Abstract
Purpose
It is unknown whether there is an association between 25-hydroxyvitamin D (25(OH)D) level and thyroid disease in postmenopausal women with type 2 diabetes. This study aimed to evaluate the relationship between blood 25(OH)D levels and thyroid function in postmenopausal women with type 2 diabetes mellitus (T2DM).
Methods
This cross-sectional study involved Chinese postmenopausal women who presented to our diabetes clinic from March 2021 to May 2022 and were diagnosed with T2DM collected via a convenience sampling method. Blood samples were obtained from each patient to detect serum thyroid-stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), free T3 (FT3), free T4 (FT4), thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TgAb) and 25(OH)D levels. Deficiency was defined as a 25(OH)D < 20 ng/mL. Comparative analysis was via t-test or chi-square test. Pearson correlation was then used to estimate the relationship between different thyroid function parameters and 25(OH)D. Multivariate logistic regression analysis was used to explore potential risk factors for 25(OH)D deficiency.
Results
In total, 157 out of 230 participants (68.26%) had 25(OH)D deficiency. Compared with patients with normal 25(OH)D levels, patients with 25(OH)D deficiency had shorter medical histories of diabetes mellitus (DM, p = 0.001) and higher rates of hyperthyroidism (p = 0.007), hypothyroidism (p < 0.001), TPOAb positive (p < 0.001) and TgAb positive (p < 0.001). Correlation analysis revealed that TSH (r = −0.144, p = 0.030), FT4 (r = −0.145, p = 0.029), TPOAb (r = −0.216, p = 0.001) and TgAb (r = −0.150, p = 0.024) levels were correlated with serum 25(OH)D levels. Further multivariable logistic regression analyses suggested that the length of DM history, presence of hyperthyroidism, presence of hypothyroidism and positive TPOAb were significantly associated with the presence of 25(OH)D deficiency in postmenopausal women with T2DM.
Conclusion
Hyperthyroidism, hypothyroidism and TPOAb positivity were significantly associated with the presence of 25(OH)D deficiency in postmenopausal women with T2DM.
Introduction
It has been reported that patients with type 2 diabetes mellitus (T2DM) are more susceptible to thyroid dysfunction.Citation1,Citation2 Thyroid dysfunction and T2DM are closely linked.Citation3 T2DM has been found to be negatively correlated with triiodothyronine (T3) and positively correlated with thyroxine (T4).Citation4 Changes in the internal environment for patients with diabetes can affect thyroid hormone levels, consequently affecting glucose and lipid metabolism. Both foreign and domestic studies have shown that the incidence of thyroid disease is higher in patients with diabetes than in healthy individuals.Citation5 Foreign epidemiological studies have shown that the proportion of thyroid dysfunction is significantly higher in patients with diabetes.Citation6,Citation7
Vitamin D is a kind of steroid molecule, in the form of vitamin D3 and D2, both of which are transported to the liver to be converted to 25-hydroxyvitamin D (25(OH)D).Citation8 This type of vitamin D is the main circulating form of vitamin D, representing the whole body’s vitamin D status.Citation8 The major biological action of vitamin D is to regulate calcium and phosphorus metabolism and preserve bone health.Citation9 Recently, the extra-skeletal actions of vitamin D and its role in the regulation of the endocrine system have been reported.Citation9 Previous studies showed that a low blood 25(OH)D level was a possible risk factor for T2DM.Citation10,Citation11 Deficiency in 25(OH)D can impair pancreatic beta-cell function, playing an important role in the onset and development of insulin resistance.Citation10 It has been reported that 25(OH)D deficiency increases the risk of autoimmune thyroid diseases such as Hashimoto’s thyroiditis and Graves’ disease.Citation12 Impaired vitamin D signalling has also been found to promote thyroid tumorigenesis.Citation13
As previously stated, 25(OH)D deficiency is thought to be a predisposing factor related to both thyroid dysfunction and glucose intolerance. This might provide the biological background needed to suggest the hypothesis that 25(OH)D levels may be associated with thyroid dysfunction in patients with T2DM. In animal models, Alrefaie et alCitation14 found that diabetes inhibited the peripheral conversion of T4 into T3 secondary to reduction in deiodinase 2 expression, while vitamin D greatly corrected thyroid profile alterations. In a cross-sectional case-control study, Bener et alCitation15 demonstrated a strong positive association between vitamin D deficiency and thyroid disease in patients with T2DM. The data from these studies supported our hypothesis.
