Abstract
Dipeptidyl peptidase-4 (DPP-4) inhibitors have recently emerged as a new class of antidiabetic that show favorable results in improving glycemic control with a minimal risk of hypoglycemia and weight gain. Teneligliptin, a novel DPP-4 inhibitor, exhibits a unique structure characterized by five consecutive rings, which produce a potent and long-lasting effect. Teneligliptin is currently used in cases showing insufficient improvement in glycemic control even after diet control and exercise or a combination of diet control, exercise, and sulfonylurea- or thiazolidine-class drugs. In adults, teneligliptin is orally administered at a dosage of 20 mg once daily, which can be increased up to 40 mg per day. Because the metabolites of this drug are eliminated via renal and hepatic excretion, no dose adjustment is necessary in patients with renal impairment. The safety profile of teneligliptin is similar to those of other available DPP-4 inhibitors. However, caution needs to be exercised when administering teneligliptin to patients who are prone to QT prolongation. One study has reported that the postprandial blood glucose-lowering effects of teneligliptin administered prior to breakfast were sustained throughout the day, and the effects observed after dinner were similar to those observed after breakfast or lunch. Thus, although clinical data for this new drug are limited, this drug shows promise in stabilizing glycemic fluctuations throughout the day and consequently suppressing the progression of diabetic complications. However, continued evaluation in long-term studies and clinical trials is required to evaluate the efficacy and safety of the drug as well as to identify additional indications for its clinical use.
Keywords:
Introduction
Incretin hormones,Citation1–Citation3 namely glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are released from enteroendocrine cells and enhance insulin secretion.Citation1,Citation2,Citation4–Citation6 Incretins are rapidly inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4), and have a very short half-life (t1/2) as a result. DPP-4 inhibitors increase the levels of active GLP-1 and GIP by inhibiting DPP-4 enzymatic activity; thus, in patients with diabetes, these inhibitors improve hyperglycemia in a glucose-dependent manner by increasing serum insulin levels and decreasing serum glucagon levels.Citation7–Citation13 Therefore, incretin-related agents such as DPP-4 inhibitors are promising drugs that can decrease glucose fluctuations in diabetic patients and have emerged as a new class of antidiabetic. The effect of these inhibitors on glycemic control when administered as monotherapy or in combination with other drugs has been investigated in multiple trials.Citation7,Citation8,Citation11,Citation12 Moreover, DPP-4 inhibitors have shown favorable results in improving glycemic control with a minimal risk of hypoglycemia and weight gain.Citation14–Citation19
In this review, a novel chemotype prolylthiazolidine-based DPP-4 inhibitor, teneligliptin (generic name: teneligliptin hydrobromide hydrate) is characterized. Teneligliptin was originally synthesized by Mitsubishi Tanabe Pharma Corporation (Osaka, Japan) and was the first drug of its kind to be synthesized in Japan. Mitsubishi Tanabe Pharma Corporation and Daiichi Sankyo Co, Ltd, (Tokyo, Japan) jointly sell the drug under the brand name TENERIA®.
Chemistry of teneligliptin
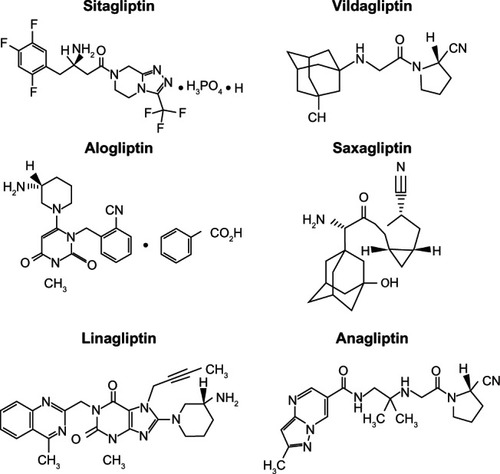
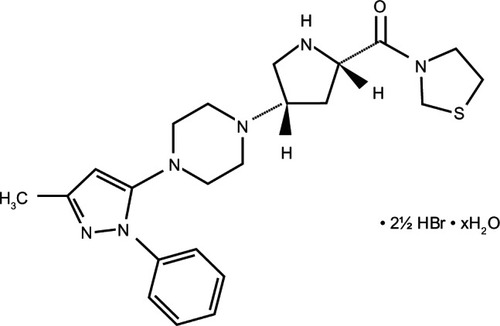
Despite their common mechanism of action, DPP-4 inhibitors show marked structural heterogeneity ().Citation20 DPP-4 inhibitors may be classified into peptidomimetic (ie, sitagliptin, vildagliptin, saxagliptin, and anagliptin) and non-peptidomimetic (ie, alogliptin and linagliptin) subtypes. Teneligliptin, {(2S,4S)-4-[4-(3-methyl-1-phenyl-1H- pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-yl} (1,3-thiazolidin-3-yl) methanone hemipentahydrobromide hydrate exhibits a unique structure that is characterized by five consecutive rings ()Citation21 and is peptidomimetic. An X-ray co-crystal structure of teneligliptin with DPP-4 demonstrates that the key interaction occurs between the phenyl ring on the pyrazole and the S2 extensive subsite of DPP-4, which not only enhances the potency of the drug but also increases its selectivity.Citation21
According to the product information provided by the pharmaceutical company, teneligliptin inhibits human plasma DPP-4 activity and recombinant human DPP-4 activity in a concentration-dependent manner with half-maximal inhibitory concentrations (IC50) of 1.75 (95% CI, 1.62–1.89) nmol/L and 0.889 (95% CI, 0.812–0.973) nmol/L, respectively. Furthermore, the IC50 values of teneligliptin for DPP-8, DPP-9, and fibroblast activation protein (FAP) are 0.189, 0.150, and >10 μmol/L, respectively, all of which are more than 160 times the value for recombinant human DPP-4.
