Abstract
Background
Previous studies have reported the diagnostic values of multiple obesity indicators for predicting the risk of non-alcoholic fatty liver disease. However, the diagnostic values of obesity indicators for predicting the risk of metabolic dysfunction-associated fatty liver disease (MAFLD) in early postmenopausal women is still unknown. Therefore, this study investigated the predictive values of common obesity indices for estimating the risk of MAFLD in early postmenopausal Chinese women.
Methods
This study enrolled 2514 early postmenopausal women, aged between 45 and 55 years, who underwent abdominal ultrasonography examination at the Health examination center of the Huadong Sanatorium between June 2021 and December 2021. The values for six obesity indices, namely, body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), body adiposity index (BAI), and Chinese visceral adiposity index (CVAI) were extracted from the medical records.
Results
Our data showed that all the six obesity indices were significantly associated with the risk of MAFLD (P < 0.05) in the obese subjects and five obesity indices except for BAI were significantly associated with the risk of MAFLD (P < 0.05) in the lean subjects. The six obesity indices showed a linear relationship with the risk of MAFLD (all P-values > 0.05). The ORs for the obesity indices with the exception of BAI showed proportional increase with the risk of MAFLD in the lean subjects. CVAI was the strongest predictor of the risk of MAFLD in both lean (AUC=0.868) and overweight/obese subjects (AUC=0.704) among the early postmenopausal women.
Conclusion
This study demonstrated that all the obesity indices were associated with an increased risk of MAFLD in the obese subjects and five obesity indices except for BAI were associated with an increased risk of MAFLD in the lean subjects among the early postmenopausal women. CVAI showed the strongest predictive performance in estimating the risk of MAFLD among early menopausal women.
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become a major public health concern, characterized by fatty infiltration of at least 5% of the hepatocytes, and affected approximately 30% of the general adult population worldwide.Citation1–3 The Asian Pacific Association for the study of the liver (APASL) re-designated NAFLD as metabolic dysfunction-associated fatty liver disease (MAFLD) in 2020.Citation4,Citation5 The diagnostic criteria of NAFLD are complex and are based primarily on excluding subjects with excess alcohol consumption, viral hepatitis, immunological liver injury, or genetic disorders of lipid metabolism.Citation6 However, the diagnosis of MAFLD includes all the mixed etiologies and is defined as the co-existence of hepatic steatosis with other metabolic disorders such as overweight or obesity, type 2 diabetes mellitus, and/or metabolic syndrome.Citation7 Recent studies have shown that the criteria for MAFLD are more accurate in identifying patients with high risk of fatty liver disease compared to the NAFLD criteria.Citation8–10 Therefore, the impact of metabolic dysfunction on the natural progression of liver diseases such as chronic viral hepatitis or alcoholic hepatitis is studied using the MAFLD criteria.Citation4
In the western countries, MAFLD is one of the leading causes of end-stage liver disease, cirrhosis, and hepatocellular carcinoma.Citation11 MAFLD affects a quarter of the entire global population. A recent systematic review and meta-analysis demonstrated that the prevalence of MAFLD in the Chinese population was 29.81% (27.78–31.93%).Citation12 Nearly 314.58 million cases are projected to be diagnosed as MAFLD by 2030 in China.Citation13 The socio-economic impact of MAFLD is significant on the health system in both developing and developed countries. Therefore, there is an urgent need to identify risk factors that can accurately predict the risk of MAFLD for improved diagnosis and preventive treatment of MAFLD.
Previous studies have demonstrated that obesity is strongly associated with the development and progression of MAFLD.Citation14 Inflammation is associated with both NAFLD and increased BMI. Most of the indices used in determination of NAFLD are somewhat associated with inflammation,Citation15,Citation16 especially obesity-related indices. Furthermore, waist circumference and visceral adipose tissue (VAT) show better association with the progression of MAFLD compared to BMI.Citation17,Citation18 These results suggested that the obesity-related indices may be used for predicting the risk of MAFLD. The body composition of early postmenopausal women is influenced by reduced estrogen expression and is characterized by changes in the distribution of fat from the subcutaneous to the visceral adipose tissues. Therefore, early postmenopausal women are more susceptible to MAFLD compared to the premenopausal women. Furthermore, MAFLD is reported in 19.2% of lean subjects with BMI < 23 kg/m2 and 40.8% of MAFLD patients are non-obese.Citation19 Moreover, histological severity of the MAFLD disease is comparable between non-obese and lean subjects. Therefore, factors that increase the risk of MAFLD in lean subjects require in-depth investigation.