Evidence of 25(OH)D inadequacy in postmenopausal women has been obtained by studies across the world.Citation16,Citation17 However, there is very limited evidence of the relationship between 25(OH)D level and thyroid disease in postmenopausal women with T2DM. Thus, in the present study, we aimed to evaluate the relationship between blood 25(OH)D levels and thyroid function variables in postmenopausal women with T2DM.
Materials and Methods
Patients
We conducted a cross-sectional study in which Chinese postmenopausal women who presented to our diabetes clinic from March 2021 to May 2022 and were diagnosed with T2DM were collected via a convenience sampling method. The inclusion criteria were as follows: 1) T2DM in accordance with the Chinese guidelines for the prevention and treatment of type 2 diabetes (2020 edition) diagnostic criteria;Citation18 and 2) menopause for more than one year. The exclusion criteria were as follows: 1) Patients with endocrine system diseases other than diabetes and thyroid diseases; 2) patients with arthritis, recent fractures, metabolic bone disease, severe infection, malignant tumours and anaemia; 3) patients who used vitamin D supplements, glucocorticoids, calcium and other drugs that affect vitamin D concentration in the past three months; and 4) patients with severe cardiac insufficiency or severe liver and kidney dysfunction. Vitamin D was mainly derived from dietary intake in all patients. This study was approved by the Ethics Committee of Yulin No.2 Hospital in accordance with the Declaration of Helsinki. All participants gave informed consent for their data to be published.
Data Collection
On the day of enrolment, a structured medical interview was performed by a trained physician to obtain information on demographic characteristics such as age, menopausal age, lifestyle habits (eg smoking status), medical history for T2DM, medical history for thyroid diseases and treatment protocols. Following this, height and weight were determined, and a physical examination was performed according to standard protocol.Citation19
The blood samples of each patient were obtained by venipuncture after an 8-hour fast. These were shipped to the central laboratory of our hospital immediately after collection. The serums were separated and stored at −80°C until examination. Serum thyroid-stimulating hormone (TSH), free T3 (FT3), free T4 (FT4), T3, T4, thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) levels were measured by electrochemiluminescence (Roche, Germany). Serum 25(OH)D levels were determined by a commercially available enzyme immunoassay kit (OCTEIA 25-[OH]D Kit, Immuno Diagnostic Systems, Boldon, UK; within-run coefficient of variations [CVs] of ≤7%, within-laboratory CVs of <9.5% and between-laboratory precision CVs of ≤10.1%).Citation20 A deficiency of 25(OH)D was defined as serum 25(OH)D levels lower than 20 ng/mL.Citation21 Levels of 25(OH)D >30 ng/mL were considered normal. The normal ranges for TPOAb and TgAb were 0–35 IU/L and 0–115 IU/L, respectively. A measured value greater than the upper limit of the normal range was defined as TPOAb or TgAb positive. The normal reference ranges for TSH, T3, T4, FT3 and FT4 were 0.27–4.20 mIU/L, 1.30–3.10 nmol/L, 66.00–181.00 nmol/L, 3.10–6.80 nmol/L and 12.00–22.00 nmol/L, respectively.
Statistical Analysis
The data in this study were analysed using SPSS (version 22.0). All data were tested for normality prior to statistical analysis using the Shapiro–Wilk normality test. Continuous variables conforming to a normal distribution were presented as . The differences in means were compared by Student’s t-test (for two groups). The qualitative data were expressed as percentages and compared by chi-square test. The relationship between different thyroid function parameters with 25(OH)D was estimated by Pearson correlation analysis. Multivariate logistic regression was used to explore potential risk factors for 25(OH)D deficiency, and stepwise regression was used to include variables for analysis. A value of p < 0.05 indicated the difference was statistically significant.
Results
Baseline Characteristics
From March 2021 to May 2022, a total of 230 postmenopausal women with an established diagnosis of T2DM were recruited for this study. The mean age of the patients was 62.4 ± 9.2 years, and the mean body mass index was 21.7 ± 1.4 kg/m2. Positive TPOAb and TgAb were observed in 99 (43.04%) and 86 (37.39%) patients, respectively, and 46.96% (108/230) of the enrolled patients had TPOAb and/or TgAb positivity. Compared with the reference range, 14.78% (34/230) and 23.04% (53/230) of patients had higher and lower TSH levels, respectively. The mean 25(OH)D level of the included patients was 18.30 ± 11.41 ng/mL. Deficiency of 25(OH)D was found in 157 patients (68.26%). They were included in the 25(OH)D deficiency group. The other 73 patients (31.74%) with normal 25(OH)D levels were included in the 25(OH)D normal group. The characteristics of the patients in both groups are shown in .