Metabolism and excretion
CYP3A4, a cytochrome P450 isozyme and flavin-containing monooxygenases (FMO1 and FMO3) play major roles in the metabolism of teneligliptin. In vitro, teneligliptin exhibits a weak inhibitory effect for CYP2D6, CYP3A4, and FMO; however, it demonstrates no inhibitory effect for CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C8/9, CYP2C19, and CYP2E1. In addition, teneligliptin does not induce the expression of CYP1A2 or CYP3A4.
About 34.4% of teneligliptin is excreted unchanged via the kidney and the remaining 65.6% teneligliptin is metabolized and eliminated via renal and hepatic excretion; 216 hours after the administration ofCitation14 C-labeled teneligliptin (20 mg), the cumulative excretion percentages of radioactive teneligliptin in urine and feces were 45.4% and 46.5%, respectively.
Clinical use
Since September 2012, teneligliptin has been commercially sold in Japan and has been used for the treatment of type 2 diabetes mellitus when patients do not show sufficient improvement after diet control and exercise or a combination of diet control, exercise, and sulfonylurea- or thiazolidine-class drugs. In adults, 20 mg of teneligliptin may be orally administered once daily. If this dosage is insufficient, the dosage is increased to 40 mg once daily.
Effects of teneligliptin on glycemic parameters
Teneligliptin is still a relatively new drug, and published clinical studies concerning this drug are sparse. However, a report by Eto et alCitation22 provides important novel data, particularly the finding that teneligliptin significantly improved 24-hour blood glucose control in Japanese patients with type 2 diabetes. Hereafter, the results of this particular report are summarized.
Effects on blood glucose level
To assess blood glucose control over 24 hours and the safety of teneligliptin at 10 and 20 mg doses, a randomized, double-blind, placebo-controlled, parallel-group study was conducted at four locations in Japan.Citation22 Japanese patients with type 2 diabetes mellitus that was inadequately controlled with diet and exercise were eligible to participate in the study. Among the 99 patients who participated, 32 were treated with a placebo, 34 were treated with teneligliptin at a dose of 10 mg, and 33 were treated with teneligliptin at a dose of 20 mg before breakfast for 4 weeks. The results revealed that both teneligliptin-treated groups showed significantly smaller 2-hour postprandial glucose (PPG), 24-hour mean glucose (MG), and fasting plasma glucose (FPG) values than the placebo group when the values at week 4 were compared to the baseline values.
The differences in the changes in 2-hour PPG after each meal between the teneligliptin (10 mg) and placebo groups were −50.7 ± 7.8, −34.8 ± 9.2, and −37.5 ± 7.5 mg/dL at breakfast, lunch, and dinner, respectively (least squares [LS] mean ± standard error [SE], P < 0.001 for all comparisons). The corresponding LS means ± SE for teneligliptin (20 mg) versus the placebo were −38.1 ± 7.8, −28.6 ± 9.2, and −36.1 ± 7.5 mg/dL, respectively (P < 0.001, P < 0.01, and P < 0.001, respectively). Importantly, the postprandial blood glucose-lowering effects of teneligliptin administered before breakfast were sustained throughout the day, and the effects observed after dinner were similar to those observed after breakfast or lunch.
The changes in the 24-hour MG level from baseline were −34.7 ± 3.9, −30.9 ± 4.0, and −5.4 ± 4.0 mg/dL in the groups receiving 10 and 20 mg of teneligliptin and the placebo groups, respectively. Therefore, the differences between the teneligliptin-treated and placebo groups were −29.3 ± 5.3 and −25.5 ± 5.3 mg/dL for teneligliptin at doses of 10 and 20 mg, respectively (LS mean ± SE). These findings demonstrate that the 24-hour MG values significantly decreased in both teneligliptin-treated groups in comparison with the placebo group (both teneligliptin-treated groups, P < 0.001). In addition, when the 24–hour MG profiles were plotted, treatment with teneligliptin suppressed the increases in blood glucose levels over a 24-hour period in comparison with the effect of the placebo at week 4.