It had been reported that obesity-related indicators such as body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), body adiposity index (BAI), and VAT are associated with accumulation of visceral fat. However, Chinese visceral adiposity index (CVAI) to estimate abdominal fat is calculated with parameters such as age, BMI, WC, total triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C), and shows better performance in predicting the risk of metabolic disorders than the VAI.Citation20,Citation21 However, currently, there is no consensus regarding the anthropometric parameters that can be used in the clinic to quantify and predict MAFLD in the early postmenopausal women. Therefore, in this study, we investigated the diagnostic performances of the obesity indices such as BMI, WC, WHR, WHtR, BAI, and CVAI for predicting the risk of MAFLD among early postmenopausal women in China.
Materials and Methods
Study Population
This cross-sectional study analyzed the clinical data of 2514 Chinese women between the ages of 45 and 55 years, and within ten years after menopause, that were evaluated at the Health Examination Center, Huadong Sanatorium between June 2021 and December 2021. Menopause was defined as at least one year since the last menstrual period. The study subjects were divided into lean group (n=1194) with BMI < 23 kg/m2 and obese group (n=1320) with BMI ≥ 23 kg/m2. All the participants underwent comprehensive anthropometric measurements, abdominal ultrasonography, and fasting blood tests. The exclusion criteria included incomplete medical records, surgical menopause, hormone replacement therapy, and severe medical diseases such as cancer or organ failure. This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics and Research Committee of Huadong Sanatorium (Approval No. ECHS2023-01). The requirement for written informed consent was waived because of the retrospective nature of the study and the data analysis was anonymous and confidential.
Anthropometric Measurements and Clinical Examination
A structured and detailed survey designed by professional physicians was used to collect the demographic and clinical parameters of the study subjects including self-reported illness and the currently used medications. The number of subjects in the smoking and alcohol consumption groups were low among early postmenopausal women, and were therefore excluded from analysis. Systolic and diastolic blood pressure was measured using an electronic brachial sphygmomanometer (T30J, OMRON, Japan). Anthropometric parameters including height, weight, waist circumference, and hip circumference were measured using standard procedures by well-trained nurses. Blood samples (8–10 mL) were collected from the antecubital vein after at least 8 h of overnight fasting and evaluated in the laboratory center within 24 h. Metabolic biomarkers and liver function parameters including fasting blood glucose (FBG), triglycerides (TGs), total cholesterol (TC), low-density-lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum uric acid (UA), serum aspartate aminotransferase (AST), and serum alanine aminotransferase (ALT) levels were measured. Furthermore, the blood counts of white blood cells (WBC) and neutrophils (NE) were also analyzed. Abdominal ultrasonography was performed using the SIEMENS ACUSON S2000 ABVS ultrasound scanner (Siemens Healthineers, Erlangen, Germany), and was operated by experienced ultra-sonographers. The data was recorded in the electronic medical system of the Health Examination Center.
Definition of MAFLD and Obesity Indices
The diagnosis of MAFLD was based on the presence of hepatic steatosis plus any one of the following three criteria:Citation5 (1) overweight and obesity defined as BMI ≥23 kg/m2; (2) type 2 diabetes mellitus; and/or (3) metabolic dysregulation. Metabolic dysregulation was defined by the occurrence of at least two of the following risks for metabolic disorders:Citation22 (1) waist circumference ≥80 cm in Asian women; (2) blood pressure ≥130/85 mmHg or specific drug treatment for controlling blood pressure; (3) plasma triglyceride ≥1.7 mmol/L or specific drug treatment; (4) plasma high-density lipoprotein cholesterol <1.3 mmol/L for women or specific drug treatment; (5) prediabetes with fasting glucose levels between 5.6 to 6.9 mmol/L; and/or (6) homeostasis model assessment of insulin resistance (HOMA-IR) score ≥2.5. Hepatic steatosis was diagnosed by the presence of hepato-renal echo contrast, ultrasound beam attenuation, liver parenchymal brightness, and vascular blurring.Citation23,Citation24
All the selected obesity-related indices were included in this study. In addition to waist circumference (WC), five other obesity indicators were calculated by following equations:
Statistical Analysis
The statistical analyses were performed using the SPSS 25.0 software and the STATA 16.0 software packages. Continuous variables were described as means ± standard deviation or medians with interquartile ranges, depending on the normality test. Categorical variables were represented as numbers (n) with percentages (%). The baseline characteristics and the biochemical parameters were compared using the Student’s t-test for normally distributed continuous variables, Mann–Whitney U-test for non-normally distributed continuous variables and the chi-square test for the categorical variables. Multivariable logistic regression analysis was performed to determine the relationship between obesity indices (per SD increase) and MAFLD. Adjusted odds ratios (ORs) were presented with 95% confidence intervals (CIs). Restricted cubic spline regression analysis was performed to estimate the association between multivariable-adjusted obesity indices and MAFLD in the lean and overweight/obese subjects and the knots were placed at the 5th, 35th, 65th, and 95th percentiles. Receiver operating characteristic (ROC) curves and area under the curve (AUC) values were used to compare the diagnostic values of the obesity indices for predicting the risk of MAFLD. A two-tailed P-value <0.05 was considered statistically significant.