Table 1 The Characteristics of Studied Patients
Univariate Analysis
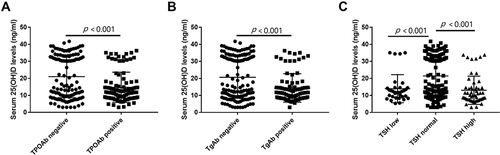
Compared with patients in the control group, patients with 25(OH)D deficiency had longer medical histories of diabetes mellitus (DM, p = 0.001) and higher rates of hyperthyroidism (p = 0.007), hypothyroidism (p < 0.001), TPOAb positivity (p < 0.001) and TgAb positivity (p < 0.001). Furthermore, TPOAb-positive patients were found to have much lower 25(OH)D levels than TPOAb-negative patients (14.76 ± 8.89 vs 21.01 ± 12.38, p < 0.001, ). The levels of 25(OH)D in TgAb-positive patients were also much lower than those in TgAb-negative patients (14.38 ± 8.36 vs 20.68 ± 12.34, p < 0.001, ). Moreover, serum 25(OH)D levels were significantly decreased in patients with lower (13.96 ± 8.24 vs 21.32 ± 12.06, p < 0.001, ) or higher (13.04 ± 8.28 vs 21.32 ± 12.06, p < 0.001, ) TSH levels compared with patients with normal TSH levels.
Figure 1 The comparisons of serum 25(OH)D levels in patients with or without TPOAb positive (A), patients with or without TgAb positive (B) and patients with different TSH levels (C).
Multivariate Analysis
Correlation analysis revealed that TSH (r = −0.144, p = 0.030), FT4 (r = −0.145, p = 0.029), TPOAb (r = −0.216, p = 0.001) and TgAb (r = −0.150, p = 0.024) levels were correlated with serum 25(OH)D levels ().
Table 2 Correlation of Different Thyroid Function Parameters with 25(OH)D
The incidence of 25(OH)D deficiency was used as the dependent variable (occurrence = 1, no occurrence = 0). Laboratory parameters with differences and baseline data were used as independent variables, which were included in the independent variables for analysis by stepwise regression. Further multivariable logistic regression analyses suggested that length of DM history (odds ratio [OR]: 1.996, 95% confidence interval [CI]: 1.239–3.214), presence of hyperthyroidism (OR: 3.563, 95% CI: 1.126–11.274), presence of hypothyroidism (OR: 4.102, 95% CI: 1.560–10.791) and TPOAb positive (OR: 2.694, 95% CI: 1.318–5.506) were significantly associated with the presence of 25(OH)D deficiency in postmenopausal women with T2DM ().
Table 3 Logistic Multivariate Regression Analysis of Risk Factors for 25(OH)D Insufficiency
Discussion
In the present study, we found that the length of DM history, presence of hyperthyroidism, presence of hypothyroidism and TPOAb positivity were significantly associated with the presence of 25(OH)D deficiency in our study population. This suggested that 25(OH)D deficiency was correlated with thyroid disorders in postmenopausal women with T2DM.
The relationships between vitamin D deficiency, T2DM and thyroid disease have been explored in previous studies. In a study conducted by Bener et al,Citation15 the authors enrolled 546 patients with T2DM and 546 control participants and showed a strong positive association between vitamin D deficiency and an increased risk of thyroid disease among patients with T2DM. One study noted that patients with T2DM are more likely to develop thyroid autoimmunity compared with the normal population, and serum 25(OH)D levels contribute to thyroid autoimmunity.Citation22 In the present study, we also found vitamin D deficiency was correlated with thyroid disorders in postmenopausal women with T2DM. Univariate analysis showed that hyperthyroidism, hypothyroidism, TPOAb positivity and TgAb positivity were closely related to serum 25(OH)D levels. Correlation analysis supported these findings. Multivariable logistic regression analysis further determined that the presence of hyperthyroidism, presence of hypothyroidism and TPOAb positivity were significantly associated with the presence of 25(OH)D deficiency. It has been reported that the prevalence of autoimmune thyroid diseases is significantly higher among patients with T2DM than in the general population.Citation23 Due to the functions of 25(OH)D deficiency and T2DM, we observed high TPOAb and TgAb positive rates in our study population. Most effects of vitamin D are mediated via the vitamin D receptor (VDR). The VDR is expressed in various cells, including immune cells. Vitamin D enhances the innate immune response, exerts immunomodulatory properties and moderates cytokines via the VDR, resulting in thyroid damage and dysfunction.Citation24 At the first stage of thyroid damage, T3 and T4 are released into the serum, resulting in the development of hyperthyroidism. As the extent of thyroid damage, the production of T3 and T4 is inhibited. Thus, hypothyroidism becomes present. Therefore, 25(OH)D deficiency was previously found to be closely related to both hyperthyroidism and hypothyroidism.Citation25,Citation26 Consistent with these findings, we also found that both hyperthyroidism and hypothyroidism were significantly associated with 25(OH)D deficiency in postmenopausal women with T2DM.