The changes in the FPG values from baseline were −20.7 ± 2.7, −20.5 ± 2.8, and −6.9 ± 2.8 mg/dL in the 10 mg and 20 mg groups of teneligliptin and the placebo groups, respectively. These results indicate the differences between the teneligliptin-treated and placebo groups, which were −13.8 ± 4.0 and −13.6 ± 4.0 mg/dL for teneligliptin at doses of 10 and 20 mg, respectively (LS mean ± SE). The decreases in the FPG values with teneligliptin at doses of 10 and 20 mg (both, P < 0.001) were statistically significant, compared with the placebo. However, there were no significant differences in the 2-hour PPG values after each meal, as well as in the 24-hour MG or FPG values between the 10 and 20 mg teneligliptin groups. These results indicate that the once-daily administration of teneligliptin before breakfast improved blood glucose control, even at dinnertime.
Effects on insulin
The area under the curve (AUC)0–2h values for the postprandial insulin levels significantly increased after dinner in the teneligliptin 10 mg group (P < 0.05), in comparison with the corresponding values in the placebo group, but not at other times in either group. The relative insulin concentrations were higher in the teneligliptin-treated groups because of the decreased blood glucose concentrations of the patients in these groups.
Effects on glucagon
In this study, the AUC0–2h for the postprandial glucagon levels significantly decreased after breakfast and lunch as well as after dinner in the 20 mg teneligliptin group compared with the corresponding values in the placebo group. In addition, there were no significant differences in the insulin or glucagon concentrations between the two teneligliptin-dosage groups, although glucagon secretion was lower with teneligliptin treatment at 20 mg, particularly after dinner. Thus, the study concluded that teneligliptin effectively suppressed postprandial glucagon secretion after meals and improved postprandial hyperglycemia.
Pharmacokinetic and pharmacodynamic properties of teneligliptin
The plasma concentrations of teneligliptin after the administration of teneligliptin at dosages of 10 or 20 mg once daily for 4 weeks revealed a median time to maximum concentration (Cmax) of 1.0 hour in both groups and a mean t1/2 of 20.8 and 18.9 hours, respectively.
The maximum percentage of the inhibition in plasma DPP-4 activity was achieved within 2 hours after administration and was 81.3% and 89.7% in the 10 and 20 mg teneligliptin groups, respectively.
The active GLP-1 concentration in the plasma in the 10 mg and 20 mg teneligliptin groups was higher than that in the placebo group throughout the day, even at 24 hours after administration. The AUC0–2h values for the active GLP-1 concentration after breakfast, lunch, and dinner were 8.0, 8.4, and 7.8 pmol ⋅ h/L, respectively, in the 10 mg teneligliptin group, and 8.3, 7.9, and 8.6 pmol ⋅ h/L, respectively, in the 20 mg teneligliptin group. Thus, the increase in AUC0–2h for the active GLP-1 concentration after dinner was slightly greater in the 20 mg teneligliptin group than in the 10 mg teneligliptin group. Differences in the AUC0–2h for the active GLP-1 concentration between both the teneligliptin-treated groups and the placebo group were statistically significant.
Safety and tolerability
The incidence of adverse events (AEs) was not significantly different between the teneligliptin and placebo groups in the study conducted by Eto et alCitation22 When AEs were rated by the investigators for intensity and potential relationship to the study drug, two drug-related AEs, increased levels of alanine aminotransferase and γ-glutamyltransferase, were observed in one patient (2.9%) treated with 10 mg of teneligliptin. No drug-related AEs occurred in the placebo or 20 mg teneligliptin groups. Furthermore, none of the patients in any of the groups experienced hypoglycemic symptoms or serious AEs. In addition, a pharmaceutical company provided information regarding domestic clinical studies that included 1183 patients, of which 118 patients (10.0%) experienced AEs, including abnormalities in clinical examination values such as levels of liver and kidney function, blood cell count, creatinine phosphokinase, and electrolytes. The main AEs included hypoglycemia (35 patients: 3.0%) and constipation (eleven patients: 0.9%). The pharmaceutical company also warned of serious AEs such as hypoglycemia, which could occur when other antidiabetic drugs were coadministered. In addition, they cautioned that intestinal obstruction could occur with an unknown frequency. GLP-1 is involved in gastrointestinal motility,Citation6 and the patients with intestinal obstruction had a past medical history of intestinal obstruction or abdominal surgery. Therefore, we should be cautious when administering incretin-related agents to patients with a history of these conditions. Continued assessment of AEs previously reported in clinical trials and post-market monitoring is required to determine the benefit/risk ratio for the drug.
According to a strict QT/QTc evaluation study and clinical studies for type 2 diabetes conducted in Japan and other countries, no AEs related to QT prolongation were detected with 40 mg/day of teneligliptin, which is the maximal dosage used in clinical practice. However, because when 160 mg/day of the drug was administered slight prolongation of the QTc interval was detected temporally at the high concentrations of the drug (around tmax level), and also because some patients with diabetes have comorbid arrhythmia or ischemic heart diseases, teneligliptin may be used for a longer period and thus special caution is required in the administration of teneligliptin to patients who are prone to QT prolongation. In addition, the coadministration of teneligliptin with drugs known to cause QT prolongation on their own, such as class IA or class III antiarrhythmic drugs, should be performed with caution.
