Abstract
Objective
The study objective was to investigate the serum levels of Klotho in patients with type 2 diabetes mellitus (T2DM) who had moderate cognitive impairment (MCI) and those without MCI and to determine its prediction of MCI in older patients with T2DM.
Methods
Patients with diabetes were examined for MCI in 292 cases (using the Montreal Cognitive Assessment: MoCA score). Biomarkers and biochemical parameter data were accumulated.
Results
Comparing T2DM patients with MCI (91 patients) and without MCI (101 patients), patients with MCI considerably reduced serum Klotho levels were observed. In all 292 hospitalized patients, serum Klotho levels were negatively correlated with age (r=−0.184, P=0.002), body mass index (BMI) (r=−0.151, P=0.010), glycosylated hemoglobin (HbA1c) (r=−0.197, P=0.001), creatinine (r=−0.178, P=0.002), and C-reactive protein (CRP) (r=−0.319, P<0.001). On the other hand, it is positively correlated with education (r=0.319, P<0.001) and high-density lipoprotein cholesterol (HDL-C) (r=0.272, P<0.001). Considering the findings of the multivariate logistic regression models, patients with type 2 diabetes who had reduced levels of serum Klotho (OR=0.987, 95% CI=0.980–0.994; P<0.001), lower HDL-C, increased levels of HbA1c, creatinine, and CRP, and limited years of formal education and a longer duration of T2DM, increase the risk of developing MCI.
Conclusion
The results showed that diabetic patients with MCI have lower serum Klotho levels than diabetic patients without MCI. It might be possible to do a more extensive population-based prospective investigation to confirm the correlation between serum Klotho levels and cognitive impairment or dementia.
Introduction
One of the most common and severe public health issues in the 21st century is type 2 diabetes mellitus (T2DM).Citation1 Over 90% of local individuals with diabetes mellitus are T2DM patients, the most prevalent type of disease in China.Citation2 The risk of dementia, cognitive impairment, and Alzheimer’s disease (AD) is correlated with T2DM.Citation3,Citation4 A progressive loss of memory and executive function is a symptom of mild cognitive impairment (MCI), a condition that occurs in people between normal ageing and dementia. As a result, the central nervous system (CNS) experiences a variety of morphological alterations, such as diminished hippocampus growth and neurogenesis, atrophy of the brain, and unexpected changes in neuronal electrical properties.Citation5–8 The major cause of blindness in people with T2DM is diabetic retinopathy (DR), which is the most prevalent microvascular consequence of T2DM.Citation9 In addition, there is a correlation between metabolic syndrome and cognitive function.Citation10 However, these disorders significantly impact the quality of life and public health. Therefore, it is critical to identify MCI early diagnostic biomarkers in T2DM patients.
The extracellular Klotho domain of Klotho, a type 1 trans-membrane protein, can be cleaved and released into the blood, urine, or cerebrospinal fluid (CSF). Klotho is mainly produced in the kidney and brain.Citation11 Membrane-bound Klotho controls the calcium and phosphate metabolism of the kidneys and is an essential co-receptor for fibroblast growth factor-23 (FGF23). Wnt and insulin-like growth factor-1 (IGF-1) are two growth factor signalling pathways where secreted Klotho participates, regulating energy metabolism and oxidative stress.Citation12 Klotho is important in controlling ageing, and animals with Klotho deficiency or silence age more quickly and have shorter lives. When Klotho is overexpressed in mice, longevity is increased.Citation13 Furthermore, irrespective of the lifespan-extension effect, an allele of the human Klotho gene is linked to improved cognition in heterozygous carriers through raising blood klotho levels.Citation14
Recent research reported how Klotho regulates glucose absorption, improves insulin sensitivity, reduces oxidative stress, and suppresses inflammation, which may impact T2DM’s severity.Citation15 Klotho expression levels were decreased in mice with STZ-induced diabetes and patients with early diabetic nephropathy. In addition, a different study found that T2DM, diabetic nephropathy, and diabetic coronary heart disease had downregulated blood levels of α-Klotho and β-Klotho.Citation16,Citation17 Klotho upregulation in the hippocampus showed an anti-neurotoxic impact in a mice model of streptozotocin-induced cognitive impairment.Citation18 However, the therapeutic effects of serum Klotho on cognitive impairment in diabetic patients remain unknown.