Results
Basic Characteristics of the Study Participants
The demographic data, general clinical characteristics, obesity-related indices, and biochemical parameters of the 2514 included study subjects are described in . Among these participants, 51 subjects (4.3%) were diagnosed with MAFLD among the 1194 lean subjects, and 407 subjects (30.8%) were diagnosed with MAFLD among the 1320 obese subjects. Obesity indicators such as BMI, WC, WHR, WHtR, BAI, and CVAI were significantly higher in the obese subjects with MAFLD compared to those without MAFLD. However, except for BAI, the other five obesity indices showed significant differences between the lean subjects with or without MAFLD. Furthermore, the proportion of subjects with chronic diseases such as diabetes and hypertension were significantly higher in the MAFLD groups than the non-MAFLD groups among both lean and obese subjects (P < 0.05). Moreover, based on the lipid profiles, we observed significant differences in the TG and the HDL-C levels between the MAFLD and non-MAFLD subjects among both the groups (P < 0.001), whereas LDL-C levels were significantly higher in the MAFLD subjects than those without MAFLD in the obesity group (P < 0.05). The obese subjects showed significantly higher ALT, UA, WBC, and NE levels compared to the lean subjects, whereas AST levels were significantly higher in the subjects with MAFLD compared to those without MAFLD in both the lean and obese groups.
Table 1 Baseline Characteristics of the Study Population
The Six Obesity Indices are Associated with the Risk of MAFLD in Early Postmenopausal Women
shows the association of six obesity indices (BMI, WC, WHR, WHtR, BAI, and CVAI) with the risk of MAFLD in early postmenopausal women. After adjusting for potential confounding factors, except for BAI, the remaining five obesity indices were significantly associated with the risk of MAFLD in the lean group and all the six obesity indices were associated with the risk of MAFLD in the obese group. Among all the obesity indices, CVAI showed the strongest association with the risk of MAFLD among all the participants (OR=5.73, 95% CI: 2.84–11.54 for lean subjects; OR=2.26, 95% CI:1.90–2.70 for overweight/obese subjects, P-value < 0.001). Furthermore, BAI showed the weakest correlation among all the obesity indices with the risk of MAFLD in the overweight/obese subjects (OR=1.20, 95% CI:1.04–1.38, P-value < 0.05).
Table 2 Association Between Six Obesity Indices and Risk of MAFLD in the Lean and Obese Subjects
The Six Obesity Indices Show Linear Relationship with the Risk of MAFLD
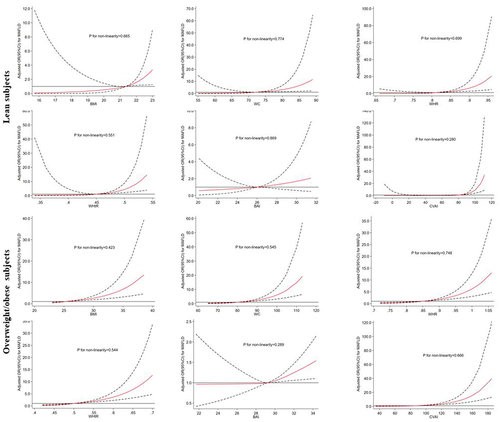
Multivariable adjusted restricted cubic spline regression analysis confirmed a linear relationship between the six obesity indices and the risk of MAFLD (all P-value > 0.05) (). The OR values for the risk of MAFLD in the lean group of early postmenopausal women increased proportionately for all the obesity indices except BAI. Furthermore, the risk of MAFLD in the overweight/obese group of early postmenopausal women also showed incremental increase with all the obesity indices. The risk of MAFLD in the early postmenopausal women increased when the CVAI values were above 80.20 and 92.01 for lean subjects and overweight/obese subjects, respectively ().