Long-term effects on glycemic control
In an independent clinical study (Phase II trial) conducted in Japan three hundred twenty-four patients with type 2 diabetes who did not achieve optimal glycemic control with diet and exercise treatment alone for more than 12 weeks were randomly allotted into the following groups: placebo, 10 mg teneligliptin, 20 mg teneligliptin, or 40 mg teneligliptin. The drugs were administered once daily for 12 weeks. After 12 weeks, the HbA1c levels in the placebo group changed by +0.11% ± 0.05%, whereas those in the 10 mg teneligliptin, 20 mg teneligliptin, and 40 mg teneligliptin groups changed by −0.77% ± 0.05%, −0.80% ± 0.05%, and −0.91% ± 0.05%, respectively (LS mean ± SE). In a Phase III trial, 20 mg of teneligliptin was administered to 151 patients with type 2 diabetes who were treated with diet control and exercise treatment alone for more than 12 weeks. The dose of teneligliptin was increased to 40 mg in patients whose HbA1c levels were greater than 7.3% at any time after week 24. At week 52, changes in the HbA1c levels (mean ± standard deviation [SD]) were −0.63% ± 0.67%, compared to baseline.
Kadowaki and KondoCitation23 conducted a double-blind placebo-controlled parallel-group study in 324 Japanese patients with type 2 diabetes randomized to receive different doses of teneligliptin or placebo once daily before breakfast for 12 weeks. The differences between the teneligliptin 10, 20, or 40 mg groups and the placebo group for the changes in HbA1c levels were −0.9 (LS mean; 95% CI: −1.0, −0.7), −0.9 (−1.1, −0.7), and −1.0 (−1.2, −0.9)%, respectively (all, P < 0.001). The respective LS means for FPG were −17.8 (−23.4, −12.1), −16.9 (−22.6, −11.2), and −20.0 (−25.7, −14.3) mg/dL (all, P < 0.001). These results indicate that treatment with teneligliptin for 12 weeks provided significant and clinically meaningful reduction in the levels of HbA1c and FPG across the dose range studied.
Continued evaluation in long-term studies and clinical practice are required to assess the long-term effects of this drug.
Effects of teneligliptin on lipid profiles
The lipid profile is an important determinant of cardiovascular risk in type 2 diabetes. It can affect antidiabetic therapy and is important in the clinical management of patients with type 2 diabetes.Citation20,Citation24–Citation26 Meta-analyses suggested a potential beneficial effect of DPP-4 inhibitors on cholesterol, which could contribute to a reduction in cardiovascular risk.Citation16,Citation24 The administration of several DPP-4 inhibitors reduces postprandial triglyceride levels in humans, mice, and hamsters; however, its effects on postprandial free fatty acid levels are a matter of debate.Citation27–Citation30 Fukuda-Tsuru et alCitation31 previously reported that a single administration of teneligliptin after an oral fat-loading test in Zucker fatty rats showed that teneligliptin at 1 mg/kg reduced the levels of postprandial triglycerides and free fatty acids and also increased the levels of GLP-1 and insulin.Citation32,Citation33 GLP-1 inhibits the secretion of gastric lipaseCitation34 and reduces intestinal triglyceride absorption and apo B and apo A-IV production,Citation28,Citation35 and insulin suppresses lipolysis in adipose tissue, resulting in a reduction of the plasma free fatty acid levels;Citation36 therefore, the study speculated that the reduction in triglyceride and free fatty acid levels could be a consequence of the elevation of active GLP-1 and insulin levels. Furthermore, in Zucker fatty rats, the repeated administration of teneligliptin for 2 weeks reduced glucose excursions in an oral carbohydrate-loading test and decreased plasma triglyceride and free fatty acid levels under nonfasting conditions. Furthermore, these results were consistent with the findings of other previous reports on DPP-4 inhibitors in various rodent models such as db/db mice and streptozocin-induced diabetic rats.Citation37–Citation40
Teneligliptin and renal impairment
The single administration of teneligliptin at 20 mg in patients with renal impairment revealed no remarkable changes in Cmax and t1/2 corresponding to the level of renal impairment. Compared with healthy adult subjects, the AUC0–∞ of subjects with mild renal impairment (50 ≤ creatinine clearance [Ccr] ≤ 80 mL/minute), moderate renal impairment (30 ≤ Ccr < 50 mL/minute), and severe renal impairment (Ccr < 30 mL/minute) was approximately 1.25 times, 1.68 times, and 1.49 times higher than that of healthy adult subjects, respectively. In addition, the AUC0–43h of patients with end-stage renal failure was approximately 1.16 times higher than that of healthy adult subjects. In addition, 15.6% of the total administration dose of teneligliptin was eliminated via hemodialysis.