In patients with type 2 diabetes, blood Klotho levels may be utilized as an early biomarker of cognitive impairment. The current study carried out cross-sectional research to investigate the following questions: Identify the predictive value of Klotho for MCI in patients with T2DM by evaluating blood levels in both with and without MCI patients with T2DM.
Methods
Study Population
From January 2017 to December 2018, this cross-sectional study was undertaken on our hospital’s Department of Endocrinology patients. The World Health Organization 1999 criteria were used to determine the diagnostic criteria for T2DM, which included 292 hospitalized patients (158 men and 134 women, ages 50 to 75).Citation19 Every case has a type 2 diabetes diagnosis. Ninety-one (91) of these people had MCI due to their diabetes, while 101 had the condition without any cognitive impairment. Serums of 150 gender and age-matched healthy donors were selected as controls. The European Alzheimer’s Association determined that the patient had moderate cognitive impairment (MCI).Citation20 The age range of 50 to 75, diabetes type 2 diagnosis made at least a year prior, and capacity to comprehend and comply with research procedures were the inclusion criteria for all diabetic patients.
The following criteria were used to exclude people:
Several diabetes consequences, including hypoglycemia, diabetic coma, and diabetic ketoacidosis.
Neurological diseases such as trauma, infection, and cerebrovascular disease history.
Severe primary diseases such as heart, liver, kidney, and lung.
Previous history of mental illness includes Parkinson’s disease, epilepsy, and major depression.
Use drugs that may cause changes in cognitive function during the month.
They were all Chinese Han people. The Department of Endocrinology at Shanghai Pudong New District Gongli Hospital was where this study was carried out. The Shanghai Pudong New District Gongli Hospital’s ethics committee approved the study, which was carried out following the Declaration of Helsinki’s ethical principles.
Clinical Data Collection and Blood Biochemistry Measurement
Procedures for internal and external quality control are used in the Gongli Hospital’s central laboratory. We acquired medical histories, including details on smoking, high blood pressure, and the duration of diabetes. Age, gender, and educational levels were recorded as demographic variables. Height and weight were used to compute the body mass index (BMI) using the following formula: [body weight (kg)]/[body height (m2)]. The levels of serum creatinine, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glycosylated hemoglobin (HbA1c), and fasting blood glucose (FBG) were measured in blood samples.
Cognitive Test
The community health service centre’s medical staff assessed general cognitive function using the Montreal Cognitive Assessment (MoCA). The MoCA looks helpful as a cognitive screening test with strong sensitivity and specificity for the early diagnosis of MCI. The MoCA test consists of seven cognitive areas: executive function, delayed memory recall, name, attention, abstraction, language, and orienting abilities.Citation21 The cut-off points used for diagnosing MCI in a prior study on the elderly Chinese population were as follows: 13/14 for those with no formal education, 19/20 for those with one to six years of schooling, and 24/25 for those with one to six years of education with seven or more. The cut-offs, as mentioned above, were sensitive and effective in identifying MCI in the elderly Chinese population.Citation22
Measurement of Circulating Klotho Level
A tube with an EDTA anticoagulant in the morning was filled with 5 mL of peripheral venous blood. Within 30 minutes of collection, blood samples were centrifuged for 15 minutes at 3000 rpm. The samples were devoid of serum and kept at −70°C for analysis. A sandwich ELISA kit was used to assess the serum Klotho level (Cloud-Clone Corp., Houston, TX, USA). At 450 nm, a microplate reader was used to calculate absorbance values.
Statistical Analysis
The relevant frequency and percentage units were used to convey the data and the mean and standard deviation (SD). The Student’s t-test examined quantitative data with normally distributed variables or with asymmetrically distributed variables using the nonparametric Mann–Whitney U and Kruskal–Wallis tests. Categorical data were analyzed by Chi-squared (χ2) test. The relationship between the MoCA test results and clinical serum markers was investigated using Pearson’s or Spearman correlation. Multiple stepwise regression analyses examined the link between cognitive abilities, demographic traits, clinical traits, and serum ACE level or activity. SPSS 20.0 was used for the statistical analysis (SPSS Inc., Chicago, IL). Statistical significance was determined by a two-sided P value of <0.05.