Based on previous studies, 25(OH)D deficiency can exacerbate positive thyroid autoantibodies such as TPOAb and TgAb.Citation27 The presence of TPOAb and TgAb in serum is characteristic of autoimmune thyroid diseases. Chen et alCitation28 discovered that serum 25(OH)D levels were lower in patients with elevated antithyroid antibodies than in those without them, and 25(OH)D levels may be an independent factor affecting the presence of TPOAb in autoimmune thyroid diseases. Fang et alCitation29 revealed that thyroid autoantibody (TPOAb and TgAb) positivity was closely associated with vitamin D deficiency, suggesting the involvement of vitamin D in the pathogenesis of autoimmune thyroid diseases. Similarly, Sulejmanovic et alCitation30 found that vitamin D deficiency was correlated with the presence of antithyroid antibodies. In the present study, we found that the presence of TPOAb and TgAb positivity was significantly higher in patients with 25(OH)D deficiency than in those without it, consistent with the findings from previous studies in other populations.Citation29,Citation31 However, of the two, only TPOAb positivity was finally included in the logistic regression equation. The exclusion of TgAb positivity might be due to the small sample size of this study.
Our study found that vitamin D levels were low in patients with high or low TSH levels. The TSH level is a sensitive indicator of thyroid function, and high and low TSH levels are associated with hypothyroidism and hyperthyroidism, respectively. The possible mechanisms of vitamin D affecting TSH levels are as follows. First, vitamin D can inhibit the Th1 cell-mediated immune response and thus play a role in protecting the thyroid gland. Under vitamin D deficiency, Th1-type cytokines induce B cells to produce thyroid antibodies, mediate immune responses, undergo lymphocyte infiltration and damage normal thyroid tissue, ultimately leading to hypothyroidism.Citation32 Second, the regulatory effect of TSH on thyroid cells, mainly through the G protein-adenylate cyclase-cyclic adenosine monophosphate system, has a certain impact on intracellular calcium levels during this process. When vitamin D deficiency leads to calcium channel closure, intracellular calcium levels change, and the TSH signalling pathway is impacted. Thyroid iodine intake and utilisation rate are reduced, resulting in compensatory enhancement of the activity of 5’ deiodinase, promoting the secretion of more T3 by the thyroid gland. The pituitary gland also secretes a large amount of compensatory TSH, resulting in hyperthyroidism.Citation33 Early animal experiments showed that the synthesis rate of 1,25(OH)2D was significantly decreased after thyroid T3 and T4 perfusion in rat kidneys. Thus, abnormal thyroid function metabolism in patients with T2DM can further exacerbate vitamin D deficiency status.
Apart from thyroid function parameters, we also found that the length of DM history was significantly associated with 25(OH)D deficiency in our study population. Patients with longer histories of DM may have longer histories of antidiabetic drug usage and therapeutic dietary restriction. Previous studies showed that antidiabetic medications, as well as therapeutic dietary restriction, could decrease vitamin D levels in patients with T2DM.Citation15 This mechanism may explain our findings on the relationship between DM history length and 25(OH)D deficiency. It is also possible that patients with a long history of T2DM are prone to thyroid disease, and concomitant thyroid dysfunction further affects vitamin D intake and metabolism. Type 2 diabetes, 25(OH)D and autoimmune thyroid disease are closely related, and a vicious cycle occurs, resulting in decreased vitamin D levels. Xiaoyan et alCitation34 studied thyroid levels in patients suffering from DM with different disease courses and found that FT3 and FT4 levels were decreased in patients with disease courses of more than five years, indicating that there is a close relationship between disease course and thyroid function diseases in patients with T2DM. This suggests that the longer the history of diabetes, the more attention should be paid to vitamin D supplementation.
