroid function with insulin resistance.Citation53 Another study in euthyroid overweight/obese individuals indicated that increased FT3 was independently associated with higher risks of insulin resistance.Citation54 FT3 and FT4 positively and negatively correlated with HOMA-IR and atherogenic lipid profiles, respectively, in a euthyroid population with obesity.Citation55 In euthyroid subjects, serum FT4 was negatively associated with and TSH was positively associated with insulin resistance. Also, FT4 was associated with risk of metabolic syndrome.Citation56 TSH and thyroid hormones were found to correlate with multiple cardiometabolic risk factors, with age- and sex-independent effects on cholesterol and glucose metabolism, both in adults and in children with diabetes.Citation57,Citation58 We also found some relationships between thyroid hormones and metabolic parameters including HOMA-IR, HOMA-β, HbA1c, serum C-peptide, and HDL-C levels. In some studies, obesity was also a risk factor of TD.Citation16,Citation17 Both FT3 and FT4 levels were positively correlated with BMI in euthyroid subjects with obesity.Citation56 Higher FT3 concentration correlated positively with markers of obesity such as BMI in euthyroid T1DM patients.Citation34 We did not find significant difference in levels of thyroid hormones between patients with obesity (BMI ≥ 28 kg/m2) and patients with relatively normal BMI values. But BMI was indicated to be positively correlated with FT3 and TSH levels. A large population-based study demonstrated that elevated TSH level within the normal range was a risk marker associated with a series of cardiometabolic changes including central obesity, insulin resistance, elevated BP, dyslipidemia, hyperuricemia, inflammation and hypercoagulability.Citation59 But in this study, except for BMI, we did not find significant relationship between TSH and other metabolic parameters.
Compared with T3 and T4, TSH seems to be a more reliable indicator of thyroid status. As aforementioned, lower T3 levels may be more indicative of underlying ill health and metabolic disorders rather than the dysregulation of thyroid function. However, the positive relationships between thyroid hormones and other complications or alterations were most commonly seen regarding T3, and sometimes T4, rather than TSH, which indicate a reverse-causation. It was reported that impaired kidney function, both eGFR and proteinuria, were associated with low FT3 and FT4 but not TSH in patients with advanced chronic kidney diseases.Citation28 In this study, urinary protein excretion was also found to increase along with the decrease of FT3 and FT4 levels (Supplementary Figure S2). But no significant difference was shown in TSH levels. Notably, there were inconsistent findings concerning with the changes of FT4. In several studies, lower FT3 and elevated FT4 concentrations were found to be linked with impaired kidney functions.Citation32,Citation60 Complicated pathogenesis mechanisms may be involved and the impact of diabetes and the associated disorders on thyroid status need to be considered in these situations. In regard to the relationships between diabetes and thyroid autoantibodies levels or autoimmune thyroid disease, the results were also inconsistent. Previous studies suggested that thyroid autoantibodies levels or autoimmune thyroid disease were not found to be significantly related with DN, both in patients with T1DMCitation61 and T2DM,Citation35 which was consistent with our finding. But a recent study in newly diagnosed T2DM patients with Hashimoto’s thyroiditis and euthyroidism showed that high TPOAb level was an independent risk factor of albuminuria.Citation62
Advanced age, long duration of diabetes and poor glycemic control were commonly indicated to be risk factors of low levels of FT3 and presence of TD in patients with T2DM.Citation16,Citation17 And the abnormalities seemed to be reversed upon restoration of metabolic control.Citation63 We also discovered higher prevalence of TD or low T3 syndrome in patients over 60 years old and patients with higher glycemic levels. TD was reported to be more common in female as compared to male patients with T2DM in many studies.Citation19,Citation64,Citation65 However, no gender difference was indicated in our study. This may be partly attributed to the different inclusion criteria. Most studies did not include low T3 syndrome as a form of TD. This may also explain the relatively higher prevalence of TD (21.2%) in our study since low T3 syndrome contributed over 50% of the disorders. Furthermore, subjects in the present study were admitted in ward for treatment of diabetes. The conditions of patients, particularly glycemic control, were generally worse than the ones in outpatient clinics. Actually, most subjects in our study had a HbA1c level over 10%. This may also contribute to the high prevalence of TD.