Results
The Baseline Characteristics of the Study Group
It was discussed the differences between type 2 diabetics with and without MCI. Student’s t-test results revealed that patients with MCI often had higher BMIs, older ages, poorer levels of education, longer durations of diabetes, and higher PBG, HbA1c, triglycerides, HDL-C, creatinine, and CRP levels as well as lower HDL-C concentrations (). The χ2 revealed substantial variations between patients with and without MCI in the presence of gender, smoking, or hypertension. Potentially decreased MoCA scores were seen in patients with MCI (23.08 ± 1.38) than in controls (28.15 ± 1.30). There were no potential differences between the groups for FBG and TC (P>0.05).
Table 1 Clinical Characteristics and Biochemical Parameters of Type 2 Diabetic Elderly Patients with and without MCI
Serum Klotho Levels Between Diabetic Patients with and without MCI
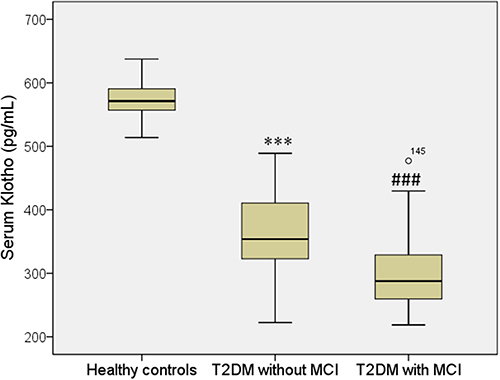
ELISA assay was performed to measure the blood Klotho levels of diabetic patients with and without MCI compared with healthy controls. The MCI group and non-MCI patients exhibited markedly serum Klotho levels lower than healthy controls. Furthermore, the MCI group (260.9 ± 33.9 pg/mL) showed significantly lower serum Klotho levels than the non-MCI patients (296.6 ± 33.5 pg/mL) (, ).
Figure 1 Serum levels of Klotho in T2DM and control cases. All data are medians and inter-quartile range (IQR); P values refer to Mann–Whitney U-tests for differences between groups. ***P<0.001 vs healthy control group (without MCI) and ###P<0.001 vs healthy control group (with MCI).
Correlation of Serum Klotho with Additional Clinical Markers in People with Diabetes
In all 292 diabetic patients, negatively associated serum Klotho level with age (r=−0.184, P=0.002), BMI (r=−0.151, P=0.010), HbA1c (r=−0.197, P=0.001), Creatinine (r=−0.178, P=0.002), CRP (r=−0.319, P<0.001), and was positively associated with Education (r=0.319, P<0.001) and HDL-C (r=0.272, P<0.001) (). There was a marginally negative connection between serum Klotho and FBG, PBG, and LDL-C.
Table 2 Relationship of Serum Level of Klotho with Other Clinical Indicators in Group of Diabetic Elderly Patients
Logistic Regression Models
To identify independent risk variables of MCI, multivariate logistic regression analysis was used. It was shown that people with type 2 diabetes who had less formal education were more likely to have MCI (OR=0.804, 95% CI=0.667–0.969; P=0.022), extended duration of T2DM (OR=2.433, 95% CI=1.628–3.638; P<0.001), increased HbA1c levels (OR=3.355, 95% CI=1.146–9.821; P=0.027), creatinine (OR=1.136, 95% CI=1.075–1.200; P<0.001) and CRP (OR=2.309, 95% CI=1.574–3.388; P<0.001), and reduced HDL-C (OR=0.203, 95% CI=0.057–0.725; P=0.014) and Klotho levels (OR=0.987, 95% CI=0.980–0.994; P<0.001) ().
