Abstract
Objective
In this pilot-study, the effects of a multispecies probiotic supplement on glycaemic control and metabolic parameters in adults with type 1 diabetes (T1DM) were explored.
Material and Methods
A total of 50 T1DM patients were enrolled and randomly placed into a group receiving capsules containing multi-probiotic strains (Bifidobacterium longum, Lactobacterium bulagricumi, Streptococcus thermophilus) and insulin (probiotics group, n = 27) or a group receiving a placebo and insulin (placebo group, n = 23). All patients underwent continuous glucose monitoring at baseline and 12 weeks after intervention. The primary outcomes were determined by comparing factors such as changes in fasting blood glucose (FBG) and haemoglobin A1c (HbA1c) between the groups.
Results
Probiotic supplementation significantly reduced FBG (−1.0 ± 4.7 vs 1.8 ± 4.7 mmol/L, p = 0.048), 30 min postprandial glucose (−0.5 ± 4.6 vs 1.9 ± 3.3 mmol/L, p = 0.0495), and low-density lipoprotein cholesterol (−0.07 ± 0.45 vs 0.32 ± 0.78 mmol/L, p = 0.0413), compared with the placebo. Although not statistically significant, probiotic supplementation also lowered HbA1c levels by 0.49% (−5.33 mmol/mol, p = 0.310). Besides, no significant difference was observed in the continuous glucose monitoring (CGM) parameters between the two groups. Further subgroup analysis revealed a significant reduction in mean sensor glucose (MSG; −0.75 (−2.11, 0.48) mmol/L vs 1.51 (−0.37, 2.74) mmol/L, p = 0.010) and time above range (TAR; −5.47 (−20.1, 3.04)% vs 18.9 (−1.11, 35.6)%, p = 0.006), as well as an greater improvement in the time in range (TIR; 9.32 (−4.84, 16.6)% vs −19.9 (−31.4, 0.69)%, p = 0.005) in male patients than female patients in the probiotics group.
Conclusion
Multispecies probiotics exerted beneficial effects on fasting and postprandial glucose and lipid profiles in adult T1DM patients, especially for male patients and those with higher baseline FBG levels.
Introduction
Type 1 diabetes mellitus (T1DM) is one of the most common autoimmune disorders in the world.Citation1 It is characterised by the progressive destruction of insulin-producing beta (β)-cells in the pancreatic islets. Managing T1DM is a challenging task that requires constant daily self-management, relying on insulin therapy. Despite considerable advances in insulin infusion techniques, T1DM patients still suffer from a high risk of both acute and chronic complications. Over the past two decades, the prevalence of T1DM among young children has increased by approximately 70%,Citation2 with an annual growth of around 3–4% globally.Citation3 This has incurred a tremendous medical, social, and economic burden worldwide.
The rapid growth in T1DM cases cannot be fully explained by genetic predisposition alone.
Accumulating evidence suggests that gut microbiota and their metabolites, deeply affected by the living environment, play a vital role in the pathophysiology of T1DM. Gut microbiota contribute to both the development and subsequent progression of islet autoimmunity, ultimately leading to hyperglycaemia and clinical disease.Citation4 Murri et al demonstrated that the alteration of bowel flora, especially with an increased number of bacteria belonging to the Bacteroidetes phylum and a relative decrease in the number of Lactobacillus, Bifidobacterium and Clostridium strains, contributed to the development of T1DM.Citation5 It has been demonstrated that due to increased intestinal permeability, immune stimulants enter the circulation to trigger systemic inflammation and aberrant immunity.Citation6 Additionally, anti-inflammatory bacteria promote gut epithelial integrity and immune homeostasis by producing short-chain fatty acids (SCFAs) such as butyrate, propionate, and acetate.Citation6
Probiotics improve the gut microbiota when administered in appropriate amounts and may confer health benefits on the host. For instance, Bifidobacterium lactis has been reported to improve insulin tolerance as well as lipid profiles.Citation7 Besides, Lactobacillus johnsonii alleviates host oxidative stress and is associated with intercellular tight junction assembly and maintenance in the gut, mitigating the development of T1DM.Citation8 Considering the limited current therapies for T1DM, probiotics hold tremendous promise for the adjuvant treatment of T1DM. However, most studies on the gut microbiome and clinical outcomes of diabetes focus on type 2 diabetes (T2DM)Citation9 and T1DM in the young.Citation10 However, more than half of T1DM cases have been reported in those over 20 years old.Citation4 Also, because of the progression in diabetes management, an unprecedented number of older adults are living with this disease. Moreover, some studies did not perform blinded experiments and very few papers have demonstrated the impact of probiotic supplementation on glycaemic variability (GV) in T1DM. Although regarded as the gold standard for glycaemic control evaluation, haemoglobin A1c (HbA1c) alone cannot provide a complete picture of glucose control. A growing body of evidence suggests that continuous glucose monitoring (CGM), which provides comprehensive blood glucose profiles using different parameters, shows great promise in improving glucose control, glucose variability, and consequently quality of life.Citation11 To the best of our knowledge, there are very few reports on the impact of commercial compound probiotics on GV in adult T1DM patients. Therefore, in this study, we evaluate the effect of the supplementation of the Bifid triple viable capsule, a multi-species compound probiotic, on fasting blood glucose (FBG), HbA1c, GV, and other metabolic parameters in adults with T1DM.