Figure 1 Dose-response relationship between obesity indices and the risk of MAFLD in the lean and overweight/obese group of early postmenopausal women after adjusting for parameters such as diabetes, hypertension, LDLC, HDLC, TG, AST, ALT WBC, NE, and UA. The solid line represents obesity index values when OR is equal to 1. The red line represents mean OR values. The area between the dashed lines represents the 95% confidence interval (CI) values of OR.
CVAI Shows the Highest Predictive Value for MAFLD
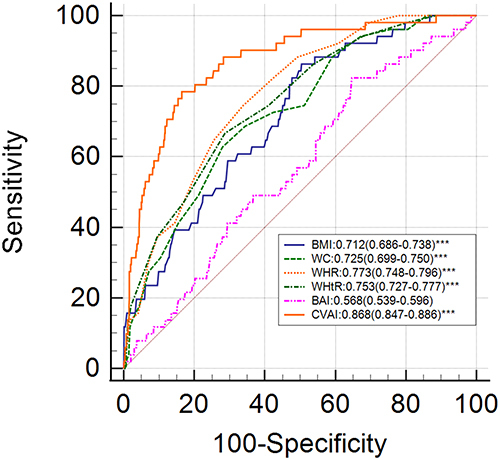
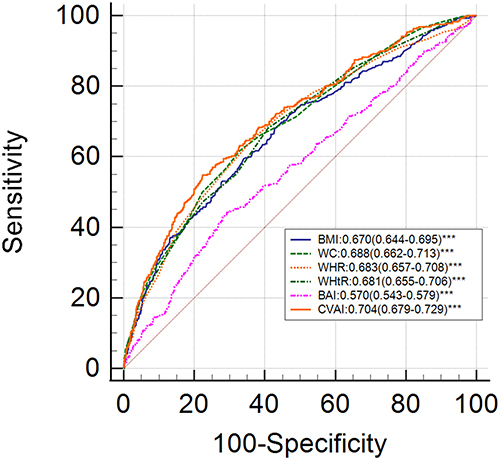
The optimal cut-off points for all the obesity indices and the corresponding ROC curves are shown in and and . The predictive values of the six obesity indices for estimating the risk of MAFLD were based on the AUC values obtained from the ROC curve analyses. CVAI showed the highest prediction value (AUC=0.820, 95% CI: 0.801–0.839) compared to the remaining five obesity parameters in all the early postmenopausal women. CVAI also showed the highest predictive value among lean subjects (AUC=0.868, 95% CI: 0.847–0.886) and overweight/obese subjects (AUC=0.704, 95% CI: 0.679–0.729). The prediction value of BAI did not show statistical significance among the lean subjects (P>0.05).
Table 3 Cut-off Points and AUC (95% CI) Values of Different Obesity Indices for Predicting MAFLD in Early Postmenopausal Women
Discussion
This cross-sectional study evaluated the diagnostic value of six commonly used, non-invasive obesity-related indicators for accurately predicting MAFLD in early postmenopausal women that belonged to lean and obese groups. In our study, CVAI showed the highest AUC value among all the obesity indices for predicting MAFLD (AUC=0.868 for lean subjects; AUC=0.704 for overweight/obese subjects).