Table 3 Assessment Results of the Risk of Having MCI in a Multivariable Logistic Regression Model in Elderly Patients with Type 2 Diabetes
Discussion
T2DM patients had a higher frequency of MCI than the control population.Citation23 Patients with T2DM with MCI had cognitive impairment more quickly than those with MCI alone.Citation24 The role of T2DM in cognitive impairment is multifaceted. It involves amyloid β (Aβ) protein deposition, hyperphosphorylation of tau protein, chronic hyperglycemia, recurrent hypoglycemia, insulin insufficiency, glucose-mediated toxicity, and chronic hyperglycemia.Citation25 These results suggested that T2DM might be a risk factor for the cognitive impairment associated with older people. However, it might be challenging to comprehend the underlying procedures. In the current study, we conducted a cross-sectional study to investigate the relationship between serum Klotho levels and cognition in Chinese diabetes patients who were not yet demented. Compared to patients with T2DM who did not have MCI, the serum Klotho level in MCI patients was significantly lower.
According to the results of the multivariate logistic regression models, patients with type 2 diabetes might be more likely to develop MCI when their serum Klotho levels are lower. In addition, blood Klotho levels were positively correlated with education and HDL-C and adversely correlated with age, BMI, HbA1c, creatinine, and CRP in all patients. These results demonstrated that decreased IL-1β levels might be linked to the decline in cognitive function in diabetes.
When compared to diabetic individuals without MCI, we discovered that diabetic patients with MCI had considerably reduced blood levels of Klotho. Moreover, our findings showed a positive correlation between Klotho levels and the MoCA score. In the crucial functional networks of the brain susceptible to ageing, higher serum klotho levels were found in healthy ageing people with Klotho homozygosity, which was also related to the greater intrinsic connection.Citation26 The result indicates that Klotho is a secretory protein containing a cognitive-improving compound effect, as evidenced by a lower risk of cognitive impairment in aged subjects with higher plasma Klotho.Citation27 Our results are consistent with the findings of several previous research that have correlated Klotho with cognitive impairment.
Klotho serum levels were lower in T2DM patients than in healthy controls,Citation17 and low levels of Klotho have been associated with an increased risk of cerebrovascular incidents and coronary artery disease.Citation28 Serum Klotho and MCI in T2DM patients are thought to be related; however, this is still unknown. However, reduced plasma levels in a group of aged people and klotho levels are correlated to vascular dementia.Citation29 The current study found that T2DM patients with MCI had lower blood levels of Klotho than T2DM patients without MCI, and a low level of Klotho is an independent cause of MCI. It is unknown if serum Klotho level is altered in the early stage of MCI in T2DM patients. The Klotho level is an independent predictor for the development of macroangiopathies in a 7-year prospective study; hence, further investigation is necessary.
The present research found a significant negative correlation of serum level of Klotho with age, BMI, HbA1c, creatinine, CRP, and a positive correlation with education and HDL-C. HbA1c level positively correlates with the duration and extent of hyperglycemia in T2DM patients. Age-related cognitive impairment and worse executive function were linked to hyperglycemia and higher HbA1c levels,Citation30 that is also evidenced by increased HbA1c in patients with MCI compared to patients without MCI. Klotho is expressed in the pancreas, and pancreatic β-cell-specific expression of Klotho could preserve β-cells in type 2 diabetes.Citation31 Whether reduced serum Klotho reflects lower Klotho expression in pancreatic β-cell, thus increasing blood glucose, remains unknown. In older people, hyperglycemia is a distinct risk factor for MCI,Citation32 and our results also showed higher triglyceride (TG) and lower HDL-C in MCI compared to non-MCI. Hyperlipidemia can cause kidney injury and decrease Klotho’s renal expression by oxidizing LDL.Citation33 As Klotho is mainly expressed in the kidney indicates that serum Klotho is influenced by hyperlipidemia, and the kidney is the main targeted organ, evidenced by the negative correlation between serum Klotho and creatinine in our results. Furthermore, reducing hyperlipidemia is a promising therapeutic method for increasing serum Klotho, which is supported by increased hippocampal klotho expression in a mice model of simvastatin-induced cognitive impairment brought on by streptozotocin.Citation18
Limitations
There were several limitations of our study. (1) We should be careful when interpreting our results because of the relatively small size of our research cohort. (2) The study was not intended to be a prospective longitudinal inquiry; instead, it was cross-sectional. The predictive role of serum Klotho should be validated. (3) This study offers crucial information on the Klotho abnormalities that underlie diabetes patients’ cognitive impairment. (4) To evaluate the cognitive cognition of the individuals, we only employed the MoCA analysis tool; other techniques should be used to obtain more accurate and dependable results. Furthermore, the specific processes driving reduced Klotho expression-related cognitive impairment brought on by T2DM have not yet been identified.