Materials and Methods
Methods and Patients
This 12-week randomised, double-blinded, controlled clinical trial was approved by the Ethics Committee of the Shenzhen People’s hospital (The Second Clinical Medical College, Jinan University, Ethics approval number: LL-KT-2018248) and was registered at clinicaltrials.gov (NCT03556631). Besides, the trial was conducted in observance of the Declaration of Helsinki and the International Conference of Harmonization - Good Clinical Practice guidelines. Subjects with T1DM were consecutively recruited between September 2020 and September 2021 at both the outpatient and inpatient clinics of the Department of Endocrinology and Metabolism of our hospital. Written informed consent was obtained from all the patients before their enrolment in the study.
The inclusion criteria were an age range of 18 to 75 years, T1DM (positive for more than one diabetes mellitus-associated autoantibody), treated with continuous subcutaneous (s.c.) insulin infusion or multiple daily injections with a stable regimen, 6.5% ≤ HbA1c ≤ 10.0%, and 7.0 ≤ FPG ≤ 13.3 mmol/L at screening. Self-monitoring of blood glucose (SMBG) at least three times a day for at least 1 month was required for all participants before enrolment in the study. The exclusion criteria included severe gastrointestinal diseases, gastrointestinal or abdominal surgery within a year, medications affecting insulin sensitivity (eg, immunosuppressants, steroids), severe hepatic, renal, cardiovascular, autoimmune, psychiatric, or infectious diseases, acute diabetic complications in the past 3 months, administration of other probiotics or probiotic products in the past 3 months, and pregnancy. All the patients were provided with the same dietary and physical activity guidance by the same dietician and diabetes educator before being randomly assigned a group.Citation12
Study Design
After preliminary screening, the randomization number was generated by a research statistician who had no contact with the participants. The corresponding author randomly assigned (1:1) the 50 patients to receive either the placebo or multispecies probiotic supplements for 3 months using a random digit table. Insulin therapy remained the same for all patients. Chief investigators and statisticians were masked to group allocation.
All participants were asked to take one supplement three times a day. Each multispecies probiotic supplement (Jinshuangqi Co., Inner Mongolia, China) consisted of three viable freeze-dried strains: Bifidobacterium longum (2 × 107 CFU), Lactobacterium bulagricumi (2 × 106 CFU), and Streptococcus thermophilus (2 × 106 CFU). The placebo containing the same substances but without the bacteria was packed into identical-looking tablets. All the supplements were coded by the producer to guarantee blinding and were distributed to participants monthly. Besides, the participants were advised to avoid consuming any other probiotic products during the study. Every participant underwent a thorough evaluation at baseline and a 12-week follow-up visit.
Laboratory Measurements
HbA1c, serum insulin, and C-peptide analyses were performed in a central laboratory at Shenzhen People’s Hospital. HbA1c was measured using an automated high-performance liquid chromatography gradient elution analyser (Arkray Adams A1c HA-8180, Arkray Inc., Japan). Additionally, a biochemical analyser (Modular Analytics, Roche, Germany) was used to measure serum glucose, total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), high sensitivity C-reactive protein (Hs-CRP), estimated glomerular filtration rate (eGFR), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). The parameters above were evaluated in all participants at baseline and the measurements were subsequently repeated after 12 weeks.