Teneligliptin and hepatic impairment
A single administration of teneligliptin 20 mg in patients with hepatic impairment revealed that the Cmax of subjects with mild hepatic impairment (Child-Pugh classification:Citation41 total score 5–6) and moderate hepatic impairment (Child–Pugh classification: total score 7–9) was approximately 1.25 times and 1.38 times that of healthy adult subjects, respectively. Compared to healthy adult subjects, the AUC0–∞ of subjects with mild and moderate hepatic impairments was approximately 1.46 times and 1.59 times higher than that of healthy adult subjects, respectively. There have been no previous clinical studies using teneligliptin in patients with severe hepatic impairment (Child–Pugh classification: total score was greater than 9). Thus, specific caution is required when the drug is administered to patients with severe hepatic impairment.
Influence on body weight
Studies on the effect of DPP-4 inhibitors on body weight demonstrated variable results; however, these results were generally considered to be neutral.Citation14,Citation16,Citation42–Citation44 In a Phase III trial, 20 mg of teneligliptin was administered to 151 patients with type 2 diabetes, who were previously treated with diet control and exercise treatment alone. The dose of teneligliptin was increased to 40 mg in patients with HbA1c levels greater than 7.3% at any time after week 24. The mean body weight change of the patients at week 52 (mean ± SD) was +0.18 ± 2.14 kg (P = 0.3254), which indicated that the effect of teneligliptin on body weight was neutral.
Gliptins in combination with other oral antidiabetic agents
Since DPP-4 inhibitors and metformin improve glycemic control via different, albeit potentially complementary, mechanisms, combination therapy with these two agents should provide effective and potentially additive glycemic control.Citation42 Studies using combination therapy of DPP-4 inhibitors and metformin (as one pill) showed favorable results in glycemic control because of favorable pharmacokinetic characteristics and complementary pharmacodynamic effects, which include enhanced incretin effect, suppressed hepatic glucose production, and improved peripheral insulin sensitivity. Moreover, in general, the combination of these drug into a single tablet improves patients’ compliance and often results in a lower cost of treatment.Citation14,Citation45,Citation46 Indeed several fixed-dose combinations have been developed and/or commercialized.Citation43,Citation45
Furthermore, α-glucosidase inhibitors (α-GIs) reportedly enhanced GLP-1 responses and reduced the total GIP responses.Citation47–Citation51 Considering the different but complementary mechanisms of action by which α-GIs and DPP-4 inhibitors lower glucose levels and increase GLP-1 action, combination therapy using these agents may provide a valuable means of treating diabetes. Using a continuous glucose-monitoring (CGM) system,Citation52,Citation53 we previously reported the efficacy of combination therapy using miglitol and sitagliptin in patients with type 2 diabetes.Citation54
Several trials have also evaluated the efficacy and safety of adding a DPP-4 inhibitor to sulfonylurea, such as glimepiride or glyburide (glibenclamide), and have indicated a significant improvement in glycemic control.Citation43,Citation55 Because hypoglycemic events may occur with the combination of DPP-4 inhibitors and sulfonylurea, caution should be taken to reduce the dose of sulfonylurea in order to minimize the risk of hypoglycemia.
When 20 mg of teneligliptin was administered to 96 patients with type 2 diabetes who were treated with glimepiride (1–4 mg per day) in addition to diet and exercise treatment, the changes in HbA1c levels and fasting and the 2-hour postprandial blood glucose levels (LS mean ± SE) were −0.71% ± 0.06%, −17.3 ± 2.2 mg/dL, and −43.1 ± 4.4 mg/dL, respectively, from baseline at week 12.
From a theoretical standpoint, combination of DPP-4 inhibitors, which stimulate insulin secretion in a glucose-dependent manner and do not promote weight gain, and thiazolidinedione, which enhances peripheral insulin action and may cause weight gain, is an attractive and rational approach.Citation43,Citation56–Citation58 When 20 mg of teneligliptin was administered to 103 patients with type 2 diabetes who were treated with pioglitazone (15–30 mg per day) in addition to diet and exercise treatment, the changes in HbA1c levels and fasting and 2-hour postprandial blood glucose levels (LS mean ± SE) were −0.94% ± 0.04%, −21.0 ± 1.9 mg/dL, and −56.9 ± 3.6 mg/dL, respectively, compared with that at baseline, at week 12.