The present study has several limitations. First, as a cross-sectional study, causality cannot be established. Second, this study was conducted in winter in northern China. The lack of sun exposure caused a high rate of 25(OH)D deficiency in postmenopausal women with T2DM. Thus, our findings need to be confirmed in other regions. Third, thyroid ultrasonography and other imageological examinations were not used to evaluate thyroid function in this study. Fourth, only one single time point of blood sample was analysed, which may have resulted in measurement error. Finally, hypoglycaemic drugs may lead to insufficient vitamin D in diabetic patients, which is not considered in this study. Therefore, further studies with adequate design are needed to confirm our findings. In future, we will conduct multicentre studies in other regions to improve the extrapolation of our findings and enable our results to be widely used.
Conclusion
Our results showed that hyperthyroidism, hypothyroidism and TPOAb positivity were significantly associated with the presence of 25(OH)D deficiency in postmenopausal women with T2DM. Postmenopausal women with T2DM should be reminded to pay attention to vitamin D supplementation to prevent possible thyroid disease.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Yulin NO.2 Hospital and informed consent was obtained from all participants.
Disclosure
The authors declare no conflicts of interest.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Zou J, Li Z, Tian F, et al. Association between normal thyroid hormones and diabetic retinopathy in patients with type 2 diabetes. Biomed Res Int. 2020;2020:8161797. doi:10.1155/2020/8161797
- Al-Geffari M, Ahmad NA, Al-Sharqawi AH, Youssef AM, Alnaqeb D, Al-Rubeaan K. Risk factors for thyroid dysfunction among type 2 diabetic patients in a highly diabetes mellitus prevalent society. Int J Endocrinol. 2013;2013:417920. doi:10.1155/2013/417920
- Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev. 2019;40(3):789–824. doi:10.1210/er.2018-00163
- Gu Y, Li H, Bao X, et al. The relationship between thyroid function and the prevalence of type 2 diabetes mellitus in euthyroid subjects. J Clin Endocrinol Metab. 2017;102(2):434–442. doi:10.1210/jc.2016-2965
- Li L, Zhongyan S. Normal thyroid function and blood lipid. Int J Endocrinol Metabol. 2010;30(5):308–310.
- Islam S, Yesmine S, Khan SA, Alam NH, Islam S. A comparative study of thyroid hormone levels in diabetic and non-diabetic patients. Southeast Asian J Trop Med Public Health. 2008;39(5):913–916.
- Zhu Y, Xu F, Shen J, et al. Prevalence of thyroid dysfunction in older Chinese patients with type 2 diabetes-A multicenter cross-sectional observational study across China. PLoS One. 2019;14(5):e0216151. doi:10.1371/journal.pone.0216151
- Kim D. The role of vitamin D in thyroid diseases. Int J Mol Sci. 2017;18(9):1949. doi:10.3390/ijms18091949
- Miteva MZ, Nonchev BI, Orbetzova MM, Stoencheva SD. Vitamin D and autoimmune thyroid diseases - a review. Folia Med (Plovdiv). 2020;62(2):223–229. doi:10.3897/folmed.62.e47794
- Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381(6):520–530. doi:10.1056/NEJMoa1900906
- Vondra K, Hampl R. Vitamin D and new insights into pathophysiology of type 2 diabetes. Horm Mol Biol Clin Investig. 2021;42(2):203–208. doi:10.1515/hmbci-2020-0055
- Zhao R, Zhang W, Ma C, et al. Immunomodulatory function of vitamin D and its role in autoimmune thyroid disease. Front Immunol. 2021;12:574967. doi:10.3389/fimmu.2021.574967
- Muscogiuri G, Tirabassi G, Bizzaro G, et al. Vitamin D and thyroid disease: to D or not to D? Eur J Clin Nutr. 2015;69(3):291–296. doi:10.1038/ejcn.2014.265
- Alrefaie Z, Awad H. Effect of vitamin D3 on thyroid function and de-iodinase 2 expression in diabetic rats. Arch Physiol Biochem. 2015;121(5):206–209. doi:10.3109/13813455.2015.1107101
- Bener A, Ozdenkaya Y, Al-Hamaq A, Barisik CC, Ozturk M. Low vitamin D deficiency associated with thyroid disease among type 2 diabetic mellitus patients. J Clin Med Res. 2018;10(9):707–714. doi:10.14740/jocmr3507w
- Chung YS, Chung DJ, Kang MI, et al. Vitamin D repletion in Korean postmenopausal women with osteoporosis. Yonsei Med J. 2016;57(4):923–927. doi:10.3349/ymj.2016.57.4.923
- Liu C, Kuang X, Li K, Guo X, Deng Q, Li D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Food Funct. 2020;11(12):10817–10827. doi:10.1039/d0fo00787k
- Chinese Diabetes Society, Chinese Medical Association. Chinese guidelines for the prevention and treatment of type 2 diabetes (2020 Edition). Int J Endocrinol Metabol. 2021;41(05):482–548.