CGM Measurements and Parameter Calculation
Glucose data were collected continuously for 7 days by professional retrospective CGM (iPro™2, Medtronic Minimed Inc., Northridge, CA, USA) in all patients at baseline and the end of the 3-month intervention. To calibrate the CGM data, all participants underwent pre-prandial SMBG four times a day, before breakfast, lunch, dinner, and bedtime, using a glucometer (Accu-Chek Mobile, Roche Diagnostics, Germany) calibrated by fasting plasma glucose, as measured by the central laboratory. These consecutive calibrated glucose profiles were recorded by the iPro™2 sensor and downloaded via Carelink iPro for further statistical analysis. To analyse GV, the standard deviation of blood glucose (SDBG), the largest amplitude of glycaemic excursions (LAGE), mean amplitude of glucose excursions (MAGE), mean of daily differences (MODD), and coefficient of variation (CV) were calculated. The parameters reflecting glycaemic control such as mean sensor glucose (MSG), time in range (TIR; glucose range of 3.9–10.0 mmol/L in 24 hours), time above range (TAR; glucose range over 10.0 mmol/L in 24 hours), and time below range (TBR; glucose range under 3.9 mmol/L in 24 hours) were also calculated using the CGM data.
Outcomes
The primary outcomes included changes in FBG and HbA1c from baseline to 12 weeks. Moreover, the secondary outcomes were changes in SD, LAGE, CV, MODD, TIR, body mass index (BMI), Hs-CRP, lipid profiles, and daily insulin dose at the 12-week follow-up. The incidence of symptomatic or biochemical hypoglycaemic events (< 2.8 mmol/L), as well as medication adverse events, were recorded for safety data analysis.
Statistical Analysis
As a pilot study, the sample size was calculated according to the feasible execution of the study. Hence, a sample size of 50 was ultimately adopted. Descriptive statistics were presented as mean ± SD for normally distributed data and median (interquartile range, IQR) for abnormally distributed data. Besides, categorical variables were presented as percentages. Continuous variables were checked for the distributional assumption of normality using the Shapiro–Wilks tests, while the Wilcoxon rank-sum test was used for abnormally distributed data. Categorical variables were compared using the chi-square test or Fisher’s exact test. Moreover, a two-tailed P-value < 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (version 23.0; SPSS Inc.).
Results
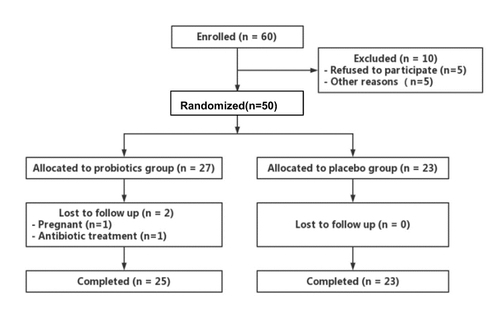
A total of 60 patients were enrolled in the study, with ten of them being excluded (five refused to participate and five did not take part for other reasons). Among the 50 eligible randomised participants, two participants in the probiotics group withdrew from the study (one became pregnant, while another required antibiotic treatment). Ultimately, 48 participants (probiotics group, n = 25; placebo group, n = 23) completed the study (). The baseline characteristics are summarised in . The average age of the participants was 39 ± 11 years, with a median diabetes duration of 10 years (interquartile range: 6–16 years). The mean daily insulin dose was 38.7 ± 18.3 U and the mean BMI was 21.1 ± 2.4 kg/m2. There was no significant difference in sex, age, duration of diabetes, BMI, blood pressure, transaminase, Hs-CRP, fasting C-peptide, or parameters of metabolic control (HbA1c level, fasting blood glucose, lipid profiles) between the probiotics group and the placebo group at baseline. Moreover, CGM parameters including SDBG, LAGE, MAGE, MODD, TIR, TAR, TBR, CV, and MSG were found to be comparable between the two groups at baseline. Furthermore, no serious adverse events were reported throughout the study.