Conclusions
In sum-up, compared to non-MCI controls, serum Klotho levels are lower in MCI diabetic patients. The independent factor raising the risk of MCI in people with type 2 diabetes is the lower level of Klotho. It needs to be made apparent what particular procedures led to this discovery. It is necessary to do a more extensive population-based prospective investigation to confirm the serum Klotho’s ability to predict cognitive deterioration in T2DM patients. Clinical benefits for the prevention of MCI may come from pharmacological treatments that specifically target the Klotho protein in diabetes.
Abbreviations
MCI, mild cognitive impairment; T2DM, type 2 diabetes mellitus; MoCA, Montreal Cognitive Assessment; BMI, body mass index; HbA1c, glycosylated hemoglobin; CRP, C-reactive protein; HDL-C, High-density lipoprotein cholesterol; CSF, cerebrospinal fluid; FGF-23, fibroblast growth factor-23; IGF-1, insulin-like growth factor-1; FBG, fasting blood glucose; PBG, postprandial blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol.
Data Sharing Statement
The datasets used/analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The Ethics Committee of Shanghai Pudong New District Gongli Hospital approved this study. All subjects provided informed consent to participate in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing of interests.
Acknowledgment
We sincerely thank all of the participants in our study.
Additional information
Funding
References
- Reed J, Bain S, Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab Syndr Obes. 2021;14:3567–3602.
- Savonitto S, Morici N, Nozza A, et al. Predictors of mortality in hospital survivors with type 2 diabetes mellitus and acute coronary syndromes. Diab Vasc Dis Res. 2018;15(1):14–23. doi:10.1177/1479164117735493
- Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942. doi:10.1212/WNL.53.9.1937
- Wang G, Li W. Diabetes as a risk factor for abnormal cognition development. J Alzheimers Dis Rep. 2020;4(1):237–242. doi:10.3233/ADR-200181
- Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi:10.1001/jama.2009.460
- Ramos-Rodriguez JJ, Molina-Gil S, Ortiz-Barajas O, et al. Central proliferation and neurogenesis is impaired in type 2 diabetes and prediabetes animal models. PLoS One. 2014;9:e89229. doi:10.1371/journal.pone.0089229
- Wrighten SA, Piroli GG, Grillo CA, et al. A look inside the diabetic brain: contributors to diabetes-induced brain aging. Biochim Biophys Acta. 2009;1792:444–453. doi:10.1016/j.bbadis.2008.10.013
- Beauquis J, Saravia F, Coulaud J, et al. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol. 2008;210:359–367. doi:10.1016/j.expneurol.2007.11.009
- Yu ZW, Liu R, Li X, et al. High serum neuron-specific enolase level is associated with mild cognitive impairment in patients with diabetic retinopathy. Diabetes Metab Syndr Obes. 2020;13:1359–1365. doi:10.2147/DMSO.S249126
- Zhan C, Wang Q, Liu J, et al. Relationship between metabolic syndrome and cognitive function: a population-based study of middle-aged and elderly adults in Rural China. Diabetes Metab Syndr Obes. 2021;14:1927–1935. doi:10.2147/DMSO.S308250
- Razzaque MS. The role of Klotho in energy metabolism. Nat Rev Endocrinol. 2012;8(10):579–587. doi:10.1038/nrendo.2012.75
- Dalton GD, Xie J, An SW, et al. New insights into the mechanism of action of Soluble Klotho. Front Endocrinol. 2017;8:323. doi:10.3389/fendo.2017.00323
- Torbus-Paluszczak M, Bartman W, Adamczyk-Sowa M. Klotho protein in neurodegenerative disorders. Neurol Sci. 2018;39(10):1677–1682. doi:10.1007/s10072-018-3496-x