Gliptin treatments in combination with insulin
The effectiveness of insulin injections has been previously established in various clinical studies; however, increasing the insulin dosage to achieve better glycemic control has been associated with an increased risk of hypoglycemia and weight gain.Citation59 To resolve these problems, combination therapy with insulin injection and oral medication is worth considering. Due to the different mechanisms and complementary effects of DPP-4 inhibitors and insulin, a combination of these agents would be a rational treatment option for insulin-treated patients. Favorable results with additional 0.3%–0.6% reductions in the HbA1c levels have been reported in patients treated with both insulin and DPP-4 inhibitors. These patients demonstrated a low rate of hypoglycemia and a slight weight reduction.Citation43,Citation55 We previously reported that the combination of sitagliptin and insulin therapy presented beneficial effects in stabilizing glycemic control by stimulating endogenous insulin secretion and suppressing glucagon secretion.Citation60 Vilsbøll et al previously reported in a 24-week study that the addition of sitagliptin to ongoing insulin therapy resulted in significant improvements in glycemic control, in comparison with the placebo treatment, by improving β-cell responsiveness.Citation61 The study concluded that, in patients with more advanced stages of the disease and β-cell failure, sitagliptin may still favorably affect glucose-dependent insulin secretion. Vildagliptin decreased HbA1c levels in patients with poorly controlled type 2 diabetes with high doses of insulin,Citation62 and vildagliptin has also been reported to be beneficial for decreasing post-meal glucagon excursion in insulinopenic patients with type 1 diabetes; this effect was not secondary to a change in endogenous insulin secretion.Citation63 Taken together, these reports encourage further study of the use of incretin-related agents in insulin-treated patients with type 2 or type 1 diabetes. Teneligliptin is not currently approved for coadministration with insulin; however, a combination therapy of teneligliptin and insulin is quite promising for these aforementioned reasons.
Case presentation
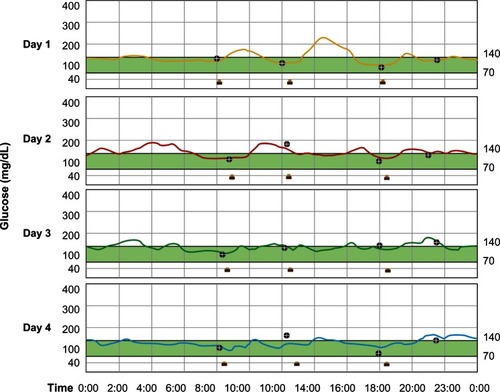
A 68-year-old Japanese woman with a 7-year history of type 2 diabetes presented at our hospital (National Center for Global Health and Medicine, Tokyo, Japan) to be equipped with a CGM device (iPro®2; Medtronic Ltd, Watford, UK).Citation64 Her height was 148.6 cm and her body weight was 59.2 kg (body mass index 26.8 kg/m2). Her hemoglobin A1c and fasting C-peptide levels were 6.6% and 3.3 ng/mL, respectively, and she was on a treatment of 20 mg of gliclazide. On the first day of the CGM period, the patient was taking gliclazide alone. From day 2 through day 4, 20 mg of teneligliptin was also taken, before breakfast, in addition to gliclazide. During this period, she did not change her lifestyle remarkably, including the timing and amounts of her meals and the levels of her daily physical activities. shows the fluctuations in glucose levels measured using CGM during treatment with gliclazide alone (day 1) and gliclazide plus teneligliptin (days 2 to 4). The average and SD values of the CGM measurements on days 1, 2, 3, and 4 were 142 ± 26, 145 ± 20, 135 ± 15, and 126 ± 16 mg/dL, respectively, reflecting the attenuation of glucose fluctuations when teneligliptin was added to gliclazide. These findings clearly demonstrate the efficacy of combinatorial therapy using sulfonylurea such as gliclazide and teneligliptin in patients with type 2 diabetes.
Figure 3 Continuous glucose monitoring results during treatment with gliclazide alone (day 1) and gliclazide plus teneligliptin (days 2 to 4).
Conclusion
Numerous clinical trials have demonstrated that DPP-4 inhibitors provide effective and consistent glycemic control with a good tolerability profile, including no severe hypoglycemia and weight gain.Citation7,Citation8,Citation11–Citation19,Citation42–Citation45 Although different DPP-4 inhibitors are distinctive in their metabolic properties, excretion, recommended dosage, and daily dosage, and head-to-head clinical trials comparing the various DPP-4 inhibitors are scarce, the available data regarding indirect comparisons suggest that all available DPP-4 inhibitors have nearly the same efficacy and safety profile.Citation14,Citation16,Citation43 Thus, we may expect a similar efficacy and safety with the novel DPP-4 inhibitor, teneligliptin, although this drug requires careful long-term postmarketing surveillance and additional clinical trials to evaluate its efficacy and safety as well as to gain additional indications for its clinical use.
Acknowledgment
The author thanks Dr Mitsuhiko Noda for his comments regarding this paper.
Disclosure
The author reports no conflicts of interest in this work.