Table 1 General Baseline Characteristics of the Study Participants
Effect of Probiotics on Glycaemic Endpoints
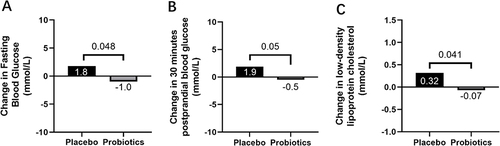
The changes in glycaemic control and GV between the probiotics group and the placebo group after the 12-week intervention are presented in . There was a significant reduction in FBG in the probiotics group, compared to the placebo group (−1.0 ± 4.7 vs 1.8 ± 4.7 mmol/L, p = 0.048, ). Additionally, 30 min postprandial blood glucose (30min-PBG) levels in the probiotics group were significantly lower than in the placebo group (−0.5 ± 4.6 vs 1.9 ± 3.3 mmol/L, p = 0.0495, ). HbA1c expression tended to decrease in both the probiotics and placebo groups, but the difference was not statistically significant (−0.49 ± 1.15% (−5.33 ± 12.60 mmol/mol) vs −0.22 ± 0.60% (−2.38 ± 6.59 mmol/mol), p = 0.310). Meanwhile, there were no noticeable differences in either the CGM parameters of glycaemic control (MSG, TIR, TAR, and TBR) or GV (SDBG, LAGE, MAGE, CV, and MODD) between the two groups.
Table 2 The Between-Group Comparisons of Metabolic Profiles, CGM Data, and Hs-CRP After Supplementation
Figure 2 Probiotics improved fasting blood glucose (FBG) and 30 minutes postprandial blood glucose (PBG) as well as low-density lipoprotein cholesterol (LDL-C). (A) Change in FBG (mmol/L) in response to Probiotics group vs Placebo group. (B) Change in 30 minutes PBG (mmol/L) in response to Probiotics group vs Placebo group. (C) Change in LDL-C (mmol/L) in response to Probiotics group vs Placebo group. Significant reductions inFBG, 30minutesPBG as well as LDL-C were observed in the patients in the Probiotics group, and the changes were significantly different compared with those in Placebo group.
Effect of Probiotics on Lipid Profile and CRP
A significant reduction in LDL-C levels was observed in the probiotics group in comparison to the placebo group (−0.07 ± 0.45 vs 0.32 ± 0.78 mmol/L, p = 0.0413, ). However, no statistical differences were detected in the other lipid indicators or Hs-CRP ().
Subgroup Analysis of Changes in the Probiotics Group
A subgroup analysis was conducted for baseline BMI, HbA1c, FBG, and sex in T1DM patients with probiotics supplementation. and list the results of the subgroup analysis, which was stratified according to the median baseline BMI (20.8 kg/m2), HbA1c (7.35%), FBG (8.62 mmol/L), and sex. The patients with a higher baseline FBG level presented a greater decrease in FBG (−2.87 ± 4.53 vs 1.51 ± 3.65 mmol/L, p = 0.013) and 30min-PBG (−2.16 ± 4.98 vs 1.69 ± 3.09 mmol/L, p = 0.027) after three months of treatment with probiotics, compared to those with a lower baseline FBG. Also, TBR decreased more significantly in patients with higher baseline FBG (−1.07 (−4.57, −0.26)% vs 2.43 (0.41, 5.19)%, p = 0.007). However, no significant differences in the changes of SDBG, LAGE, MAGE, MODD, CV, MSG, TIR, TAR, and C-peptide were found between the two subgroups. Regarding sex, male patients showed a significant reduction in MSG (−0.75 (−2.11, 0.48) mmol/L vs 1.51 (−0.37, 2.74) mmol/L, p = 0.010) and TAR (−5.47 (−20.1, 3.04)% vs 18.9 (−1.11, 35.6)%, p = 0.006) and greater improvement in TIR (9.32 (−4.84, 16.6)% vs −19.9 (−31.4, 0.69)%, p = 0.005), compared to the female patients in the probiotics group. Meanwhile, a significant increase in TIR (1.09 (0.09, 4.59)% vs −1.07 (−4.57, 1.39)%, p = 0.039) was identified in patients with lower basal HbA1c levels in the probiotics group. However, there were no major differences in the changes in blood glucose, SDBG, LAGE, MAGE, MODD, CV, MSG, TAR, TBR, and C-peptide between patients with different levels of HbA1c. Also, no significant differences in glycaemic control and GV were found in patients with different baseline BMI values.