There are several limitations of the present study. First, only limited number of patients in a single center were involved. The samples were derived from an inpatient setting and most patients were in poor glycemic control. Therefore, they may not be representative of the true population newly diagnosed with T2DM. Researches involving large numbers of outpatients or community-based studies are needed to confirm our findings. Second, since the patients were not followed up for thyroid tests after hospital discharge, whether the abnormalities of thyroid function could get resolved with remission of diabetic conditions remains undefined. Third, the present study did not evaluate the iodine status which might influence FT4/FT3 ratio and TSH level. Fourth, chronic diabetic complications besides DN were not comprehensively assessed. Lastly, due to the cross-sectional nature of this study, definite cause-and-effect relationships between TD and other abnormalities or factors could not be established. Therefore, our results should be interpreted with caution.
Conclusion
The present study showed that TD was not rare in hospitalized patients with newly diagnosed T2DM. Low T3 syndrome was the most common subtype. Low FT3 level was strongly associated with the presence of diabetic complications including DK/DKA and DN. Metabolic and demographic factors, including age, glycemic control and insulin resistance also correlated with levels of thyroid hormones. In the future, large prospective studies are needed to further investigate the prevalence of TD and to determine the association between thyroid hormones and diabetes-related conditions, especially diabetic complications.
Data Sharing Statement
The data used to support the findings of this study are included within the article. All the data related to this work are available from the corresponding author upon reasonable request.
Disclosure
All authors have no conflicts of interest in this work.
Additional information
Funding
References
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;2013:390534. doi:10.1155/2013/390534
- Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res. 2011;2011:439463. doi:10.4061/2011/439463
- Vondra K, Vrbikova J, Dvorakova K. Thyroid gland diseases in adult patients with diabetes mellitus. Minerva Endocrinol. 2005;30(4):217–236.
- Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev. 2019;40(3):789–824. doi:10.1210/er.2018-00163
- Dueñas O, Burgh A, Ittermann T, et al. Thyroid function and the risk of prediabetes and type 2 diabetes. J Clin Endocrinol Metab. 2022;2022:dgac006. doi:10.1210/clinem/dgac006
- Hussein S, AbdElmageed R. The relationship between type 2 diabetes mellitus and related thyroid diseases. Cureus. 2021;13(12):e20697. doi:10.7759/cureus.20697
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine. 2019;98(20):e15631. doi:10.1097/MD.0000000000015631
- Rashidi H, Ghaderian SB, Latifi SM, Hoseini F. Impact of diabetic ketoacidosis on thyroid function tests in type 1 diabetes mellitus patients. Diabetes Metab Syndr. 2017;11(Suppl 1):S57–S59. doi:10.1016/j.dsx.2016.12.001
- Wang J, Li H, Tan M, et al. Association between thyroid function and diabetic nephropathy in euthyroid subjects with type 2 diabetes mellitus: a cross-sectional study in China. Oncotarget. 2019;10(2):88–97. doi:10.18632/oncotarget.26265
- Zou J, Li Z, Tian F, et al. Association between normal thyroid hormones and diabetic retinopathy in patients with type 2 diabetes. Biomed Res Int. 2020;2020:8161797. doi:10.1155/2020/8161797
- Zhang S, Feng G, Kang F, et al. Hypothyroidism and adverse endpoints in diabetic patients: a systematic review and meta-analysis. Front Endocrinol. 2020;10:889. doi:10.3389/fendo.2019.00889