- Dubal DB, Yokoyama JS, Zhu L, et al. Life extension factor klotho enhances cognition. Cell Rep. 2014;7(4):1065–1076. doi:10.1016/j.celrep.2014.03.076
- Donate-Correa J, Martín-Núñez E, Delgado NP, et al. Implications of Fibroblast growth factor/Klotho system in glucose metabolism and diabetes. Cytokine Growth Factor Rev. 2016;28:71–77. doi:10.1016/j.cytogfr.2015.12.003
- Asai O, Nakatani K, Tanaka T, et al. Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81(6):539–547. doi:10.1038/ki.2011.423
- Nie F, Wu D, Du H, et al. Serum klotho protein levels and their correlations with the progression of type 2 diabetes mellitus. J Diabetes Complications. 2017;31(3):594–598. doi:10.1016/j.jdiacomp.2016.11.008
- Adeli S, Zahmatkesh M, Tavoosidana G, et al. Simvastatin enhances the hippocampal klotho in a rat model of streptozotocin-induced cognitive decline. Prog Neuropsychopharmacol Biol Psychiatry. 2017;72:87–94. doi:10.1016/j.pnpbp.2016.09.009
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
- Portet F, Ousset PJ, Visser PJ, et al. MCI Working Group of the European Consortium on Alzheimer’s Disease (EADC). Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J Neurol Neurosurg Psychiatry. 2006;77(6):714–718. doi:10.1136/jnnp.2005.085332
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
- Lu J, Li D, Li F, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol. 2011;24(4):184–190. doi:10.1177/0891988711422528
- Umegaki H, Hayashi T, Nomura H, et al. Cognitive dysfunction: an emerging concept of a new diabetic complication in the elderly. Geriatr Gerontol Int. 2013;13(1):28–34. doi:10.1111/j.1447-0594.2012.00922.x
- Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369(19):1863–1864. doi:10.1056/NEJMoa1215740
- McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. doi:10.1016/S0140-6736(12)60360-2
- Yokoyama JS, Marx G, Brown JA, et al. Systemic klotho is associated with KLOTHO variation and predicts intrinsic cortical connectivity in healthy human aging. Brain Imaging Behav. 2017;11(2):391–400. doi:10.1007/s11682-016-9598-2
- Shardell M, Semba RD, Rosano C, et al. Plasma Klotho and cognitive decline in older adults: findings from the InCHIANTI Study. J Gerontol a Biol Sci Med Sci. 2016;71(5):677–682. doi:10.1093/gerona/glv140
- Brombo G, Bonetti F, Ortolani B, et al. Lower Plasma Klotho concentrations are associated with vascular dementia but not late-onset alzheimer’s disease. Gerontology. 2018;64(5):414–421. doi:10.1159/000488318
- Pan HC, Chou KM, Lee CC, et al. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis. 2018;276:83–90. doi:10.1016/j.atherosclerosis.2018.07.006
- Pappas C, Small BJ, Andel R, et al. Blood glucose levels may exacerbate executive function deficits in older adults with cognitive impairment. J Alzheimers Dis. 2019;67(1):81–89. doi:10.3233/JAD-180693
- Lin Y, Sun Z. In vivo pancreatic β-cell-specific expression of antiaging gene Klotho: a novel approach for preserving β-cells in type 2 diabetes. Diabetes. 2015;64(4):1444–1458. doi:10.2337/db14-0632
- Wang F, Zhao M, Han Z, et al. Long-term subclinical hyperglycemia and hypoglycemia as independent risk factors for mild cognitive impairment in elderly people. Tohoku J Exp Med. 2017;242(2):121–128. doi:10.1620/tjem.242.121
- Sastre C, Rubio-Navarro A, Buendía I, et al. Hyperlipidemia-associated renal damage decreases Klotho expression in kidneys from ApoE knockout mice. PLoS One. 2013;8(12):e83713. doi:10.1371/journal.pone.0083713