Table 3 Subgroup Analysis of C-Peptide and Glucose Level in the Probiotics Group After Intervention
Table 4 Subgroup Analysis of Glycaemic Variability and Glycaemic Control in the Probiotics Group After Intervention
Safety Data
Among the 50 randomized participants, only 3 participants (6%) failed to complete the study protocol. Among these patients, 2 was dropped from the study due to the initiation of antibiotics and 1 was pregnant. No severe adverse events were reported during the study in two groups.
Discussion
In the analysis, we observed slight declines in the FBG and 30min-PBG levels in participants who consumed multispecies probiotic supplements, compared to those who had the placebo. Additionally, LDL-C expression was significantly lower in the probiotics group. However, there were no statistically significant differences regarding HbA1c, CGM parameters (SDBG, LAGE, MAGE, MODD, CV, MSG, TAR, TBR, and TIR), and Hs-CRP between the two groups after the 12-week trial.
The effect of probiotics on glycaemic control in individuals with diabetes has received increased attention in recent years. Researchers speculate that amelioration of gut dysbiosis using specific probiotics is associated with a decline in the risk of islet β-cell autoimmunity, reduction of Toll-like receptor 4 signalling (a type of inflammatory signalling), and improvement in gut integrity. This subsequently leads to the amelioration of T1D pathology.Citation13 Furthermore, beneficial gut bacteria may increase the synthesis of glucagon-like peptide 1 (GLP-1), which contributes to the improvement of glucose homeostasis.Citation14 For instance, as the most commonly-found gut microbiota in probiotic supplements, Bifidobacterium and Lactobacillus species have been reported to improve glucose homeostasis in several clinical trials.Citation15,Citation16 However, some studies were not clearly blinded and the bacterial strains used in other studies were not used routinely in commercial probiotics preparations.
As far as we know, this is the first double-blinded randomised controlled trial that evaluates the effect of commercial probiotics on glycaemic control in T1DM patients. Existing studies have provided either inconclusive results or reported only modest effects of probiotics supplementation on glycaemic control in diabetes patients. Several meta-analyses have shown a significant reduction in FBG with probiotics or synbiotics supplementation compared with placebos. Besides, HbA1c levels exhibited a downward trend, but without statistical significance.Citation9,Citation17 It is noteworthy that most studies included in the aforementioned meta-analyses focused on T2DM, while only one paper investigated T1DM with synbiotics as a supplement.Citation10 Interestingly, an observational study conducted by Ailaet al witnessed a significant reduction in HbA1c levels in T1DM using probiotics after 12 weeks.Citation18 They inferred that the discrepant results of different trials concerning HbA1c could be explained by different intervention lengths, since those less than 8 weeks may be too short to reveal significant changes in HbA1c expression. Further subgroup analyses revealed that probiotics were more effective in T2DM patients who were not on insulin therapy or those with poor glycaemic control. Likewise, Firouzi et al reported that a 12-week probiotics intervention resulted in greater improvements in HbA1c levels in T2DM patients with a normal weight than in those who were overweight or obese.Citation19 The similar result was also revealed by Kumar et al in children with new-onset T1DM. They found that patients treated with high dose multi-strain probiotics had a significant decrease in HbA1c as well as a significant decline in daily insulin requirement compared with those in the placebo group after 3-month intervention.Citation20 However, in our 12-week trial, the HbA1c levels in the probiotics group tended to decrease over time, but the difference was statistically insignificant.
Interestingly, both FBG and 30min-PBG levels decreased significantly in the T1DM patients who used probiotics in this study. This is an aspect that has only been examined by a limited number of other studies. Accepted as an independent predictor of cardiovascular events, PBG is marked by postprandial glycaemic peaks that result in inflammation, oxidative stress, and endothelial dysfunction. Besides, it is related to the development of insulin resistance and T2DM.Citation21 Indeed, an early study reported that gut microbiota was a co-determinant of PBG response.Citation22 Similarly, Oh et al found that daily administration of L. plantarum HAC01 over eight weeks significantly reduced 2h-PBG levels in a population with impaired glucose tolerance (IGT), compared to the placebo group.Citation23 One possible hypothesis for this is that selective increases of bifidobacterial in gut microflora improve glucose tolerance, glucose-induced insulin secretion and normalised inflammatory tone through decreasing endotoxaemia, plasma and adipose tissue proinflammatory cytokines in high-fat-diet-induced diabetes in mice.Citation24 Another mechanism may be found in the association between gut bacterial metabolism and intestinal transit time. Roager et al found that increased gut microbiota richness due to decreased intestinal transit time is accompanied by a shift in colonic metabolism from carbohydrate fermentation to protein catabolism.Citation25 However, the detailed mechanism remains largely undetermined and needs to be further explored.