- Chinese Diabetes Society. Guideline for Type 2 Diabetes (2010). Beijing: Beijing University Medical Press; 2011.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi:10.7326/0003-4819-130-6-199903160-00002
- Li X, Zhou ZG, Qi HY, Chen XY, Huang G. Replacement of insulin by fasting C-peptide in modified homeostasis model assessment to evaluate insulin resistance and islet beta cell function. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29(4):419–423.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi:10.2337/dc09-9032
- Khassawneh AH, Al-Mistarehi A, Alaabdin AMZ, et al. Prevalence and predictors of thyroid dysfunction among type 2 diabetic patients: a case-control study. Int J Gen Med. 2020;13:803–816. doi:10.2147/IJGM.S273900
- Qin K, Zhang F, Wu Q, et al. Thyroid hormone changes in euthyroid patients with diabetes. Diabetes Metab Syndr Obes. 2020;13:2533–2540. doi:10.2147/DMSO.S260039
- Palma CC, Pavesi M, Nogueira VG, et al. Prevalence of thyroid dysfunction in patients with diabetes mellitus. Diabetol Metab Syndr. 2013;5(1):58. doi:10.1186/1758-5996-5-58
- Jali MV, Kambar S, Jali SM, Pawar N, Nalawade P. Prevalence of thyroid dysfunction among type 2 diabetes mellitus patients. Diabetes Metab Syndr. 2017;11(Suppl 1):S105–108. doi:10.1016/j.dsx.2016.12.017
- Ogbonna SU, Ezeani IU. Risk factors of thyroid dysfunction in patients with type 2 diabetes mellitus. Front Endocrinol. 2019;10:440. doi:10.3389/fendo.2019.00440
- Ozair M, Noor S, Raghav A, Siddiqi SS, Chugtai AM, Ahmad J. Prevalence of thyroid disorders in North Indian Type 2 diabetic subjects: a cross sectional study. Diabetes Metab Syndr. 2018;12(3):301–304. doi:10.1016/j.dsx.2017.12.016
- Peters KE, Chubb SP, Bruce D, Davis W, Davis TM. Prevalence and incidence of thyroid dysfunction in type 1 diabetes, type 2 diabetes and latent autoimmune diabetes of adults: the Fremantle Diabetes Study Phase II. Clin Endocrinol. 2020;92(4):373–382. doi:10.1111/cen.14164
- Tudor RM, Garrahy A, Woods C, et al. The prevalence and incidence of thyroid dysfunction in patients with diabetes – a longitudinal follow-up study. Ir J Med Sci. 2020;189(1):171–175. doi:10.1007/s11845-019-02082-9
- Barmpari M, Kokkorou M, Micheli A, et al. Thyroid dysfunction among Greek patients with type 1 and type 2 diabetes mellitus as a disregarded comorbidity. J Diabetes Res. 2017;2017:6505814. doi:10.1155/2017/6505814
- Mebis L, Van den Berghe G, Van den Berghe G. Thyroid axis function and dysfunction in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25(5):745–757. doi:10.1016/j.beem.2011.03.002
- Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol. 1993;39(5):499–518. doi:10.1111/j.1365-2265.1993.tb02401.x
- de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol. 2015;225(3):R67–81. doi:10.1530/JOE-15-0133
- Peters J, Roumeliotis S, Mertens P, Liakopoulos V. Thyroid hormone status in patients with impaired kidney function. Int Urol Nephrol. 2021;53(11):2349–2358. doi:10.1007/s11255-021-02800-2
- Hu YY, Li GM, Wang W. Euthyroid sick syndrome in children with diabetic ketoacidosis. Saudi Med J. 2015;36(2):243–247. doi:10.15537/smj.2015.2.10304
- Yang W, Sheng F. Factors associated with thyroid dysfunction in children with newly diagnosed type 1 diabetes mellitus. Minerva Pediatr. 2021;73(4):324–329. doi:10.23736/S2724-5276.19.05484-7
- Fatourechi A, Ardakani HM, Sayarifard F, Sheikh M. Hypothyroidism among pediatric patients with type 1 diabetes mellitus, from patients’ characteristics to disease severity. Clin Pediatr Endocrinol. 2017;26(2):73–80. doi:10.1297/cpe.26.73