Previous studies showed that obesity, abnormal metabolic features, and environmental factors contributed significantly to the risk of developing MAFLD.Citation25 Rapid changes in lifestyle and dietary culture have resulted in widespread prevalence of obesity in most Asian countries and caused serious socio-economic burden on the society and human health resources.Citation26 Therefore, screening of relevant risk factors of MAFLD is of great importance for early detection and preventive treatment. Liver biopsy is an invasive measure that is commonly used to diagnose MAFLD in suspected patients, but is not widely recommended for large-scale routine healthcare check-ups. Therefore, alternative non-invasive indicators are used to predict the occurrence of MAFLD in high-risk patients.Citation26 MAFLD is confirmed in high-risk patients using a liver biopsy or imaging method to verify the histopathological and imaging features, respectively. Excessive accumulation of visceral adipose tissue is associated with increased risk of MAFLD in Asians. The early postmenopausal women are more vulnerable to MAFLD because of significant changes in the sex hormones, increase in the abdominal adipose tissue deposits, abnormal lipid metabolism, and the onset of insulin resistance.Citation27
Previous studies have recommended BMI as an indicator of visceral fat accumulation and is widely used to evaluate fatty liver disease.Citation28,Citation29 BMI is a better predictor of NAFLD compared with WC and WHtR. Moreover, the AUC of BMI in subgroup analysis was greater than 0.8, and represented its good predictive performance in most populations.Citation30 However, previous study had not explored its predictive value of MAFLD among early postmenopausal women, and BMI may not reflect the abdominal adipose tissue accumulation among women after menopause. Besides, the validity of BMI as a valuable indicator of regional adipose distribution remains controversial because of its inability to distinguish between fat mass and lean body mass.Citation31 Additionally, studies also indicated that WHR and WHtR had been suggested as most useful predictor of MAFLD only in relatively young Chinese males; Furthermore, BAI represents the overall adipose accumulation and does not reflect the characteristics of abdominal fat accumulation. Although previous studies demonstrated the relative association of BMI and BAI with fatty liver disease,Citation30 the diagnostic performance of both BMI and BAI was inferior compared to the other visceral obesity indices in the early postmenopausal women. Furthermore, characteristics such as sex, age or ethnicity can influence the prediction of MAFLD.
Our findings demonstrated that CVAI was a superior predictor of MAFLD compared to five other obesity indices in early postmenopausal women. The AUC and Youden’s index values were higher for CVAI than the other obesity indices for predicting MAFLD among all the participants. CVAI which includes BMI, WC, TG, and HDL-C is used to estimate the visceral adiposity index and predict the occurrence of metabolic disorders. CVAI is widely used in predicting the incidence of diabetes, hypertension, and cardiovascular diseases.Citation20,Citation32–36 Multivariable regression analysis in our study also showed increased risk of MAFLD with every standard deviation (SD) increase in the CVAI value in the lean subjects (adjusted OR=5.73) and overweight/obese subjects (adjusted OR=2.26) among early postmenopausal women. This suggested that lean subjects with low BMI but higher visceral adipose deposits were at an increased risk of developing MAFLD than the overweight/obese subjects.
Chronic low-grade inflammation promotes development and progression of MAFLD, and also plays a role in the hepatic and extrahepatic complications.Citation37,Citation38 Our results demonstrated that the level of white blood cells and neutrophils were significantly higher in those with MAFLD group compared to those without MAFLD among the early postmenopausal women (all P-value < 0.05). Visceral adipose tissues upregulate the expression of inflammatory factors and influence the development of metabolic disorders such as chronic inflammation and insulin resistance.Citation39 Moreover, macrophage infiltration is observed in the abdominal adipose in subjects with metabolic syndrome. Furthermore, infiltration of inflammatory cells and the production of pro-inflammatory cytokines by the macrophages adversely affect insulin-dependent tissues and and pancreatic beta cells, and promote glycometabolic disorders.
Early postmenopausal women are more susceptible to metabolic dysfunction because of sex hormones disturbances. Estrogen has been shown to regulate insulin sensitivity and metabolic homeostasis, decreases inflammation in the white adipose tissues, and reduces immune cell infiltration and oxidative stress in the adipose tissues caused by the ectopic lipid accumulation in the skeletal muscle and the liver.Citation40,Citation41 Furthermore, women show higher rates of non-oxidative free fatty acid disposal and lower level of basal fatty acid oxidation than men. This increases the levels of very low-density lipoprotein (VLDL) and TG and accelerates the progression of various metabolic disorders.Citation42,Citation43 This may explain the increased risk of MAFLD in early postmenopausal women with higher CVAI. CVAI is the strongest non-invasive predictor of MAFLD among common obesity indicators in early postmenopausal women. Therefore, CVAI can be recommended as an early screening indicator for identifying large health check-up populations among early postmenopausal women with high risk of developing MAFLD.
This study has several limitations. Firstly, this was a single-center, cross-sectional, and retrospective study that could not confirm the causal association between the obesity indices and the risk of MAFLD. Therefore, in the future, a large-cohort, multi-center, prospective study is required to confirm our findings. Secondly, this study included early postmenopausal women from China. This may have resulted in bias and may not be applicable to subjects from other countries. Finally, abdominal ultrasonography is a reliable and non-invasive approach to diagnose MAFLD. However, it does not adequately estimate the levels of hepatic steatosis and fibrosis. Therefore, we could not verify if the obesity indicators increased the risk of hepatic steatosis or fibrosis.