Glycaemic variability, another key metric of the glucose profile other than HbA1c, has been associated with hypoglycaemia, chronic complications of diabetes, and reduced quality of life.Citation26 However, the association between gut microbiota and GV has hardly been discussed before. In this study, we observed no improvements in GV metrics in the overall study population with probiotics supplementation. However, further subgroup analysis based on gender indicated that, compared with female patients, probiotic supplements were more effective in reducing MSG and TAR and enhancing TIR in males. Popularised as an intuitive and valid measure of glucose control, TIR has been proved to be a marker of microvascular risk. For every 10% decrease in TIR, the risk of retinopathy and microalbuminuria increases by 64% and 40%, respectively.Citation27 Thus, it seems that men benefit more from probiotics supplementation. A previous study reported that men had lower gut microbial diversity and a lower abundance of multiple species conferring beneficial effects on host metabolism compared to women.Citation28 Also, in an animal experiment, Zhang et al stated that male mice had a smaller transition in the gut microbiota than female mice during the development of T1DM.Citation29 Further correlation network analysis demonstrated that gut microbiota exerted a significant effect on host metabolic changes induced by T1DM in a sex-specific manner, mainly through four different pathways: short-chain fatty acid (SCFA) metabolism, energy metabolism, amino acid metabolism, and choline metabolism.Citation29 Therefore, we speculate that probiotics, which are live microorganisms that help maintain host physiology and immune homeostasis and shape the host immune system,Citation30 have a more potent effect on men than women.
Additionally, in this study, probiotics were more effective than the placebo in reducing serum LDL-C from the baseline, but they did not affect TC, HDL-C, and TG levels. Although several pioneering studies have demonstrated the lipid-lowering effect of probiotics administration to maintain cardiovascular health,Citation31 some reported results were inconsistent or contradictory. Zarezadeh et al reported that supplementation with probiotics enhanced TC, LDL-C, and TG levels but had no effect on HDL-C, and these effects occurred in a time-dependent manner.Citation32 Furthermore, probiotics had the greatest modifying effect on the lipid profile of T2DM and non-alcoholic fatty liver disease (NAFLD) patients.Citation32 Furthermore, Aila et al found that the TG and the TG-HDL-cholesterol ratio was lower in T1DM patients with self-reported use of probiotics.Citation18 Several papers have proposed the mechanisms that are potentially responsible for the lipid-regulating effect of probiotics. The first possible mechanism is the deconjugation of bile salts, which involves the coprecipitation of intestinal cholesterol. Alternatively, lipometabolism regulation may allow the cell membrane of the probiotic to incorporate and assimilate cholesterol. Another potential mechanism is the reduction in intestinal cholesterol absorption through various pathways, which inhibits the expression of the intestinal cholesterol transporter Niemann–Pick C1-Like 1 (NPC1L1) in the enterocytes.Citation33
Low-grade chronic inflammation is a critical factor in the pathogenesis of numerous chronic conditions such as diabetes and cardiovascular disease. Although Hs-CRP did not improve with the supplementation of probiotics in this study, several other studies have observed a reduction in inflammatory biomarkers such as Hs-CRP, IL-6, and the complement system, by using probiotics in patients with diabetes.Citation34,Citation35 The reasons for these discrepant findings are unclear. We speculate the following reasons. Firstly, Type 1 diabetes and type 2 diabetes share different pathogenesis. Secondly, the relationship between inflammation and type 2 diabetes has long been established. Nevertheless, the mechanisms behind the aforementioned effects remain to be investigated.