References
- Drucker DJ The role of gut hormones in glucose homeostasis J Clin Invest 2007 117 24 32 17200703
- Meier JJ Nauck MA Incretins and the development of type 2 diabetes Curr Diab Rep 2006 6 194 201 16898571
- Holst JJ The physiology of glucagon-like peptide 1 Physiol Rev 2007 87 1409 1439 17928588
- Kreymann B Williams G Ghatel MA Bloom SR Glucagon-like peptide-1 7–36: a physiological incretin in man Lancet 1987 2 1300 1304 2890903
- McIntosh CH Widenmaier S Kim SJ Glucose-dependent insulinotropic polypeptide (Gastric Inhibitory Polypeptide; GIP) Vitam Horm 2009 80 409 471 19251046
- Seino Y Fukushima M Yabe D GIP and GLP-1, the two incretin hormones: similarities and differences J Diabet Invest 2010 1 8 23
- Herman GA Bergman A Stevens C Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes J Clin Endocrinol Metab 2006 91 4612 4619 16912128
- Karasik A Aschner P Katzeff H Davies MJ Stein PP Sitagliptin, a DPP-4 inhibitor for the treatment of patients with type 2 diabetes: a review of recent clinical trials Curr Med Res Opin 2008 24 489 496 18182122
- Xu L Man CD Charbonnel B Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach Diabetes Obes Metab 2008 10 1212 1220 18476982
- Brazg R Xu L Dalla Man C Cobelli C Thomas K Stein PP Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes Diabetes Obes Metab 2007 9 186 193 17300594
- Drucker DJ Nauck MA The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes Lancet 2006 368 1696 1705 17098089
- Deacon CF Ahrén B Holst JJ Inhibitors of depeptidyl peptidase IV: a novel approach for the prevention and treatment of type 2 diabetes? Expert Opin Investig Drugs 2004 13 1091 1102
- Drucker DJ Therapeutic potential of depeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes Expert Opin Investig Drugs 2003 12 87 100
- Dicker D DPP-4 inhibitors. Impact on glycemic control and cardiovascular risk factors Diabetes Care 2011 34 Suppl 2 S276 S278 21525468
- Davidson JA Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors Cleve Clin J Med 2009 76 Suppl 5 S28 S38 19952301
- Amori RE Lau J Pittas AG Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis JAMA 2007 298 194 206 17622601
- Balas B Baig MR Watson C The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients J Clin Endocrinol Metab 2007 92 4 1249 1255 17244786
- Williams-Herman D Round E Swern AS Safety and tolerability of sitagliptin in patients with type 2 diabetes: a pooled analysis BMC Endocr Disord 2008 8 14 18954434
- Karagiannis T Paschos P Paletas K Matthews DR Tsapas A Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis BMJ 2012 344 e1369 22411919
- Baetta R Corsini A Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences Drugs 2011 71 1441 1467 21812507
- Yoshida T Akahoshi F Sakashita H Discovery and preclinical profile of teneligliptin(3-[(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl]thiazolidine): a highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes Bioorg Med Chem 2012 20 19 5705 5719 22959556
- Eto T Inoue S Kadowaki T Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial Diabetes Obes Metab 2012 14 1040 1046 22776014
- Kadowaki T Kondo K Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus Diabetes Obes Metab Epub 3 6 2013
- Monami M Lamanna C Desideri CM Mannucci E DPP-4 inhibitors and lipids: systematic review and meta-analysis Adv Ther 2012 29 14 25 22215383
- Poitout V Robertson RP Glucolipotoxicity: fuel excess and beta-cell dysfunction Endocrine Rev 2008 29 351 366 18048763
- Sone H Tanaka S Tanaka S Japan Diabetes Complications Study Group Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS) J Clin Endocrinol Metab 2011 96 3448 3456 21865372
- Ansar S Koska J Reaven PD Postprandial hyperlipidemia, endothelial dysfunction and cardiovascular risk: focus on incretins Cardiovasc Diabetol 2011 10 61 21736746
- Hsieh J Longuet C Baker CL The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice Diabetologia 2010 53 552 561 19957161
- Matikainen N Mänttäri S Schweizer A Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes Diabetologia 2006 49 2049 2057 16816950
- Tremblay AJ Lamarche B Deacon CF Weisnagel SJ Couture P Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes Diabetes Obes Metab 2011 13 366 373 21226820
- Fukuda-Tsuru S Anabuki J Abe Y Yoshida K Ishii S A novel, potent, and long-lasting dipeptidyl peptidase-4 inhibitor, teneligliptin, improves postprandial hyperglycemia and dyslipidemia after single and repeated administrations Eur J Pharmacol 2012 696 1–3 194 202 23022337
- Ionescu E Sauter JF Jeanrenaud B Abnormal oral glucose tolerance in genetically obese (fa/fa) rats Am J Physiol 1985 248 E500 E506 3887938
- Pederson RA White HA Schlenzig D Pauly RP McIntosh CH Demuth HU Improved glucose tolerance in Zucker fatty rats by oral administration of the dipeptidyl peptidase IV inhibitor isoleucine thiazolidide Diabetes 1998 47 1253 1258 9703325
- Wøjdemann M Wettergren A Sternby B Inhibition of human gastric lipase secretion by glucagon-like peptide-1 Dig Dis Sci 1998 43 799 805 9558037