Conclusion
This study of early postmenopausal women demonstrated that five of the six obesity indices (except BAI) were significantly associated with the risk of MAFLD in the lean subjects and all the six obesity indices were significantly associated with the risk of MAFLD in the obese subjects. CVAI was the strongest predictor of MAFLD among all the six obesity indicators. Therefore, our study demonstrates that CVAI is a promising non-invasive diagnostic biomarker for identifying subjects with high risk of developing MAFLD.
Data Sharing Statement
The raw data used for this study is available from the first author upon reasonable request.
Informed Consent Statement
Written informed consent was waived due to the anonymous retrospective analysis of the electronic health check-up records and the advance removal of personal private information.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi:10.1002/hep.28431
- European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi:10.1016/j.jhep.2015.11.004
- Pouwels S, Sakran N, Graham Y, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63.
- Eslam M, Sarin SK, Wong VW, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14(6):889–919. doi:10.1007/s12072-020-10094-2
- Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi:10.1053/j.gastro.2019.11.312
- Davis T. Diabetes and metabolic dysfunction-associated fatty liver disease. Metabolism. 2021;123:154868. doi:10.1016/j.metabol.2021.154868
- Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
- Yamamura S, Eslam M, Kawaguchi T, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLDJ. Liver Int. 2020;40(12):3018–3030. doi:10.1111/liv.14675
- Liu Q, Zhao G, Li Q, et al. A comparison of NAFLD and MAFLD diagnostic criteria in contemporary urban healthy adults in China: a cross-sectional study. BMC Gastroenterol. 2022;22(1):471. doi:10.1186/s12876-022-02576-4
- Lin S, Huang J, Wang M, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089. doi:10.1111/liv.14548
- Lin H, Zhang X, Li G, et al. Epidemiology and clinical outcomes of metabolic (dysfunction)-associated fatty liver disease. J Clin Transl Hepatol. 2021;9(6):972–982. doi:10.14218/JCTH.2021.00201
- Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi:10.1016/S2468-1253(19)30039-1
- Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi:10.1016/j.jhep.2018.05.036
- Huh JH, Kim KJ, Kim SU, et al. Obesity is an important determinant of severity in newly defined metabolic dysfunction-associated fatty liver disease. Hepatobiliary Pancreat Dis Int. 2022;21(3):241–247. doi:10.1016/j.hbpd.2022.03.009
- Kosekli MA, Kurtkulagii O, Kahveci G, et al. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: the abund study. Rev Assoc Med Bras. 2021;67:549–554. doi:10.1590/1806-9282.20201005
- Aktas G, Kocak MZ, Duman TT, et al. Mean Platelet Volume (MPV) as an inflammatory marker in type 2 diabetes mellitus and obesity. Bali Med J. 2018;7:3. doi:10.15562/bmj.v7i3.806
- Kuk JL, Church TS, Blair SN, et al. Measurement site and the association between visceral and abdominal subcutaneous adipose tissue with metabolic risk in women. Obesity. 2010;18(7):1336–1340. doi:10.1038/oby.2009.414
- Demerath EW, Reed D, Rogers N, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levelsJ. Am J Clin Nutr. 2008;88(5):1263–1271. doi:10.3945/ajcn.2008.26546
- Lee S, Kim KW, Lee J. Sex-specific Cutoff Values of Visceral Fat Area for Lean vs overweight/obese nonalcoholic fatty liver disease in Asians. J Clin Transl Hepatol. 2022;10(4):595–599. doi:10.14218/JCTH.2021.00379
- Han M, Qin P, Li Q, et al. Chinese visceral adiposity index: a reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab Res Rev. 2021;37(2):e3370. doi:10.1002/dmrr.3370
- Xia MF, Lin HD, Chen LY, et al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: a prospective cohort study. Diabetes Metab Res Rev. 2018;34(7):e3048. doi:10.1002/dmrr.3048
- Kang SH, Cho Y, Jeong SW, et al. From nonalcoholic fatty liver disease to metabolic-associated fatty liver disease: big wave or ripple? Clin Mol Hepatol. 2021;27(2):257–269. doi:10.3350/cmh.2021.0067