There are several strengths and limitations of note in this study. First, this paper was a carefully designed and well-conducted clinical study and was the first paper to evaluate the effect of probiotics administration on GV in adult T1DM patients. Besides, we chose commercially available probiotic supplements as the intervention, to realise a cost-effective method that utilised probiotics in the management of T1DM. Also, the patients of this study were recruited from both the inpatient and outpatient departments of our hospital, so our findings could be generalised to include all patients in a primary care setting. Finally, the patients in both groups adhered closely to the CGM procedures with a high follow-up rate. However, this study has certain limitations. It was an exploratory clinical study conducted in a single centre and the sample size was relatively small, due to a relatively low prevalence of T1DM compared with T2DM. Moreover, although a prolonged duration (2–4 weeks) of CGM is recommended by current guidelines to reduce the likelihood of statistical bias, we only collected the CGM data for 7 days due to restrictions related to the CGM recorder.Citation36 Additionally, the impact of dietary factors and exercise on GV cannot be underestimated. To minimise such a bias, the participants received advice regarding diet and physical activity and were urged to follow a consistent diet and exercise regularly during the CGM periods.
Conclusion
This randomised, double-blind, placebo-controlled trial confirmed for the first time that the intervention with a multispecies probiotic supplement containing Bifidobacterium longum, Lactobacterium bulagricumi, and Streptococcus thermophilus for 12 weeks exerted a beneficial effect on fasting and postprandial glucose and lipid profiles in adult T1DM patients without any serious adverse events, especially for male patients and those with higher baseline FBG levels. However, this compound probiotic did not improve GV in adult T1DM patients. In conclusion, probiotics supplementation may serve as a safe adjuvant strategy for the amelioration of cardiometabolic health parameters in adult T1DM patients.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Statement of Ethics
The study was approved by the Ethics Committee of the Shenzhen People’s hospital (The Second Clinical Medical College, Jinan University, China. Ethics approval number: LL-KT-2018248). All patients provided written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no personal or financial conflicts of interest.
Acknowledgments
The authors thank all the patients who participated in this study. The authors also thank the colleagues who devoted their efforts to this study, including dieticians, diabetes educators, nurses, and technicians.
Additional information
Funding
References
- Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–20. doi:10.2337/diacare.26.2007.s5
- Lipman TH, Levitt Katz LE, Ratcliffe SJ, et al. Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia pediatric diabetes registry. Diabetes Care. 2013;36(6):1597–1603. doi:10.2337/dc12-0767
- Paun A, Yau C, Danska JS. Immune recognition and response to the intestinal microbiome in type 1 diabetes. J Autoimmun. 2016;71:10–18. doi:10.1016/j.jaut.2016.02.004
- Vatanen T, Franzosa EA, Schwager R, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562(7728):589–594. doi:10.1038/s41586-018-0620-2
- Murri M, Leiva I, Gomez-Zumaquero JM, et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. doi:10.1186/1741-7015-11-46
- Harbison JE, Roth-Schulze AJ, Giles LC, et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: a prospective cohort study. Pediatr Diabetes. 2019;20(5):574–583. doi:10.1111/pedi.12865
- Kim SH, Huh CS, Choi ID, et al. The anti-diabetic activity of Bifidobacterium lactis HY8101 in vitro and in vivo. J Appl Microbiol. 2014;117(3):834–845. doi:10.1111/jam.12573
- Valladares R, Sankar D, Li N, et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS One. 2010;5(5):e10507. doi:10.1371/journal.pone.0010507
- Rittiphairoj T, Pongpirul K, Janchot K, Mueller NT, Li T. Probiotics contribute to glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Adv Nutr. 2021;12(3):722–734. doi:10.1093/advances/nmaa133
- Zare Javid A, Aminzadeh M, Haghighi-Zadeh MH, Jamalvandi M. The effects of synbiotic supplementation on glycemic status, lipid profile, and biomarkers of oxidative stress in type 1 Diabetic Patients. A placebo-controlled, double-blind, randomized clinical trial. Diabetes Metab Syndr Obes. 2020;13:607–617. doi:10.2147/DMSO.S238867
- Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(5):1146–1156. doi:10.2337/dc19-1459
- Zhang X, Xu D, Xu P, et al. Metformin improves glycemic variability in adults with type 1 diabetes mellitus: an open-label randomized control trial. Endocr Connect. 2021;10(9):1045–1054. doi:10.1530/EC-21-0146
- Uusitalo U, Liu X, Yang J, et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. 2016;170(1):20–28. doi:10.1001/jamapediatrics.2015.2757
- Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288(35):25088–25097. doi:10.1074/jbc.M113.452516
- Toshimitsu T, Gotou A, Sashihara T, et al. Effects of 12-week ingestion of yogurt containing lactobacillus plantarum OLL2712 on glucose metabolism and chronic inflammation in prediabetic adults: a randomized placebo-controlled trial. Nutrients. 2020;12(2). doi:10.3390/nu12020374
- Sabico S, Al-Mashharawi A, Al-Daghri NM, et al. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(4):1561–156910. doi:10.1016/j.clnu.2018.08.009
- Bock PM, Telo GH, Ramalho R, et al. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2021;64(1):26–41. doi:10.1007/s00125-020-05295-1
- Aila JA, Valma H, Carol F, Riitta F, Sari M, Groop PH. The self-reported use of probiotics is associated with better glycaemic control and lower odds of metabolic syndrome and its components in type 1 diabetes. J Probiotics Health. 2017;5(4). doi:10.4172/2329-8901.1000188
- Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. 2017;56(4):1535–1550. doi:10.1007/s00394-016-1199-8
- Kumar S, Kumar R, Rohilla L, Jacob N, Yadav J, Sachdeva N. A high potency multi-strain probiotic improves glycemic control in children with new-onset type 1 diabetes mellitus: a randomized, double-blind, and placebo-controlled pilot study. Pediatr Diabetes. 2021;22(7):1014–1022. doi:10.1111/pedi.13244
- Sargsyan A, Herman MA. Regulation of glucose production in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2019;19(9):77. doi:10.1007/s11892-019-1195-5
- Sondertoft NB, Vogt JK, Arumugam M, et al. The intestinal microbiome is a co-determinant of the postprandial plasma glucose response. PLoS One. 2020;15(9):e0238648. doi:10.1371/journal.pone.0238648
- Oh MR, Jang HY, Lee SY, et al. Lactobacillus plantarum HAC01 supplementation improves glycemic control in prediabetic subjects: a randomized, double-blind, placebo-controlled trial. Nutrients. 2021;13(7):2337. doi:10.3390/nu13072337
- Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi:10.1007/s00125-007-0791-0
- Roager HM, Hansen LB, Bahl MI, et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1(9):16093. PMID: 27562254. doi:10.1038/nmicrobiol.2016.93
- Wilmot EG, Choudhary P, Leelarathna L, Baxter M. Glycaemic variability: the under-recognized therapeutic target in type 1 diabetes care. Diabetes Obes Metab. 2019;21(12):2599–2608. doi:10.1111/dom.13842
- Lu J, Home PD, Zhou J. Comparison of multiple cut points for time in range in relation to risk of abnormal carotid intima-media thickness and diabetic retinopathy. Diabetes Care. 2020;43(8):e99–e101. doi:10.2337/dc20-0561
- Zhang X, Zhong H, Li Y, Shi Z, Ren H, Ji L. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat Aging. 2021;1(1):87–100. doi:10.1038/s43587-020-00014-2
- Zhang X, Wang D, Zheng Y, et al. Sex-dependent effects on the gut microbiota and host metabolome in type 1 diabetic mice. Biochim Biophys Acta Mol Basis Dis. 2021;1867(12):166266. doi:10.1016/j.bbadis.2021.166266
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–8803. doi:10.3748/wjg.v21.i29.8787
- Dong Y, Xu M, Chen L, Bhochhibhoya A. Probiotic foods and supplements interventions for metabolic syndromes: a systematic review and meta-analysis of recent clinical trials. Ann Nutr Metab. 2019;74(3):224–241. doi:10.1159/000499028
- Zarezadeh M, Musazadeh V, Faghfouri AH, Roshanravan N, Dehghan P. Probiotics act as a potent intervention in improving lipid profile: an umbrella systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021;1–14. doi:10.1080/10408398.2021.2004578
- Reis SA, Conceicao LL, Rosa DD, Siqueira NP, Peluzio MCG. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutr Res Rev. 2017;30(1):36–49. doi:10.1017/S0954422416000226
- Raygan F, Rezavandi Z, Bahmani F, et al. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr. 2018;10:51. doi:10.1186/s13098-018-0353-2
- Raygan F, Ostadmohammadi V, Asemi Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(4):1594–1598. doi:10.1016/j.clnu.2018.07.017
- Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi:10.2337/dci19-0028