- Chen Y, Zhang W, Wang N, et al. Thyroid parameters and kidney disorder in type 2 diabetes: result from the METAL Study. J Diabetes Res. 2020;2020:4798947. doi:10.1155/2020/4798947
- Zhao W, Li X, Liu X, Lu L, Gao Z. Thyroid function in patients with type 2 diabetes mellitus and diabetic nephropathy: a single center study. J Thyroid Res. 2018;2018:9507028. doi:10.1155/2018/9507028
- Han Q, Zhang J, Wang Y, et al. Thyroid hormones and diabetic nephropathy: an essential relationship to recognize. Nephrology. 2019;24(2):160–169. doi:10.1111/nep.13388
- Zou J, Tian F, Zhang Y, et al. Association between thyroid hormone levels and diabetic kidney disease in euthyroid patients with type 2 diabetes. Sci Rep. 2018;8(1):4728. doi:10.1038/s41598-018-22904-7
- Yang Z, Duan P, Li W, et al. The correlation between thyroid hormone levels and the kidney disease progression risk in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2022;15:59–67. doi:10.2147/DMSO.S347862
- Falkowski B, Rogowicz-Frontczak A, Grzelka A, et al. Higher free triiodothyronine concentration is associated with lower prevalence of microangiopathic complications and better metabolic control in adult euthyroid people with type 1 diabetes. Endocrine. 2018;60(3):458–465. doi:10.1007/s12020-018-1582-8
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2019;160(4):503–515. doi:10.1530/EJE-08-0837
- Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23(1):22–26. doi:10.1681/ASN.2010070766
- Lin Y, Sun Z. Thyroid hormone ameliorates diabetic nephropathy in a mouse model of type II diabetes. J Endocrinol. 2011;209(2):185–191. doi:10.1530/JOE-10-0340
- Schalkwijk CG, Stehouwer CDA. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci. 2005;109(2):143–159. doi:10.1042/CS20050025
- You AS, Budoff M, Zeb I, et al. Elevated serum thyrotropin levels and endothelial dysfunction in a prospective hemodialysis cohort. Hemodial Int. 2021;26(1):57–65. doi:10.1111/hdi.12964
- Yang JK, Liu W, Shi J, Li YB. An association between subclinical hypothyroidism and sight-threatening diabetic retinopathy in type 2 diabetic patients. Diabetes Care. 2010;33(5):1018–1020. doi:10.2337/dc09-1784
- El-Sehrawy AA, Elkhamisy EM, Badawi AE. Subclinical hypothyroidism in patients with diabetic retinopathy: role of vascular endothelial growth factor. Endocr Metab Immune Disord Drug Targets. 2021;21. doi:10.2174/1871530321666210809151258
- Reddy N, Pradeep T, Tirupati S, Sarathi V, Kumar D. Thyroid dysfunction and its association with microvascular complications in patients with type 2 diabetes mellitus in south India. Diabetes Metab Syndr. 2020;14(4):615–617. doi:10.1016/j.dsx.2020.05.005
- Allam MA, Nassar YA, Shabana HS, et al. Prevalence and clinical significance of subclinical hypothyroidism in diabetic peripheral neuropathy. Int J Gen Med. 2021;14:7755–7761. doi:10.2147/IJGM.S337779
- Li MF, Ke JF, Li S, Wang JW, Zhu ZH, Li JB. Serum free triiodothyronine is inversely associated with diabetic peripheral neuropathy but not with carotid atherosclerotic lesions in euthyroid patients with type 2 diabetes. Diabetol Metab Syndr. 2021;13(1):142. doi:10.1186/s13098-021-00760-2
- Ramis JN, Artigas CF, Santiago MAA, Mañes FJV, Canonge RS, Comas LM. Is there a relationship between TSH levels and diabetic retinopathy in the Caucasian population? Diabetes Res Clin Pract. 2012;97(3):e45–47. doi:10.1016/j.diabres.2012.05.015
- tefanowicz-Rutkowska M, Baranowska-Jurkun A, Matuszewski W, Bandurska-Stankiewicz E. Thyroid dysfunction in patients with diabetic retinopathy. Endokrynol Pol. 2020;71(2):176–183. doi:10.5603/EP.a2020.0013