- Qin X Shen H Liu M GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats Am J Physiol Gastrointest Liver Physiol 2005 288 G943 G949 15677555
- Mahler R Statford WS Tarrant ME Ashmore J The effect of insulin on lipolysis Diabetes 1964 13 297 302 14166680
- Moritoh Y Takeuchi K Asakawa T Kataoka O Odaka H Combining a dipeptidyl peptidase-4 inhibitor, alogliptin, with pioglitazone improves glycaemic control, lipid profiles and beta-cell function in db/db mice Br J Pharmacol 2009 157 415 426 19371350
- Mu J Woods J Zhou YP Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes Diabetes 2006 55 1695 1704 16731832
- Pospisilik JA Martin J Doty T Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats Diabetes 2003 52 741 750 12606516
- Sudre B Broqua P White RB Chronic inhibition of circulating dipeptidyl peptidase IV by FE 999011 delays the occurrence of diabetes in male zucker diabetic fatty rats Diabetes 2002 51 1461 1469 11978643
- Pugh RN Murray-Lyon IM Dawson JL Pietroni MC Williams R Transection of the esophagus for bleeding esophageal varices Br J Surg 1973 60 646 654 4541913
- Goldstein BJ Feinglos MN Lunceford JK Johnson J Williams-Herman DE Sitagliptin 036 Study Group Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes Diabetes Care 2007 30 1979 1987 17485570
- Scheen AJ A review of gliptins in 2011 Expert Opin Pharmacother 2012 13 81 99 22149369
- Barnett A Allsworth J Jameson K Mann R A review of the effects of antihyperglycaemic agents on body weight: the potential of incretin targeted therapies Curr Med Res Opin 2007 23 1493 1507 17559747
- Koliaki C Doupis J Linagliptin/Metformin fixed-dose combination treatment: a dual attack to type 2 diabetes pathophysiology Adv Ther 2012 29 12 993 1004 23184570
- Genovese S Tedeschi D Effects of vildagliptin/metformin therapy on patient-reported outcomes: work productivity, patient satisfaction, and resource utilization Adv Ther 2013 30 2 152 164 23430354
- Narita T Katsuura Y Sato T Miglitol induces prolonged and enhanced glucagon-like peptide-1 and reduced gastric inhibitory polypeptide responses after ingestion of a mixed meal in Japanese type 2 diabetic patients Diabet Med 2009 26 187 188 19236625
- Arakawa M Ebato C Mita T Miglitol suppresses the postprandial increases in interleukin 6 and enhances active glucagon-like peptide 1 secretion in viscerally obese subjects Metabolism 2008 57 1299 1306 18702958
- Lee A Patrick P Wishart J Horowitz M Morley JE The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics Diabetes Obes Metab 2002 4 329 335 12190996
- Narita T Yokoyama H Yamashita R Comparison of the effects of 12-week administration of miglitol and voglibose on the responses of plasma incretins after a mixed meal in Japanese type 2 diabetic patients Diabetes Obes Metab 2012 14 283 287 22051162
- Aoki K Kamiyama H Yoshimura K Shibuya M Masuda K Terauchi Y Miglitol administered before breakfast increased plasma active glucagon-like peptide-1 (GLP-1) levels after lunch in patients with type 2 diabetes treated with sitagliptin Acta Diabetol 2012 49 225 230 21898126
- Gross TM Bode BW Einhorn D Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use Diabetes Technol Ther 2000 2 49 56 11467320
- Kaufman FR Gibson LC Halvorson M Carpenter S Fisher LK Pitukcheewanont P A pilot study of glucose measurements using the glucose sensor Diabetes Care 2002 25 1185 1191 12087017
- Kishimoto M Noda M A pilot study of the efficacy of miglitol and sitagliptin for type 2 diabetes with a continuous glucose monitoring system and incretin-related markers Cardiovasc Diabetol 2011 10 115 22189184
- Scheen AJ DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials Diabetes Metab 2012 38 2 89 101 22197148
- Defronzo RA Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus Diabetes 2009 58 773 795 19336687
- Mikhail N Combination therapy with DPP-4 inhibitors and pioglitazone in type 2 diabetes: theoretical consideration and therapeutic potential Vasc Health Risk Manag 2008 4 1221 1227 19337535
- Rosenstock J Kim SW Baron MA Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes Diabetes Obes Metab 2007 9 2 175 185 17300593
- Mäkimattila S Nikkilä K Yki-Järvinen H Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus Diabetologia 1999 42 406 412 10230643
- Kishimoto M Noda M Effect of the addition of sitagliptin and miglitol on insulin-treated type 2 diabetes Diabetes Ther 2012 3 1 11 23055336
- Vilsbøll T Rosenstock J Yki-Järvinen H Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes Diabetes Obes Metab 2010 12 167 177 20092585
- Fonseca V Schweizer A Albrecht D Baron MA Chang I Dejager S Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes Diabetologia 2007 50 1148 1155 17387446
- Foley JE Ligueros-Saylan M He YL Effect of vildagliptin on glucagon concentration during meals in patients with type 1 diabetes Horm Metab Res 2008 40 727 730 18597213
- iPro®2 Professional CGM [homepage on the internet] Medtronic MiniMed Inc 2013 Available from: http://www.professional.medtronicdiabetes.com/hcp-products/ipro2 Accessed March 29, 2013