- Rajindrajith S, Pathmeswaran A, Jayasinghe C, et al. Non-alcoholic fatty liver disease and its associations among adolescents in an urban, Sri Lankan community. BMC Gastroenterol. 2017;17(1):135. doi:10.1186/s12876-017-0677-7
- Chen YL, Li H, Li S, et al. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21(1):212. doi:10.1186/s12876-021-01782-w
- Sheng G, Lu S, Xie Q, et al. The usefulness of obesity and lipid-related indices to predict the presence of Non-alcoholic fatty liver disease. Lipids Health Dis. 2021;20(1):134. doi:10.1186/s12944-021-01561-2
- Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281. doi:10.1053/j.gastro.2018.12.036
- Khalfa A, Tiali A, Zemour L, et al. Prevalence of metabolic syndrome and its association with lifestyle and cardiovascular biomarkers among postmenopausal women in western Algeria. Int J Gynaecol Obstet. 2017;138(2):201–206. doi:10.1002/ijgo.12206
- Almeida NS, Rocha R, Cotrim HP, et al. Anthropometric indicators of visceral adiposity as predictors of non-alcoholic fatty liver disease: a review. World J Hepatol. 2018;10(10):695–701. doi:10.4254/wjh.v10.i10.695
- Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–544. doi:10.1016/j.jhep.2018.10.033
- Cai J, Lin C, Lai S, et al. Waist-to-height ratio, an optimal anthropometric indicator for metabolic dysfunction associated fatty liver disease in the Western Chinese male population. Lipids Health Dis. 2021;20(1):145. doi:10.1186/s12944-021-01568-9
- Agbim U, Carr RM, Pickett-Blakely O, et al. Ethnic disparities in adiposity: focus on non-alcoholic fatty liver disease, visceral, and generalized obesity. Curr Obes Rep. 2019;8(3):243–254. doi:10.1007/s13679-019-00349-x
- Li B, Lai X, Yan C, et al. The associations between neutrophil-to-lymphocyte ratio and the Chinese Visceral Adiposity Index, and carotid atherosclerosis and atherosclerotic cardiovascular disease risk. Exp Gerontol. 2020;139:111019. doi:10.1016/j.exger.2020.111019
- Li B, Wang J, Zhou X, et al. Chinese visceral adiposity index is more closely associated with hypertension and prehypertension than traditional adiposity indices in Chinese population: results from the REACTION Study. Front Endocrinol. 2022;13:921997. doi:10.3389/fendo.2022.921997
- Pan L, Xu Q, Liu J, et al. Dose-response relationship between Chinese visceral adiposity index and type 2 diabetes mellitus among middle-aged and elderly Chinese. Front Endocrinol. 2022;13:959860. doi:10.3389/fendo.2022.959860
- Shang L, Li R, Zhao Y, et al. Association between Chinese visceral adiposity index and incident type 2 diabetes mellitus in Japanese adults. Diabetes Metab Syndr Obes. 2021;14:3743–3751. doi:10.2147/DMSO.S322935
- Han M, Qie R, Li Q, et al. Chinese visceral adiposity index, a novel indicator of visceral obesity for assessing the risk of incident hypertension in a prospective cohort study. Br J Nutr. 2021;126(4):612–620. doi:10.1017/S0007114520004298
- Masarone M, Rosato V, Dallio M, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. doi:10.1155/2018/9547613
- Theofilis P, Vordoni A, Nakas N, et al. Endothelial dysfunction in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Life. 2022;12:5. doi:10.3390/life12050718
- Lu Y, Yang H, Xu Z, et al. Association between different obesity patterns and the risk of developing type 2 diabetes mellitus among adults in Eastern China: a cross-sectional study. Diabetes Metab Syndr Obes. 2021;14:2631–2639. doi:10.2147/DMSO.S309400
- Camporez JP, Lyu K, Goldberg EL, et al. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J Physiol. 2019;597(15):3885–3903. doi:10.1113/JP277270
- Nickelson KJ, Stromsdorfer KL, Pickering RT, et al. A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice. Exp Diabetes Res. 2012;2012:859395. doi:10.1155/2012/859395
- Koutsari C, Basu R, Rizza RA, et al. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab. 2011;96(2):541–547. doi:10.1210/jc.2010-1651
- Lewis GF, Uffelman KD, Szeto LW, et al. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95(1):158–166. doi:10.1172/JCI117633