- Gomez-Zamudio JH, Mendoza-Zubieta V, Ferreira-Hermosillo A, et al. High thyroid-stimulating hormone levels increase proinflammatory and cardiovascular markers in patients with extreme obesity. Arch Med Res. 2016;47(6):476–482. doi:10.1016/j.arcmed.2016.10.007
- Zantut-Wittmann DE, Parisi MCR, Tambascia MA, Pavin EJ, Alegre SM, Zantut-Wittmann DE. Relationship of thyroid hormone levels and cardiovascular events in patients with type 2 diabetes. Endocrine. 2014;45(1):84–91. doi:10.1007/s12020-013-9938-6
- Hu Y, Yan Z, Pan C. Associations of thyroid hormone levels and macrovascular complications in euthyroid type 2 diabetic patients. Diabetes Metab Syndr Obes. 2021;14:2683–2691. doi:10.2147/DMSO.S313803
- Wang CY, Yu TY, Shih SR, Huang KC, Chang TC. Low total and free triiodothyronine levels are associated with insulin resistance in non-diabetic individuals. Sci Rep. 2018;8(1):10685. doi:10.1038/s41598-018-29087-1
- Ma D, Zeng J, Huang B, et al. Independent associations of thyroid-related hormones with hepatic steatosis and insulin resistance in euthyroid overweight/obese Chinese adults. BMC Gastroenterol. 2021;21(1):431. doi:10.1186/s12876-021-02011-0
- Temizkan S, Balaforlou B, Ozderya A, et al. Effects of thyrotrophin, thyroid hormones and thyroid antibodies on metabolic parameters in a euthyroid population with obesity. Clin Endocrinol. 2016;85(4):616–623. doi:10.1111/cen.13095
- Mehran L, Amouzegar A, Tohidi M, Moayedi M, Azizi F. Serum free thyroxine concentration is associated with metabolic syndrome in euthyroid subjects. Thyroid. 2014;24(11):1566–1574. doi:10.1089/thy.2014.0103
- Le TN, Celi FS, Wickham EP. 3rd Thyrotropin levels are associated with cardiometabolic risk factors in euthyroid adolescents. Thyroid. 2016;26(10):1441–1449. doi:10.1089/thy.2016.0055
- Yuan CJ, Sun XM, Liu Y, Wu J. The thyroid hormone levels and glucose and lipid metabolism in children with type 1 diabetes: a correlation analysis. Transl Pediatr. 2021;10(2):276–282. doi:10.21037/tp-20-204
- Chang YC, Hua SC, Chang CH, et al. High TSH levels within normal range is associated with obesity, dyslipidemia, hypertension, inflammation, hypercoagulability, and the metabolic syndrome: a novel cardiometabolic marker. J Clin Med. 2019;8(6):817. doi:10.3390/jcm8060817
- Schultheiss UT, Daya N, Grams ME, et al. Thyroid function, reduced kidney function and incident chronic kidney disease in a community-based population: the Atherosclerosis Risk in Communities study. Nephrol Dial Transplant. 2017;32(11):1874–1881. doi:10.1093/ndt/gfw301
- Stefanowicz-Rutkowska MM, Matuszewski W, Gontarz-Nowak K, Bandurska-Stankiewicz EM. Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease? Open Life Sci. 2021;16(1):611–619. doi:10.1515/biol-2021-0064
- Zhu W, Dong X, Pan Q, Hu Y, Wang G. The association between albuminuria and thyroid antibodies in newly diagnosed type 2 diabetes mellitus patients with Hashimoto’s thyroiditis and euthyroidism. BMC Endocr Disord. 2020;20(1):172. doi:10.1515/biol-2021-0064
- Moura Neto A, Zantut-Wittmann DE. Abnormalities of thyroid hormone metabolism during systemic illness: the low T3 syndrome in different clinical settings. Int J Endocrinol. 2016;2016:2157583. doi:10.1155/2016/2157583
- Papazafiropoulou A, Sotiropoulos A, Kokolaki A, Kardara M, Stamataki P, Pappas S. Prevalence of thyroid dysfunction among Greek type 2 diabetic patients attending an outpatient clinic. J Clin Med Res. 2010;2(2):75–78. doi:10.4021/jocmr2010.03.281w
- Zhu Y, Xu F, Shen J, et al. Prevalence of thyroid dysfunction in older Chinese patients with type 2 diabetes-A multicenter cross-sectional observational study across China. PLoS One. 2019;14(5):e0216151. doi:10.1371/journal.pone.0216151