- Chen H. Effect of Changes in Physical Measurement Index on Urinary 8-Hydroxydeoxyguanosine Levels [D]. Huazhong University of Science and Technology; 2018.
- Zhang R, Muyiduli X, Su D, et al. Effect of low-dose vitamin D supplementation on serum 25(Oh)D in school children and white-collar workers. Nutrients. 2017;9(5):505. doi:10.3390/nu9050505
- Ferreira PP, Cangussu L, Bueloni-Dias FN, et al. Vitamin D supplementation improves the metabolic syndrome risk profile in postmenopausal women. Climacteric. 2020;23(1):24–31. doi:10.1080/13697137.2019.1611761
- Nan X, Li X, Xiang Y, et al. Thyroid autoantibody distribution in patients with latent autoimmune diabetes in youth: a multicenter, national survey. Ann Transl Med. 2022;10(16):851. doi:10.21037/atm-22-423
- Sarfo-Kantanka O, Sarfo FS, Ansah EO, et al. Frequency and determinants of thyroid autoimmunity in Ghanaian type 2 diabetes patients: a case-control study. BMC Endocr Disord. 2017;17(1):2. doi:10.1186/s12902-016-0152-4
- Dutta D, Sharma M, Aggarwal S, Mohindra R, Bhattacharya S, Kalra S. Vitamin D, thyroid autoimmunity and cancer: an interplay of different factors. Indian J Endocrinol Metab. 2019;23(5):507–513. doi:10.4103/ijem.IJEM_526_19
- Appunni S, Rubens M, Ramamoorthy V, et al. Association between vitamin D deficiency and hypothyroidism: results from the National Health and Nutrition Examination Survey (NHANES) 2007–2012. BMC Endocr Disord. 2021;21(1):224. doi:10.1186/s12902-021-00897-1
- Zhou Y, Wang X, Xin M, Zhuang H. Changes in bone mineral density, 25-hydroxyvitamin D(3) and inflammatory factors in patients with hyperthyroidism. Exp Ther Med. 2021;21(6):617. doi:10.3892/etm.2021.10049
- Taheriniya S, Arab A, Hadi A, Fadel A, Askari G. Vitamin D and thyroid disorders: a systematic review and meta-analysis of observational studies. BMC Endocr Disord. 2021;21(1):171. doi:10.1186/s12902-021-00831-5
- Chen Y, Han B, Zhu C, et al. Bidirectional Mendelian randomization analysis for vitamin D and thyroid peroxidase antibody. Int J Endocrinol. 2022;2022:2260388. doi:10.1155/2022/2260388
- Fang F, Chai Y, Wei H, et al. Vitamin D deficiency is associated with thyroid autoimmunity: results from an epidemiological survey in Tianjin, China. Endocrine. 2021;73(2):447–454. doi:10.1007/s12020-021-02688-z
- Sulejmanovic M, Begić A, Mujaric-Bousbia F, Salkic S, Ramas A, Ramas A. The relationship between thyroid antibodies and vitamin D level in primary hypothyroidism. Medical Archiv. 2020;74(5):359–362. doi:10.5455/medarh.2020.74.359-362
- Jiang H, Chen X, Qian X, Shao S. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto’s thyroiditis-a meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2022;47(6):767–775. doi:10.1111/jcpt.13605
- Hongyan L, Xiangfeng M, Shoujing L, Lingyu H, Haixia L. Effect of 25 (OH) D on TSH level in patients with type 2 diabetes mellitus. J Weifang Med Coll. 2021;43(05):352–354. doi:10.16846/j.issn.1004-3101.2021.05.009
- Li W, Huanfa Z, Huirong Z. Correlation between serum 25-hydroxyvitamin D level and thyroid function in patients with type 2 diabetes. J Hubei Univ National. 2021;38(03):7–10. doi:10.13501/j.cnki.42-1590/r.2021.03.002
- Xiaoyan W, Yangyang L, Rui L. Analysis of thyroid function status and related factors in patients with type 2 diabetes of different course. Clin Educ General Pract. 2014;12(02):169–171. doi:10.13558/j.cnki.issn1672-3686.2014.02.016
